Abstract
Prebiotic oligosaccharides are thought to provide beneficial effects in the gastrointestinal tract of humans and animals by stimulating growth of selected members of the intestinal microflora. Another means by which prebiotic oligosaccharides may confer health benefits is via their antiadhesive activity. Specifically, these oligosaccharides may directly inhibit infections by enteric pathogens due to their ability to act as structural mimics of the pathogen binding sites that coat the surface of gastrointestinal epithelial cells. In this study, the ability of commercial prebiotics to inhibit attachment of microcolony-forming enteropathogenic Escherichia coli (EPEC) was investigated. The adherence of EPEC strain E2348/69 on HEp-2 and Caco-2 cells, in the presence of fructooligosaccharides, inulin, galactooligosaccharides (GOS), lactulose, and raffinose was determined by cultural enumeration and microscopy. Purified GOS exhibited the greatest adherence inhibition on both HEp-2 and Caco-2 cells, reducing the adherence of EPEC by 65 and 70%, respectively. In addition, the average number of bacteria per microcolony was significantly reduced from 14 to 4 when GOS was present. Adherence inhibition by GOS was dose dependent, reaching a maximum at 16 mg/ml. When GOS was added to adhered EPEC cells, no displacement was observed. The expression of BfpA, a bundle-forming-pilus protein involved in localized adherence, was not affected by GOS, indicating that adherence inhibition was not due to the absence of this adherence factor. In addition, GOS did not affect autoaggregation. These observations suggest that some prebiotic oligosaccharides may have antiadhesive activity and directly inhibit the adherence of pathogens to the host epithelial cell surface.
Prebiotic oligosaccharides are defined as nondigestible food ingredients that provide beneficial effects to the host by stimulating the growth of selected microbial members of the gastrointestinal tract (16). Among the colonic bacteria capable of metabolizing prebiotic oligosaccharides and whose growth is stimulated are species of Lactobacillus and Bifidobacterium. The presence of these bacteria in the gastrointestinal tract has long been associated with various health benefits (53). For example, lactobacilli and bifidobacteria, by virtue of their ability to produce organic acids and other antagonistic agents, may directly inhibit opportunistic pathogens (18, 48, 58). Also, enrichment of lactobacilli and bifidobacteria by prebiotics may indirectly result in the displacement of less-desirable members of the competing microflora (14, 17).
Recently, another mechanism by which prebiotics might interfere with and inhibit infectious bacteria has been proposed (5, 17, 22, 26). Specifically, this model is based on the observation that certain exogenous oligosaccharides structurally resemble the receptor sites coating the intestinal epithelial cells to which intestinal pathogens recognize and adhere (31). Accordingly, these oligosaccharides may act as molecular receptor decoys or antiadhesives that can competitively inhibit bacterial adherence. Simply stated, rather than binding to host cell surface oligosaccharides and initiating the infection process, the pathogen would instead bind to the soluble decoy oligosaccharides and be displaced or flushed from the gastrointestinal tract.
Some intestinal pathogens, such as enteropathogenic Escherichia coli (EPEC), express multiple oligosaccharide-binding proteins or adhesins that enable them to adhere to distinct oligosaccharide receptor sites located at the host cell surface. In particular, EPEC strains are model organisms for adherence studies due to the distinctive manner in which they attach. Ultimately, the multistep infection process generates attaching-and-effacing lesions on the brush border surface of the small intestine (35). Lesion formation by EPEC involves an initial nonintimate “localized adherence” (LA) characterized by the formation of three-dimensional microcolonies, type III secretion, and effector protein translocation to the host cell, followed by microvillus effacement, and finally intimate attachment and pedestal formation (15, 27, 50). Recently, Cleary et al. (8) suggested that bundle-forming pili (BFP) are primarily responsible for initial brush border cell attachment and that EspA filaments are probably involved in atypical EPEC attachment when BFP is absent. Thus, the initial nonintimate adherence is a key aspect of EPEC pathogenesis, since it is the first step in infection. Preventing this initial adherence may ultimately inhibit the infection process.
Several different oligosaccharides have been shown to have antiadhesive activity (2, 6, 13). Many of these oligosaccharides have been isolated from natural sources, such as human breast milk, whereas others have been synthesized based on the known oligosaccharide components of glycolipids and glycoproteins that line the cell surface of the gastrointestinal tract. However, the antiadhesive potential of commercially available prebiotic oligosaccharides, some of which resemble those found in human breast milk, have not yet been studied. Therefore, the aim of the present study was to examine the ability of various prebiotic oligosaccharides to inhibit the adherence of an EPEC strain to HEp-2 and Caco-2 tissue culture cell lines.
MATERIALS AND METHODS
Prebiotic oligosaccharides.
The prebiotic oligosaccharides used in the present study are described in Table 1. They include galactooligosaccharides (GOS; purified from Oligomate 55 [Yakult, Tokyo, Japan]), raffinose (Sigma Chemical Co., St. Louis, MO), and four fructan-type oligosaccharides: inulin (Sigma), Raftiline HP (an inulin-containing product produced by Orafti Active Food Ingredients, Tienen, Belgium), Nutraflora (FOS; GTC Nutrition Company, Westminster, CO), and Raftilose P95 (Orafti). The inulin obtained from Sigma (Inu-S) and from Orafti (Inu-O) are both extracted from chicory root and vary in the degree of polymerization only slightly, with both averaging more than 23 fructose monomers per molecule. Nutraflora is synthesized enzymatically to generate fructooligosaccharide (FOS) species consisting of a glucose (G) monomer linked to two, three, or four fructose (F) molecules. This product is referred to as the GFn type of FOS. In contrast, Raftilose P95 is derived by partial hydrolysis of inulin, yielding an FOS product containing ca. 75% of fructose-only chains (FFn) with degrees of polymerization ranging from 2 to 7. The balance (ca. 25%) is of the GFn form. The disaccharide, lactulose (Sigma), and the trisaccharide, raffinose (Sigma), were also used due to their known activity as prebiotics (9).
TABLE 1.
Structure and composition of commercial oligosaccharides used in this study
| Oligosaccharide | Chemical structure | Composition (%)a |
|---|---|---|
| Inulin (Inu-S) | α-Glu-(1→2)-[β-Fru-(1→2)]>20 | 95 |
| Raftiline HP (Inu-O) | α-Glu-(1→2)-[β-Fru-(1→2)]>20 | 100 |
| Nutraflora (GFn) | α-Glu-(1→2)-[β-Fru-(1→2)]2-4 | 97 |
| Raftilose P95 (FFn) | β-Fru-(1→2)-[β-Fru-(1→2)]1-10 and α-Glu-(1→2)-[β-Fru-(1→2)]2-10 | 75 and 25 |
| Lactulose | β-Gal-(1→4)-β-Fru | 95 |
| Raffinose | α-Gal-(1→6)-α-Glu-(1→2)-β-Fru | 98 |
| Oligomate (GOS) | α-Glu-(1→4)-[β-Gal-(1→6)]2-4 | 100b |
From the manufacturer.
After purification.
GOS purification.
Commercial GOS, Oligomate 55, contains 55% GOS with glucose, galactose, and lactose making up the remaining 45%. Because the latter sugars could interfere with adherence assays, the Oligomate 55 was purified by size exclusion chromatography using Sephadex G-10 (Sigma). Fractions were eluted with distilled water, and those that contained carbohydrate were identified by using a refractometer (Bausch & Lomb, Rochester, NY). Portions were applied to aluminum-backed silica gel 60 thin-layer chromatography plates (Whatman, Ltd., Kent, England). The plates were developed by using 1-butanol-2-propanol-water (3:12:4, by volume) and sprayed with 50% ethanolic sulfuric acid and heated to 115°C for 5 min to detect carbohydrates. The oligosaccharide-containing fractions (retention factor [Rf] of ca. 0.30 to 0.67), free of mono- and disaccharides (Rf of >0.75), were then pooled and lyophilized (FTS Systems, Inc., Stone Ridge, NY).
Bacterial strains and culture conditions.
EPEC strain E2348/69 (O127:H6) and its isogenic bfpA mutant, UMD901, were obtained from M. Donnenberg (University of Maryland School of Medicine, Baltimore). Prior to each experiment, a freezer stock was plated onto tryptic soy agar (TSA; Difco, Detroit, MI) with glucose and grown overnight at 37°C. A single colony was inoculated into 4 ml of tryptic soy broth without glucose (TSB; Difco) and incubated overnight at 37°C without shaking. After the overnight incubation, the cultures were inoculated at 1% (vol/vol) into Dulbecco modified Eagle medium (DMEM; Gibco-BRL, Grand Island, NY) supplemented with HEPES buffer that was preequilibrated overnight in tissue culture conditions (CO2 incubator set at 5% CO2 and 95% air and 95% relative humidity). Cells were incubated 80 min in DMEM prior to the start of each experiment to induce the genes necessary for localized adherence (57).
Tissue culture conditions.
HEp-2 (CCL-23) and Caco-2 cells (HTB37) were obtained from the American Type Culture Collection (Manassas, Virginia) and maintained under tissue culture conditions in minimal essential medium (Gibco-BRL) supplemented with 10% fetal bovine serum (Gibco-BRL), and minimal essential medium supplemented with 20% fetal bovine serum, respectively. For the inhibition assays, subconfluent monolayers of HEp-2 cells were harvested with 0.25% (vol/vol) trypsin in FC buffer (0.14 M NaCl, 5.0 mM KCl, 20.0 mM Tris-HCl, 5.0 Tris base, 0.5 mM EDTA [pH 7.2]) and seeded onto 12-mm-diameter glass coverslips in 24-well tissue culture plates at approximately 5 × 104 HEp-2 cells per well. Plates were incubated under tissue culture conditions at 37°C for use in experiments the following day. Fourteen- to fifteen-day-postconfluent monolayers of Caco-2 cells were prepared in 24-well tissue culture dishes, as described previously (8, 33), with or without 12-mm-diameter coverslips, except that seeding was at 3.6 × 105 viable cells per well. The growth medium was replaced every other day until their use in assays.
HEp-2 inhibition assays.
EPEC E2348/69 and subconfluent HEp-2 cells were prepared as described above. Inhibition assays were modified slightly from those reported previously (55). Briefly, mannose was added to 4 ml of a previously prepared bacterial culture at a final concentration of 1% (vol/vol) to inhibit type I-mediated bacterial attachment (9). Each prebiotic was added at a final concentration of 16 mg/ml immediately before the assay, unless otherwise indicated. After washing the tissue culture monolayers with phosphate-buffered saline (PBS), 900 μl of the supplemented bacterial culture (containing ca. 107 cells) was added to each well. Tissue culture plates were incubated at 37°C in a CO2 incubator for 30 min. Preliminary experiments revealed that a 30-min incubation time was sufficient to measure initial adherence, as indicated by the formation of microcolonies (12, 19). The wells were then washed five times with PBS to remove nonadherent EPEC. Coverslips were fixed with methanol for 10 min, allowed to dry, stained with Giemsa stain for 20 min, and observed under a phase contrast microscope with the 100× objective. A minimum of 100 consecutive HEp-2 cells were observed. Cells with microcolonies of EPEC consisting of four or more bacteria were considered positive for having an LA phenotype (57). The number of HEp-2 cells with LA, the number of EPEC per LA microcolony, and the number of microcolonies per HEp-2 cell were determined. Experiments were performed in triplicate and replicated at least three times. For displacement assays, bacteria were incubated with HEp-2 cells for various times (0 to 30 min) before the addition of GOS. Three wells were sacrificed every 10 min after the addition of GOS for a total of 30 min. The results were reported as a percentage of LA on 100 consecutive HEp-2 cells counted. The experiment was performed in triplicate and replicated three times.
Caco-2 inhibition assays.
EPEC E2348/69 and 14- to 15-day-postconfluent Caco-2 cells were prepared as described above. These assays were performed with slight modifications from above. First, the original inoculum size was determined by plating on TSA. Second, the assays were incubated at 37°C under tissue culture conditions for 60 min. Lastly, plate count data using wells without coverslips and microscopy data using wells with coverslips were recorded. Caco-2 cells without coverslips were treated with 0.1% Triton X-100 (Sigma) for 20 min, disrupted by vigorous pipetting, serially diluted, and plated on TSA. Microscopy data was collected by examining five fields of view at ×100 magnification to determine the number of LA microcolonies (≥4 bacteria) present and the number of EPEC organisms per microcolony. Experiments were performed three times, each time in duplicate.
Determination of BfpA expression.
Overnight cultures of EPEC E2348/69 in TSB without glucose were inoculated at 1% into DMEM and incubated under tissue culture conditions for 80 min. GOS was added at various concentrations, followed by incubation for 1 h. As a negative control, the bfpA mutant, UMD901 (10), was grown in LB with nalidixic acid (100 μg/ml), pelleted by centrifugation, resuspended in DMEM, and incubated for 1 h under tissue culture conditions along with the other samples. Cultures were then pelleted by centrifugation at 2,500 × g for 5 min and resuspended in 350 μl of 1% sodium dodecyl sulfate (SDS). Next, 150 μl was added to 50 μl of 4× SDS gel electrophoresis sample buffer (0.25 M Tris-HCl [pH = 6.8], 8% SDS, 40% [vol/vol] glycerol, 0.0008% bromophenol blue, 50 mM dithiothreitol). Samples were denatured by boiling for 10 min. Then, 5 μg of total protein (per lane) was loaded onto a 15% polyacrylamide gel and separated by electrophoresis. Protein concentrations were determined by the bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Samples were transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, MA). The blots were blocked with PBS containing 0.05% (vol/vol) Tween 20 (PBST) and 5% nonfat dried milk and then incubated with polyclonal anti-BfpA (1:15,000 dilution; provided by M. Donnenberg) for 2 h at room temperature. The membranes were washed three times with PBST and incubated with donkey anti-rabbit peroxidase-conjugated antibodies (1:30,000 dilution) for 1.5 h at room temperature. After three washes in PBST, the membranes were developed with enhanced chemiluminescence color development reagents according to the manufacturer (Amersham Biosciences, Pittsburgh, PA). Protein bands were visualized by exposing the membrane to Kodak X-Omat Blue XB-1 film (Eastman-Kodak, Rochester, NY).
Autoaggregation assay.
Aggregation assays were performed as previously described with slight modifications (1). Overnight cultures of E2348/69 and UMD901 grown in TSB and LB with nalidixic acid (100 μg/ml), respectively, were inoculated at 1% into 4 ml of DMEM supplemented with 15 mM HEPES (Gibco-BRL) and incubated under tissue culture conditions for 80 min. Various concentrations of GOS were added, and the cultures were incubated until bacterial aggregates were visible in BFP-expressing strain E2348/69. A 1-ml aliquot of each culture was removed, and the optical density (OD) was measured at 620 nm. The samples were vigorously pipetted for 30 s each, and the OD was measured again. The percentage increase in the OD after pipetting was reported as a quantitative aggregation index.
Scanning electron microscopy.
HEp-2 inhibition assays were performed through the wash step to remove nonadherent bacteria as described above. After being washed with PBS, the cells were fixed with 3% glutaraldehyde in 0.1 M phosphate buffer for 1 h and then rinsed with PBS three times for 10 min each time. A second fixative step was performed by incubating the cells with 1% osmium tetroxide in 0.1 M phosphate buffer for 1 h, followed by another series of three rinses in PBS. The sample was then dehydrated with a series of graded ethanols (25% for 10 min, 50% for 10 min, 75% for 10 min, 95% for 10 min, and 100% ethanol for two 10-min sessions). The samples were dried by critical point drying and sputter coated with gold particles for viewing.
Statistical analysis.
Significant differences between treatments were determined by the general linear model with the least-significant-difference comparison of means at 95% confidence by using SAS version 9.1.
RESULTS
Prebiotic oligosaccharides reduce adherence of EPEC to HEP-2 cells.
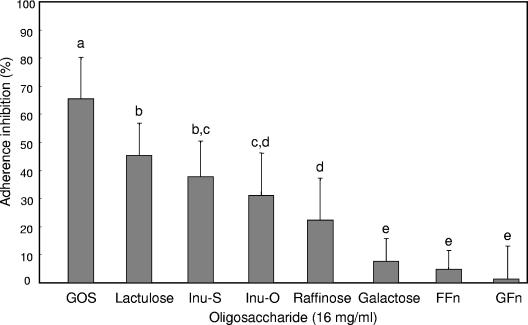
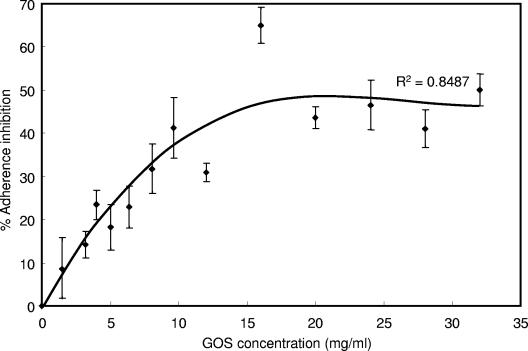
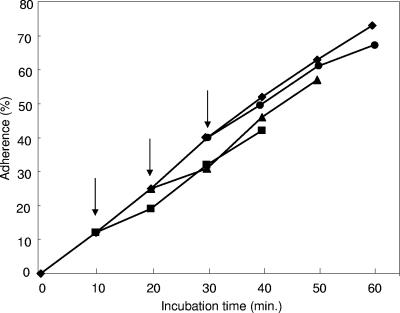
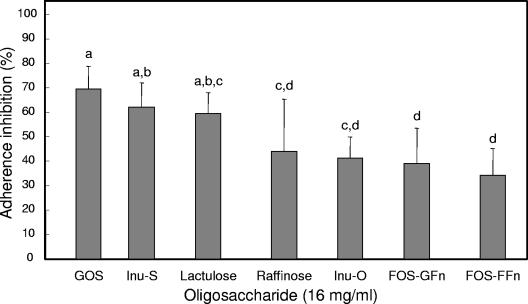
Although several prebiotic sugars inhibited adherence of EPEC to HEp-2 cells, purified GOS was the most inhibitory (P < 0.05) compared to the other oligosaccharides tested (Fig. 1). Treatment with GOS resulted in an adherence inhibition of 65%, compared to 45% by lactulose, 38 and 31% by the two inulin products, respectively, and 22% by raffinose. Little inhibition (<5%) was observed for the two FOS products, Raftilose (FFn type) and Nutraflora (GFn type). However, unpurified GOS gave a slightly higher adherence inhibition, which may be attributed to the presence of galactose (data not shown). When tested alone, galactose showed ca. 8% adherence inhibition. In addition, as the concentration of GOS was increased from 0 to 32 mg/ml, adherence inhibition was saturable, reaching a plateau after 16 mg/ml (Fig. 2). In contrast, GOS did not significantly reduce the presence of EPEC microcolonies when added 10, 20, or 30 min after infection (Fig. 3).
FIG. 1.
Inhibition of E2348/69 adherence to HEp-2 tissue culture cells by prebiotic oligosaccharides. Bacteria were incubated with HEp-2 cells for 30 min in the presence of 16 mg of GOS, lactulose, Inu-S, Inu-O, raffinose, galactose, FOS-FFn, or FOS-GFn/ml. The data were collected by counting the number of HEp-2 cells of 100 with localized adherence. Different letters above the bars indicate statistically significant differences (P < 0.05) in means (n ≥ 9). Lines represent the standard deviations of the means.
FIG. 2.
Effect of GOS concentration on the adherence inhibition of EPEC strain E2348/69 to HEp-2 cells. Lines represent the standard errors of the means (n ≥ 9).
FIG. 3.
Displacement of EPEC strain E2348/69 by GOS. Bacteria were incubated with HEp-2 cells for 60 min (⧫). To parallel mixtures, GOS was added after 10 (▪), 20 (▴), and 30 min (•), as indicated by the arrows, and incubated for an additional 30 min. The percent adherence was determined from the number of HEp-2 cells out of 100 with localized adherence.
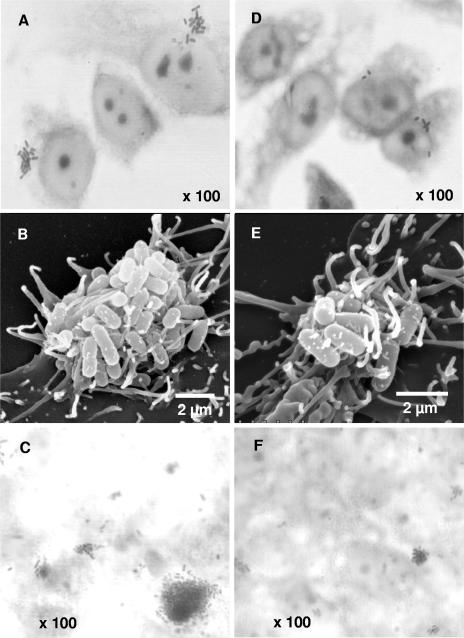
The ability of prebiotic oligosaccharides to reduce the number of EPEC per microcolony on HEp-2 cells was also observed (Table 2 and Fig. 4). Without any additions, the average EPEC microcolony (as observed by phase-contrast microscopy) contained about 15 bacteria. Whereas Nutraflora (GFn), Raftilose (FFn), and galactose had no significant effect on the number of bacteria per microcolony, Inu-O, lactulose, and Inu-S significantly reduced microcolony size to about eight cells per microcolony (P < 0.05). In contrast, the GOS treatment gave the greatest reduction (>70%), with only about four EPEC organisms per microcolony. Although scanning electron microscopy also revealed fewer cells per microcolony in the GOS treatment, the microcolonies did not appear to be structurally different from the control cells (Fig. 4).
TABLE 2.
Effect of oligosaccharides on EPEC strain E2348/69 microcolony size and formation on HEp-2 cellsa
| Oligosaccharide | Mean no. of EPEC organisms/microcolony ± SD | Mean no. of microcolonies/HEp-2 cell ± SD |
|---|---|---|
| Control | 14.5 ± 2.2A | 2.0 ± 0.4A |
| Nutraflora (GFn) | 13.2 ± 2.1A,B | 1.9 ± 0.4A |
| Raftilose (FFn) | 12.4 ± 1.9A,B | 2.1 ± 0.3A |
| Galactose | 12.2 ± 1.5A,B | 1.5 ± 0.1B |
| Raffinose | 11.6 ± 3.9B | 1.4 ± 0.1B |
| Raftiline (Inu-O) | 8.6 ± 3.3C | 1.5 ± 0.3B |
| Inulin (Inu-S) | 7.6 ± 2.6C | 1.5 ± 0.1B |
| Lactulose | 8.0 ± 3.8C | 1.4 ± 0.2B |
| GOS | 4.2 ± 1.8D | 0.9 ± 0.3C |
Values shown are means±the standard deviations (n = 9). Different superscript letters associated with values in the same column indicate statistically significant differences (P < 0.05).
FIG. 4.
Phase-contrast (A, C, D, and F) and scanning electron (B and E) micrographs showing representative fields of tissue culture cells challenged with EPEC strain E2348/69 in the absence (A to C) and presence (D to F) of GOS (16 mg/ml). HEp-2 cells are shown in panels A, B, D, and E; Caco-2 cells are shown in panels C and F.
The total number of microcolonies observed per HEp-2 cell was also measured (Table 2). These results were similar to those described above in that GOS exhibited the greatest decrease in microcolonies per HEp-2 cell. Once again, GOS was significantly different from the control and the remaining prebiotic oligosaccharides.
Prebiotic oligosaccharides also reduce adherence of EPEC to Caco-2 cells.
Adherence inhibition on Caco-2 cell was determined by cultural enumeration and microscopic methods. By the former method, all of the oligosaccharides tested reduced the adherence of EPEC to Caco-2 cells, with inhibition activities ranging from 40 to 70% (Fig. 5). As for the HEp-2 cells, adherence inhibition on Caco-2 cells was greatest when GOS was present. However, inhibition by Inu-S and lactulose were not significantly different from that by GOS. Also, the two FOS products were the least inhibitory.
FIG. 5.
Inhibition of EPEC strain E2348/69 adherence to Caco-2 tissue culture cells by GOS, Inu-S, lactulose, raffinose, Inu-O, FOS-GFn, and FOS-FFn. Bacteria were incubated with Caco-2 cells plus 16 mg of oligosaccharide/ml for 60 min. The data were collected by plate counting. Different letters above bars indicate statistically significant differences (P < 0.05) in means (n = 6). Lines represent the standard deviations of the means.
As expected, the microscopic results for Caco-2 inhibition assays were similar to the cultural enumeration data, except that the overall extent of inhibition was somewhat lower. Again, GOS had the greatest ability to reduce EPEC localized adherence. However, all of the prebiotics except raffinose significantly reduced the ability of EPEC to adhere in a LA pattern to Caco-2 tissue culture cells compared to the control (Table 3). In addition to reducing the number of EPEC adhered to Caco-2 cells, GOS also reduced the average number of bacteria per microcolony from about 14 to 6, a finding similar to that observed for HEp-2 cells (data not shown).
TABLE 3.
Inhibition of localized adherence of EPEC strain E2348/69 on Caco-2 cells by oligosaccharides
| Oligosaccharide | Mean total EPEC ± SDa | % Adherence inhibitionb |
|---|---|---|
| Control | 160.5 ± 23.8A | |
| Raffinose (FFn) | 143.3 ± 15.1A,B | 11 |
| Raftiline (Inu-O) | 131.0 ± 27.3B | 18 |
| Nutraflora (GFn) | 109.7 ± 15.6C | 32 |
| Lactulose | 99.3 ± 21.7C,D | 38 |
| Raftilose (FFn) | 93.9 ± 6.4C,D | 42 |
| Inulin (Inu-S) | 92.8 ± 11.3C,D | 42 |
| GOS | 83.3 ± 10.3D | 48 |
That is, the mean number of microcolonies±the standard deviation (n = 6) in five microscopic fields. Different superscript letters associated with values indicate statistically significant differences (P < 0.05).
Adherence inhibition was calculated using five fields of view, as follows: [(mean number of microcolonies of the control − mean number of microcolonies of the treatment group)/mean number of microcolonies of the control] × 100.
GOS does not affect autoaggregation of EPEC.
When EPEC stains that express BFP are grown under tissue culture conditions, they aggregate to form large clusters that can be viewed microscopically and effectively quantified (1, 4). The wild-type E2348/69 and the bfpA mutant UMD901 strains were grown under optimal conditions for BFP expression and tested for aggregation by measuring the OD at 620 nm of a 3-h culture before and after vigorous agitation. The percent increase after agitation represents the aggregation index. Table 4 shows that the aggregation index for the wild-type strain E2348/69 was only slightly affected by GOS. As expected, the bfpA strain was unable to form aggregates.
TABLE 4.
Effect of GOS on the autoaggregation of EPEC strain E2348/69
| Strain | GOS concn (mg/ml) | Aggregation index ± SDa | % of wild type |
|---|---|---|---|
| E2348/69 (WT)b | 0 | 49.8 ± 0.03 | 100 |
| 4 | 53.2 ± 0.7 | 106.8 | |
| 8 | 52.2 ± 0.3 | 104.9 | |
| 12 | 52.9 ± 2.3 | 106.1 | |
| 16 | 56.4 ± 3.3 | 113.2 | |
| 20 | 55.0 ± 1.1 | 110.5 | |
| 24 | 55.3 ± 1.4 | 111.0 | |
| 28 | 49.6 ± 0.6 | 99.5 | |
| UMD901 (bfpA) | 0 | 1.80 ± 2.5 | 3.6 |
The values shown are mean values from replicates ± the standard deviation.
WT, wild type.
Expression of EPEC BfpA is not affected by GOS.
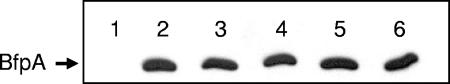
The possibility that adherence inhibition by oligosaccharides may be due to modulation at the genetic level, rather than physical inhibition, has been suggested (28, 56). That is, the putative antiadhesive oligosaccharides may repress the synthesis of adhesins, thereby accounting for the observed reduced adherence rates. To test this hypothesis for GOS, the expression of BfpA was examined via Western blot analysis for cells grown in the presence or absence of GOS. No difference in BfpA expression was found for EPEC cells, even when GOS was added at high concentrations (Fig. 6). In addition, when HEp-2 cells that had been infected with EPEC (grown in the presence of GOS) were examined by scanning electron microscopy, adherence still occurred (albeit at lower rates than for the control) for the GOS-treated samples (Fig. 4).
FIG. 6.
Effect of GOS on expression of BfpA. The bfpA mutant strain, UMD901, was used as a negative control (lane 1). EPEC E2348/69 was grown in DMEM with GOS at concentrations of 0 mg/ml (lane 2), 4 mg/ml (lane 3), 8 mg/ml (lane 4), 12 mg/ml (lane 5), and 16 mg/ml (lane 6). Protein separation and immunoblot procedures were performed as described in the text.
DISCUSSION
The selective manipulation of the colonic microbial population by prebiotic oligosaccharides and subsequent inhibition of enteric pathogens within the gastrointestinal tract is generally thought to be mediated by one of two mechanisms. First, the metabolism of these oligosaccharides by resident lactobacilli and bifidobacteria results in the production of organic acids and other antagonistic agents that are inhibitory to many enteric pathogens (14, 16, 18). Second, prebiotic oligosaccharides provide selective advantages within the highly competitive gastrointestinal tract for organisms that have the metabolic capacity to utilize these nutrients at the expense of organisms that do not (53). However, prebiotics may have a more direct effect on pathogens within this environment. Specifically, it has been suggested that oligosaccharides may act as antiadhesives against bacterial adherence by mimicking the host cell receptor sites to which intestinal pathogens recognize and adhere (31). Our results indicate that prebiotic oligosaccharides can significantly inhibit the adherence of EPEC to tissue culture cells due to their antiadhesive activity.
The observed differences in adherence inhibition among the various oligosaccharides were likely due to their structural differences. The prebiotic that had the greatest inhibitory effect in both the HEp-2 and Caco-2 cell assays was GOS. Commercial GOS is synthesized from lactose via a transgalactosylation reaction, yielding several GOS species containing two, three, four, or five galactose units and a terminal glucose (11). Naturally occurring GOS with galactosyl backbones have previously been shown to inhibit adherence of pathogens (36, 40, 51). For example, human breast milk oligosaccharides have been shown to inhibit the adherence of Campylobacter jejuni, E. coli, Helicobacter pylori, and other pathogens to tissue culture cells (7, 38-42). These milk-borne oligosaccharides are comprised of 12 core structures that are derived from glucose, galactose, and N-acetylglucosamine and are often fucosylated or sialylated (52). Several studies have attributed antiadherence activity to these fucosylated or sialylated milk oligosaccharide fractions (10, 34, 47). In the present study, relatively high concentrations of free GOS were necessary to obtain significant adherence inhibition. Therefore, further chemical or enzymatic modification of GOS by fucosylation or sialylation would be expected to enhance adherence inhibition. However, other unmodified oligosaccharides, including globotriose (Galα1-4Galβ1-4Glu) and globotetraose (GalNAcβ1-3Galα1-4Galβ1-4Glu), have been reported to inhibit the adherence of Shiga toxins produced by enterohemorrhagic E. coli strains that infect both humans and pigs and thus are potential anti-infective agents against these toxins (43, 44). Large-scale production of globotriose for therapeutic use against Shiga toxins has recently been reported (59). Considering the observed inhibitory effects of GOS, the true EPEC receptor may, therefore, contain multiple galactose (or lactose) residues, although N-acetyllactosamine, N-acetylgalatosamine, and other modified sugar moieties are also probably involved (24, 49, 56).
Lactulose, which also reduced adherence of EPEC to both HEp-2 and to Caco-2 cells (by 45 and 65%, respectively), is a nondigestable disaccharide containing a single galactose moiety. However, it appears that it still may act as a molecular mimic. Galactose, itself, had no effect, indicating that the chain length and/or the nature of sugar linkage are important factors. In contrast, the oligosaccharides that had the lowest adherence inhibition for both HEp-2 and Caco-2 cells were of the FOS types (GFn and FFn). Although an in vivo study using mice showed that dietary oligofructose (FFn) and inulin protected female mice from the enteric pathogens Salmonella enterica serovar Typhimurium and Listeria monocytogenes, this effect was attributed to the enhanced immune functions generated by the resident microflora rather than to an antiadhesive activity (6).
EPEC are known for their initial adherence to tissue culture cells, which manifests in localized adherent microcolonies that contain four or more bacteria per microcolony (57). Our data indicated that GOS not only reduced the adherence of EPEC microcolonies to HEp-2 and Caco-2 cells but also had the greatest ability to reduce the number of bacteria per microcolony. This suggests that GOS may be targeted to the virulence factor that is responsible for microcolony formation. BFP have been shown to mediate bacterium-bacterium interactions and microcolony formation (20), in addition to microcolony dispersion (4, 30). Interestingly, BFP have also been implicated in the initial adherence of EPEC to host epithelial cells (12, 20, 54), while other studies have suggested, at least for human intestinal organ tissue, that BFP are not involved in initial adherence (23). Other adherence factors, such as the type III secretion system protein EspA, flagella, the toxin lymphostatin, and translocated protein intimin have been implicated in the initial adherence of EPEC (3, 12, 20, 21, 29, 54). However, our data support the role of BFP as a mediator of microcolony formation and also as an initial adherence factor in EPEC pathogenesis, because GOS not only reduced the number of bacteria per microcolony, it also reduced the overall adherence of EPEC to tissue culture cells.
Although it was expected that aggregate formation would be reduced by GOS, autoaggregation rates of EPEC were not affected by GOS, at least in the absence of tissue culture cells. However, when examined microscopically, the aggregates formed in the presence of GOS were considerably smaller and appeared less dense, albeit more abundant, than those aggregates formed in the absence of GOS. Thus, although the expected decrease in autoaggregation by GOS did not occur, it remains possible that GOS changed the manner in which aggregate formation occurred such that BFP-mediated adherence of EPEC was still inhibited. The possibility also exists that adherence inhibition by GOS does occur via a decrease in aggregate formation, but only in the presence of tissue culture cells.
To examine the ability of GOS to displace already adhered EPEC microcolonies, we challenged EPEC-infected HEp-2 cells with GOS at various times after infection. No matter the length of the infection process, only slight reductions in adherence occurred. Thus, GOS may not be effective at dislodging previously attached EPEC. In contrast, when HEp-2 cells were incubated with GOS 30 min prior to infection with EPEC, adherence was inhibited, albeit at a somewhat lower level than with GOS addition at the time of infection (data not shown).
Despite the numerous reports that support the role of glycolipids, glycoproteins, and soluble oligosaccharides as molecular decoys to host cell surface oligosaccharides (2, 10, 25, 31, 32, 34, 37-39, 41, 43, 45, 46), there are also reports that suggest that reduced adherence is due to reduced levels of adherence proteins rather than by physical inhibition (28, 56). Our results demonstrated, however, that the expression of BfpA was not affected by the presence of GOS, even at high concentrations. In addition, when tissue culture cells that had been infected with EPEC were observed by scanning electron microscopy, the manner in which EPEC adhered to target cells was the same in the presence or in the absence of GOS. Noticeably, however, the GOS-supplemented specimen showed a reduced number of bacteria per microcolony, indicating that GOS affected the amount of adherent bacteria but not the manner in which the adherence occurred. Thus, although we did not measure the expression of other putative adhesins, such as EspA and intimin, our data support the role of GOS as an antiadhesive rather than affecting the phenotypic regulation of BfpA-mediated adherence. Moreover, in experiments with bfpA and espA mutants, a reduction in adherence with GOS was observed only for the espA mutant, suggesting that adherence inhibition by GOS may be mediated via BfpA (data not shown).
In summary, the results of the present study indicate that GOS and perhaps other galactose-containing prebiotic oligosaccharides can act as antiadhesives against EPEC adherence to intestinal epithelial cells. In addition, our data support the role of BFP as an initial adherence factor in EPEC pathogenesis but do not rule out other possible adherence factors.
Acknowledgments
This paper is a contribution from the University of Nebraska Agricultural Research Division, supported, in part, by funds provided through Hatch Act Funds from the U.S. Department of Agriculture (USDA).
This study was supported, in part, by grant 2004-35503-14118 from the USDA National Research Initiative Competitive Grants Program and by a USDA National Needs Fellowship to K.S.
We thank Yakult, GTC Nutrition Company, and Orafti Active Food Ingredients for their donations of the prebiotic oligosaccharides. We are grateful to Michael Donnenberg and his lab for providing the EPEC strains and anti-BFP sera used in this study. We also thank Andy Benson and Rod Moxley for helpful suggestions.
Editor: F. C. Fang
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Anantha, R. P., K. D. Stone, and M. S. Donnenberg. 2000. Effects of bfp mutations on biogenesis of functional enteropathogenic Escherichia coli type IV pili. J. Bacteriol. 182:2498-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson, M., O. Medalia, L. Schori, and I. Ofek. 1979. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking bacterial adherence with methyl alpha-mannopyranoside. J. Infect. Dis. 139:329-332. [DOI] [PubMed] [Google Scholar]
- 3.Badea, L. S., S. Doughty, L. Nicholls, J. Sloan, R. M. Robins-Browne, and E. L. Hartland. 2003. Contribution of Efa1/LifA to the adherence of enteropathogenic Escherichia coli to epithelial cells. Microb. Pathog. 34:205-215. [DOI] [PubMed] [Google Scholar]
- 4.Bieber, D., S. W. Ramer, C. Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 5.Bruck, W. B., S. Kelleher, G. R. Gibson, K. E. Nielsen, D. E. W. Chatterton, and B. Lonnerdal. 2003. rRNA probes used to quantify the effects of glycomacropeptide and alpha-lactalbumin supplementation on the predominant groups of intestinal bacteria of infant rhesus monkeys challenged with enteropathogenic Escherichia coli. J. Pediatr. Gastroenterol. Nutr. 37:273-280. [DOI] [PubMed] [Google Scholar]
- 6.Buddinton, K. K., J. B. Donahoo, and R. K. Buddinton. 2002. Dietary oligosaccharides and inulin protect mice from enteric and systemic pathogens and tumor inducers. J. Nutr. 132:472-477. [DOI] [PubMed] [Google Scholar]
- 7.Cervantes, L., D. Newburg, and G. Ruiz-Palacios. 1995. 1-2 Fucosylated chains (H-2 and Lewis) are the main human milk receptor analogs for Campylobacter. Pediatr. Res. 37:171A. [Google Scholar]
- 8.Cleary, J., L.-C. Lai, R. K. Shaw, A. Straatman-Iwanowska, M. S. Donnenberg, G. Frankel, and S. Knutton. 2004. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments, and intimin. Microbiology 150:527-538. [DOI] [PubMed] [Google Scholar]
- 9.Cravioto, A., R. J. Gross, S. M. Scotland, and B. Rowe. 1979. An adhesive factor found in strains of Escherichia coli belonging to the traditional infantile enteropathogenic serotypes. Curr. Microbiol. 3:95-99. [Google Scholar]
- 10.Cravioto, A., A. Tello, H. Villafan, J. Ruiz, S. del Vedovo, and J. R. Neeser. 1991. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J. Infect. Dis. 163:1247-1255. [DOI] [PubMed] [Google Scholar]
- 11.Crittenden, R. G., and M. J. Playne. 1996. Production, properties, and applications of food-grade oligosaccharides. Trends Food Sci. Tech. 7:353-361. [Google Scholar]
- 12.Donnenberg, M. S., J. A. Giron, J. P. Nataro, and J. B. Kaper. 1992. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol. Microbiol. 6:3427-3437. [DOI] [PubMed] [Google Scholar]
- 13.Firon, N., S. Ashkenazi, D. Mirelman, I. Ofek, and N. Sharon. 1987. Aromatic alpha-glycosides of mannose are powerful inhibitors of the adherence of type 1 Escherichia coli to yeast and intestinal epithelial cells. Infect. Immun. 55:474-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fooks, L. J., and G. R. Gibson. 2002. Probiotics as modulators of the gut flora. Br. J. Nutr. 88:S39-S49. [DOI] [PubMed] [Google Scholar]
- 15.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 16.Gibson, G., and M. Roberfroid. 1995. Dietary modulation of human colonic microbiota-introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 17.Gibson, G. R., A. L. McCartney, and R. A. Rastall. 2005. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 93:S31-S34. [DOI] [PubMed] [Google Scholar]
- 18.Gibson, G. R., and X. Wang. 1994. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 77:412-420. [DOI] [PubMed] [Google Scholar]
- 19.Giron, J. A., A. S. Ho, and G. K. Schoolnik. 1993. Characterization of fimbriae produced by enteropathogenic Escherichia coli. J. Bacteriol. 175:7391-7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giron, J. A., A. S. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 21.Giron, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 22.Heerze, L. D., M. A. Kelm, J. A. Talbot, and G. D. Armstrong. 1994. Oligosaccharide sequences attached to an inert support (SYNSORB) as potential therapy for antibiotic-associated diarrhea and pseudomembranous colitis. J. Infect. Dis. 169:1291-1296. [DOI] [PubMed] [Google Scholar]
- 23.Hicks, S., G. Frankel, J. B. Kaper, G. Dougan, and A. D. Phillips. 1998. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect. Immun. 66:1570-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyland, R. M., T. P. Griener, G. L. Mulvey, P. I. Kitov, O. P. Srivastava, P. Marcato, and G. D. Armstrong. 2006. Basis for N-acetyllactosamine-mediated inhibition of enteropathogenic Escherichia coli localized adherence. J. Med. Microbiol. 55:669-675. [DOI] [PubMed] [Google Scholar]
- 25.Jagannatha, H. M., U. K. Sharma, T. Ramaseshan, A. Surolia, and T. S. Balganesh. 1991. Identification of carbohydrate structures as receptors for localized adherent enteropathogenic Escherichia coli. Microb. Pathog. 11:259-268. [DOI] [PubMed] [Google Scholar]
- 26.Jayaraman, N., S. A. Nepogodiev, and J. F. Stoddart. 1997. Synthetic carbohydrate-containing dendimers. Chem. Eur. J. 3:1193-1199. [Google Scholar]
- 27.Kaper, J. B. 1996. Defining EPEC. Rev. Microbiol. Sao Paulo 27:130-133. [Google Scholar]
- 28.Knutton, S., J. Adu-Bobie, C. Bain, A. Phillips, G. Dougan, and G. Frankel. 1997. Down regulation of intimin expression during attaching and effacing enteropathogenic Escherichia coli adhesion. Infect. Immun. 65:1644-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, S. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knutton, S., R. K. Shaw, R. P. Anantha, M. S. Donnenberg, and A. A. Zorgani. 1999. The type IV bundle-forming pilus of enteropathogenic Escherichia coli undergoes dramatic alterations in structure associated with bacterial adherence, aggregation and dispersal. Mol. Microbiol. 33:499-509. [DOI] [PubMed] [Google Scholar]
- 31.Kunz, C., S. Rudloff, W. Baier, N. Klein, and S. Strobel. 2000. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu. Rev. Nutr. 20:699-722. [DOI] [PubMed] [Google Scholar]
- 32.Laegreid, A., A.-B. K. Otnaess, and J. Fuglesang. 1986. Human and bovine milk: comparison of ganglioside composition and enterotoxin-inhibitory activity. Pediatr. Res. 20:416-421. [DOI] [PubMed] [Google Scholar]
- 33.Lee, Y.-K., K.-Y. Puong, A. C. Ouwehand, and S. Salminen. 2003. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J. Med. Microbiol. 52:925-930. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Sosa, S., M.-J. Martin, and P. Hueso. 2002. The sialylated fraction of milk oligosaccharides is partially responsible for binding to enterotoxigenic and uropathogenic Escherichia coli human strains. J. Nutr. 132:3067-3072. [DOI] [PubMed] [Google Scholar]
- 35.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Biannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulvey, G., P. I. Kitov, P. Marcato, D. R. Bundle, and G. D. Armstrong. 2001. Glycan mimicry as a basis for novel anti-infective drugs. Biochimie 83:841-847. [DOI] [PubMed] [Google Scholar]
- 37.Nagahori, N., R. Lee, S. Nishimura, D. Page, R. Roy, and Y. Lee. 2002. Inhibition of adhesion of type 1 fimbriated Escherichia coli to highly mannosylated ligands. Chem. Biochem. 3:836-844. [DOI] [PubMed] [Google Scholar]
- 38.Nascimento de Araujo, A., and L. G. Giugliano. 1999. Human milk fractions inhibit the adherence of diffusely adherent Escherichia coli (DAEC) and enteroaggregative E. coli (EAEC) to HeLa cells. FEMS Microbiol. Lett. 184:91-94. [DOI] [PubMed] [Google Scholar]
- 39.Newburg, D. S. 1997. Do the binding properties of oligosaccharides in milk protect human infants from gastrointestinal bacteria? J. Nutr. 127:980S-984S. [DOI] [PubMed] [Google Scholar]
- 40.Newburg, D. S. 1999. Human milk glycoconjugates that inhibit pathogens. Curr. Med. Chem. 6:117-127. [PubMed] [Google Scholar]
- 41.Otnaess, A. B., A. Laegreid, and K. Ertresvag. 1983. Inhibition of enterotoxin from Escherichia coli and Vibrio cholerae by gangliosides from human milk. Infect. Immun. 40:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmeira, P., S. Carbonare, M. Silva, L. Trabulsi, and M. Carneiro-Sampaio. 2001. Inhibition of enteropathogenic Escherichia coli (EPEC) adherence to HEp-2 cells by bovine colostrum and milk. Allergol. Immunopathol. 29:229-237. [DOI] [PubMed] [Google Scholar]
- 43.Paton, A. W., R. Morana, and J. C. Paton. 2001. Neutralization of Shiga toxins Stx1, Stx2, and Stx2e by recombinant bacteria expressing mimics of globotriose and globotetraose. Infect. Immun. 69:1967-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paton, A. W., R. Morona, and J. C. Paton. 2000. A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nat. Med. 6:265-270. [DOI] [PubMed] [Google Scholar]
- 45.Payne, D., M. O'Reilly, and D. Williamson. 1993. The K88 fimbrial adhesin of enterotoxigenic Escherichia coli binds to B1-linked galactosyl residues in glycosphingolipids. Infect. Immun. 61:3673-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts, J. A., B.-I. Marklund, D. Ilver, D. Haslam, M. B. Kaack, G. Baskin, M. Louis, R. Mollby, J. Winberg, and S. Normark. 1994. The Gal(a1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl. Acad. Sci. USA 91:11889-11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruiz-Palacios, G. M., L. E. Cervantes, P. Ramos, B. Chavez-Munguis, and D. S. Newburg. 2003. Campylobacter jejuni binds intestinal H(O) antigen (Fuc α1, 2Gal β1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J. Biol. Chem. 278:14112-14120. [DOI] [PubMed] [Google Scholar]
- 48.Sanders, M. E. 1993. Effect of consumption of lactic cultures on human health. Adv. Food Nutr. Res. 37:67-130. [DOI] [PubMed] [Google Scholar]
- 49.Scaletsky, I. C. A., S. R. Milani, L. R. Trabulsi, and L. R. Travassos. 1988. Isolation and characterization of the localized adherence factor of enteropathogenic Escherichia coli. Infect. Immun. 56:2979-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scaletsky, I. C. A., M. L. M. Silva, and L. R. Trabulsi. 1984. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect. Immun. 45:534-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharon, N., and I. Ofek. 2000. Safe as mother's milk: carbohydrates as future anti-adhesion drugs for bacterial diseases. Glycoconj. J. 17:659-664. [DOI] [PubMed] [Google Scholar]
- 52.Stahl, B., S. Thurl, J. Zeng, M. Karas, F. Hillenkamp, M. Steup, and G. Sawatzki. 1994. Oligosaccharides from human milk as revealed by matrix-assisted laser desorption/ionization mass spectrometry. Anal. Biochem. 223:218-226. [DOI] [PubMed] [Google Scholar]
- 53.Tannock, G. W. 2002. Probiotics and prebiotics: where are we going?, p. 1-40. In G. W. Tannock (ed.), Probiotics and prebiotics: where are we going? Caister Academic Press, London, England.
- 54.Tobe, T., and C. Saskawa. 2002. Species-specific cell adhesion of enteropathogenic Escherichia coli is mediated by type IV bundle-forming pili. Cell Microbiol. 4:29-42. [DOI] [PubMed] [Google Scholar]
- 55.Vanmaele, R. P., and G. Armstrong. 1997. Effect of carbon source on localized adherence of enteropathogenic Escherichia coli. Infect. Immun. 65:1408-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanmaele, R. P., L. D. Heerze, and G. D. Armstrong. 1999. Role of lactosyl glycan sequences in inhibiting enteropathogenic Escherichia coli attachment. Infect. Immun. 67:3302-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vuopio-Varkila, J., and G. K. Schoolnik. 1991. Localized adherence by enteropathogenic Escherichia coli is an inducible phenotype associated with the expression of new outer membrane proteins. J. Exp. Med. 174:1167-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yildirim, Z., and M. G. Johnson. 1998. Characterization and antimicrobial spectrum of bifidocin B, a bacteriocin produced by Bifidobacterium bifidum NCFB 1454. J. Food Prot. 61:47-51. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, J., P. Kowal, X. Chen, and P. G. Wang. 2003. Large-scale synthesis of globotriose derivatives through recombinant Escherichia coli. Org. Biomol. Chem. 1:3048-3053. [DOI] [PubMed] [Google Scholar]