Abstract
The recombinant protective antigen (rPA) of Bacillus anthracis is a promising anthrax vaccine. We compared serum immunoglobulin G levels and toxin-neutralizing antibody titers in rabbits following delivery of various doses of vaccine by microneedle-based intradermal (i.d.) delivery or intramuscular (i.m.) injection using conventional needles. Intradermal delivery required less antigen to induce levels of antibody similar to those produced via i.m. injection during the first 2 weeks following primary and booster inoculation. This dose-sparing effect was less evident at the later stages of the immune response. Rabbits immunized i.d. with 10 μg of rPA displayed 100% protection from aerosol spore challenge, while i.m. injection of the same dose provided slightly lower protection (71%). Groups immunized with lower antigen doses were partially protected (13 to 29%) regardless of the mode of administration. Overall, our results suggest rPA formulated with aluminum adjuvant and administered to the skin by a microneedle-based device is as efficacious as i.m. vaccination.
In the autumn of 2001, anthrax spores were intentionally released through the U.S. mail. This bioterror attack resulted in 11 cases of cutaneous anthrax and 11 cases of inhalational anthrax, 5 of which were fatal (7, 9). There has been an unprecedented level of public and private support for the development of new means of preventing and treating anthrax during the years following these attacks. Although antibiotics are nearly 100% effective in treating the cutaneous form of the disease, the case fatality rate for inhalational anthrax was estimated to be 75% or higher, even in the presence of supportive care and postexposure antibiotic treatment (information found at the CDC website [http://www.bt.cdc.gov/agent/anthrax/faq/signs.asp]). A recombinant form of the Bacillus anthracis protective antigen (rPA) is a candidate for replacement of Anthrax Vaccine Adsorbed (BioThrax), the currently licensed anthrax vaccine. Proposed applications of the rPA vaccine include prophylactic vaccination as well as therapeutic postexposure use in combination with antibiotics (5). Numerous preclinical studies have demonstrated that the rPA vaccine can provide complete protection against lethal inhalational anthrax (4, 8, 11, 13, 14, 18, 23). Results of phase I clinical trials suggest that the vaccine is safe and immunogenic following intramuscular (i.m.) injection in humans (6).
Most licensed and new vaccines under clinical development, including rPA, are administered by i.m. or subcutaneous injection using conventional needles and syringes. However, recent studies demonstrate that vaccine delivery to the skin can increase the magnitude of the immune response and, in some cases, do so using less vaccine than required with i.m. injection (1, 2, 10, 12, 18-20, 22). For example, clinical studies evaluating intradermal (i.d.) delivery of influenza vaccine have suggested that dose sparing relative to i.m. administration can be achieved (1, 10). Although conventional needles can be used for i.d. delivery, the injection method (the Mantoux technique) requires extensive training and is difficult to perform. Furthermore, it is difficult to precisely control the injection depth using this technique, which often results in the misdirection of a portion of the administered dose into the poorly immune-reactive subcutaneous tissue underlying the skin or leakage of the dose from the injection site after removal of the large-bore needle. We are developing microneedle-based delivery systems for epidermal and dermal administration of vaccines (3, 16-18). These microneedle-based devices accurately deposit the vaccine to a defined depth within the skin. Using these devices, we previously reported that rabbits were completely protected against inhalational anthrax following i.d. administration of three 50-μg doses of rPA (18). Here, we compared microneedle-based i.d. delivery to i.m. injection using graded doses of rPA. We used a dose range (10, 0.2, or 0.08 μg of rPA) that was previously shown to provide 100% survival at the highest dose, 83% survival at the intermediate dose, and 33% survival at the lowest dose following two i.m. inoculations of rPA plus adjuvant (13). Our results suggest that i.d. delivery enables vaccine dose sparing during the early stages of the immune response and that similar levels of protection against aerosol spore challenge can be achieved by this new route of administration and by conventional i.m. injection.
MATERIALS AND METHODS
Animals and immunizations.
Rabbit studies were conducted in accordance with U.S. Department of Agriculture and National Institutes of Health guidelines for the care and use of animals and under Institutional Animal Care and Use Committee-approved protocols. Rabbits were housed at Provident Preclinical, Inc. (Doylestown, PA) for immunizations before being transferred to the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID; Fort Detrick, MD) for spore challenge.
Female New Zealand White rabbits (Charles River Laboratories, Wilmington, MA) were immunized with 10, 0.2, or 0.08 μg of rPA via either i.d. or i.m. injection. The rPA protein was provided by VaxGen, Inc. (Brisbane, CA) and was mixed with Alhydrogel (E.M. Sergeant Pulp and Chemical Co., Inc, Clifton, NJ) just before injection. The amount of Alhydrogel per dose of vaccine was constant across all groups (35 μl of Alhydrogel containing 10 mg/ml Al = 0.35 mg Al per dose), while the amount of rPA and diluent per dose of vaccine varied by condition (rPA stock solution was provided at 3.1 mg/ml). The total dosing volume across all groups was 100 μl per rabbit. Immunizations were performed on day 0 (d0) and d28, and blood samples were collected on days 0, 14, 28, 42, and 56 from the marginal ear vein. i.d. injections were performed using a stainless steel 1-mm, 34-gauge microneedle and a 1-ml syringe (BD Technologies, Research Triangle Park, NC); the procedure was performed as described previously (3, 18). i.m. injections were administered into the quadriceps muscle by use of a 27-guage needle (1/2-in. length) and a 1-ml syringe (BD, Franklin Lakes, NJ).
Immune response assays and aerosol challenge.
A quantitative enzyme-linked immunosorbent assay kit for rabbit immunoglobulin G (IgG) (Bethyl Laboratories, Montgomery, TX) was used with modifications. The first two columns of Maxisorp 96-well plates (Nalge Nunc, Rochester, NY) were coated with the provided capture antibody for the IgG standard curve. The remaining wells were coated with 1 μg/ml rPA in 0.05 M carbonate coating buffer, pH 9.6, for sample analysis. The plates were then incubated overnight at 4°C. Plates were blocked for 1 h at room temperature (RT) in blocking buffer (50 mM Tris, 0.14 M NaCl, 1% bovine serum albumin, pH 8.0) and then washed three times with wash solution (50 mM Tris, 0.14 M NaCl, 0.05% Tween 20, pH 8.0). IgG standards were prepared using the provided primary standard solution in a range from 7.8 to 500 ng/ml. Twofold serial dilutions of serum samples were performed (ranging from 1:50 to 1:6,400). Plates were incubated for 1 h at RT and washed three times. Plates were incubated for 1 h at RT following the addition of horseradish peroxidase-conjugated detection antibody (1:100,000) and then developed by 30 min of RT incubation with 3,3′,5,5′tetramethylbenzidine (TMB; Sigma, St. Louis, MO) substrate. The enzymatic reaction was stopped with 0.5 M H2SO4, and the optical densities were read at 450 nm (Tecan U.S., Research Triangle Park, NC). The rabbit IgG calibration curve was used to semiquantitatively determine the PA-specific IgG concentrations in the samples. A four-parameter logistic fit model was used to predict sample concentrations from the calibration curve. Toxin neutralizing antibody (TNA) titers were determined as described previously (15, 18). Rabbits were challenged with Ames strain anthrax spores as described previously (18, 21). The mean inhaled dose was equivalent to 263 ± 97 50% lethal doses (LD50) of Ames spores, calculated according to methods described previously (18, 21).
Statistics.
Antibody titers between groups were compared statistically by t test. Values reported represent two-tailed P values. The relationship between d56 anti-PA antibody levels and survival was determined by logistic regression.
RESULTS
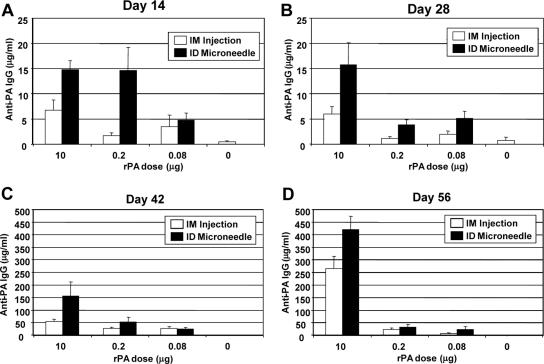
Rabbits were immunized with various doses of rPA (10, 0.2, or 0.08 μg) plus a constant amount of Alhydrogel (350 μg) on d0 and d28. The serum antibody response was assessed after primary immunization and also following booster inoculation. i.d. delivery of 10-μg or 0.2-μg doses of rPA induced PA-specific IgG at levels approaching 15 μg/ml 2 weeks following the initial dose (Fig. 1A). The i.d. induced responses at d14 were significantly greater than the corresponding responses induced by i.m. injection at the 10-μg and 0.2-μg dosage levels (P values were 0.01 and 0.02, respectively). In addition, the primary response induced by i.d. delivery of 0.2 μg rPA was statistically equivalent to that generated using a 50-fold excess of antigen (10 μg) administered i.m. (P = 0.14). There was no increase in the antibody response between d14 and d28; in fact, response levels decreased in the group immunized i.d. with 0.2 μg of antigen. Nonetheless, the i.d. induced responses were generally greater than the corresponding responses elicited by i.m. injection at each dosage level at d28 after priming (Fig. 1B).
FIG. 1.
PA-specific serum IgG levels following immunization with anthrax rPA vaccine. Rabbits (eight per group) were immunized on d0 and d28. Displayed are antibody response levels from rabbits at d14 (A), d28 (B), d42 (C), and d56 (D). Data represent mean PA-specific IgG levels ± standard errors of the mean.
A strong booster response was observed across all animal groups following secondary immunization on d28 (Fig. 1C and D). PA-specific IgG levels were highest in the group immunized i.d. with 10 μg of rPA, with antibody levels greater than 155 μg/ml at d42 and further increasing to above 420 μg/ml by d56. Dose sparing was evident after boosting at d42, as the response in the i.d. group receiving 0.2 μg of rPA was equivalent to the response achieved by i.m. injection of a 10-μg dose (P = 0.98) (Fig. 1C). This dose-sparing effect was not evident at d56 (Fig. 1D). The antibody response appeared to be more durable in rabbits immunized with 10 μg of rPA, since PA-specific IgG levels increased between d42 and d56 in these groups, whereas it decreased slightly in groups immunized with 0.2 μg of antigen and in the group immunized i.m. with 0.08 μg of rPA (Fig. 1C and D).
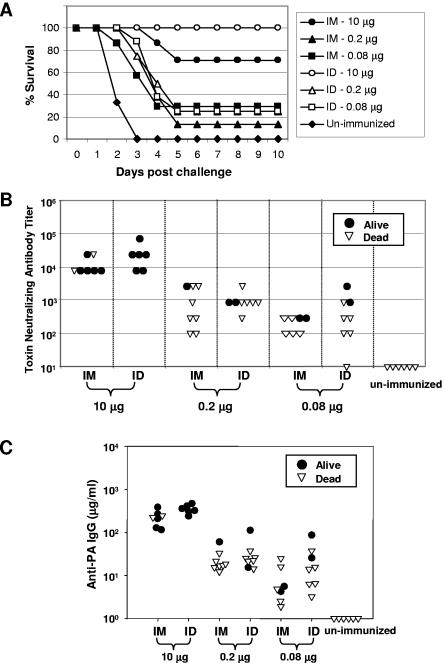
Rabbits were given an aerosol challenge with Ames strain anthrax spores at approximately d80. Survival rates are depicted in Fig. 2A. Complete protection was evident in the group immunized with 10 μg of rPA via the i.d. route, while the corresponding group immunized i.m. displayed 71% survival. Considerably lower levels of protection, ranging from 13 to 29%, were observed across all groups immunized using lower antigen doses. All of the unimmunized control rabbits died within 2 to 3 days postchallenge (Fig. 2A). Although only partial protection was observed in groups immunized with low doses (0.2 or 0.08 μg) of rPA, there was a modest 1- to 3-day delay in the time to death in these groups compared to controls. Nonsurviving rabbits in the group immunized i.m. with 10 μg of antigen also showed a 2- to 3-day delay in the time to death relative to controls (Fig. 2A).
FIG. 2.
Relationship between antibody response levels and survival following aerosol challenge with 263 ± 97 LD50 of Ames strain anthrax spores. (A) Survival versus time. Rabbits were challenged at approximately d80 and monitored daily for morbidity and mortality. Eight animals were challenged per group except for the following groups: i.d., 10 μg (six animals); i.m., 10 μg (seven animals); i.m., 0.08 μg (seven animals); and unimmunized (six animals). In these groups, fatalities had occurred before challenge. These fatalities did not appear to be due to the vaccine or the method of delivery, since they occurred in unimmunized control rabbits as well as in animals immunized by the i.d. and i.m. routes. (B) TNA titers at d56 for individual rabbits that survived or died. (C) Anti-PA IgG levels at d56 for individual rabbits that survived or died.
Serum TNA titers from individual rabbits were determined at d56 and are plotted versus survival in Fig. 2B. Similar TNA titers of 10,000 or greater were observed in rabbits immunized either i.d. or i.m. with 10 μg of rPA. TNA titers were more variable in groups immunized at the lower rPA doses. Overall response levels across these groups were similar, with TNA titers generally ranging from around 100 to greater than 1,000. Although a general pattern of higher TNA (Fig. 2B) and PA-specific serum IgG (Fig. 2C) levels at d56 was evident in surviving rabbits compared to nonsurvivors, there were several notable exceptions. For example, some rabbits with TNA titers of ≥10,000 and PA-specific IgG levels of ≥200 μg/ml did not survive, while other animals with more-than-10-fold-reduced levels of TNA titers and PA-specific IgG survived. Nonetheless, d56 serum IgG levels were a significant predictor of survival by logistic regression analysis (P < 0.0025).
DISCUSSION
There is growing interest in new approaches to induce more-potent and more-rapid immune responses to anthrax and other biodefense vaccines. Here, we observed that microneedle-based i.d. delivery required up to 50-fold less antigen to induce levels of antibody similar to those induced via i.m. injection during the first 2 weeks following primary and booster doses of vaccine. This dose-sparing effect dissipated within 4 weeks and did not result in increased survival of i.d. vaccinated rabbits at the lower vaccine dosage levels following aerosol spore challenge. It is notable that the PA-specific IgG levels increased between d42 and d56 in rabbits immunized with 10 μg of rPA, whereas the response decreased in most groups immunized with lower doses of vaccine. This reduction in circulating antibody levels may have contributed to the lower survival rates observed following aerosol challenge in rabbits immunized with low doses of rPA. Additional studies using rPA doses within the 10-μg- to 0.2-μg range will be required to further elucidate the potential dose-sparing benefits of the i.d. route, as have been observed for other vaccines (1, 2, 10, 12, 20, 22).
The survival rates we observed following i.d. and i.m. vaccinations were substantially lower per dose of antigen administered than those in a previously reported study, in which 83% survival was observed following two i.m. administered doses of 0.2 μg rPA, and 33% survival was found with the use of 0.08 μg of antigen (13). These differences may be due, in part, to differences in the amounts of Alhydrogel (500 μg in previous studies versus 350 μg per injection in this study) or in the biopotencies of the vaccines used in the two studies. Overall, the results highlight the inherent variability associated with in vivo challenge models that make it difficult to directly compare results between separate studies conducted at different times in different laboratories. In general, rabbits with the highest levels of PA-specific serum IgG and the most elevated TNA titers survived lethal aerosol challenge, whereas those with lower responses died. Consistent with the results of others (13, 21), we observed that anti-PA antibody levels were a significant predictor of survival.
The use of minimally invasive, easy-to-use delivery devices such as the microneedle-based system described herein could potentially reduce the burden on highly skilled medical practitioners for biodefense vaccination. In addition, increased ease of use may enable biodefense vaccines to be administered at numerous decentralized locations rather than at large, centralized vaccination centers that could represent targets for terrorist attack and facilitate the spread of infection. Our results suggest that i.d. delivery induces a level of protection against inhalational anthrax in a rabbit model that is comparable to that achieved via i.m. injection using conventional needle and syringe technology. On a dose-by-dose basis, i.d. delivery provided increased immune responses over i.m. injection during the early stages of the immune response. In addition, dose sparing was evident at the early time points. These improvements relative to i.m. injection, if recapitulated in humans, could potentially be of importance to biodefense vaccination in both prophylactic and postexposure therapeutic settings. Future studies will involve clinical evaluation of the i.d. route for anthrax immunization as a possible alternative to the standard i.m. route.
Acknowledgments
We thank VaxGen, Inc. for kindly providing rPA antigen; Pat McCutchen for administrative assistance in preparing the manuscript; M. Ishaq Haider, Tommy Robinson, Frank Martin, and Scott O'Connor for device fabrication; Perry Haaland and Dan Samarov for statistical analyses; Mary Ann Guetthoff for technical assistance; and M. Louise Pitt for aerosol challenge. We also thank Marc Gurwith and Mike Lock of VaxGen, Inc. for critical review of the manuscript.
Financial support was provided, in part, by funding from the U.S. Army Medical Research and Materiel Command, agreement number DAMD17-03-2-0037 (J.A.M.), and from the Defense Threat Reduction Agency, agreement number C.2 X001 04 RD B (R.G.U.).
Editor: D. L. Burns
Footnotes
Published ahead of print on 9 October 2006.
REFERENCES
- 1.Belshe, R. B., F. K. Newman, J. Cannon, C. Duane, J. Treanor, C. Van Hoecke, B. J. Howe, and G. Dubin. 2004. Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 351:2286-2294. [DOI] [PubMed] [Google Scholar]
- 2.Carcaboso, A. M., R. M. Hernandez, M. Igartua, J. E. Rosas, M. E. Patarroyo, and J. L. Pedraz. 2004. Enhancing immunogenicity and reducing dose of microparticulated synthetic vaccines: single intradermal administration. Pharm. Res. 21:121-126. [DOI] [PubMed] [Google Scholar]
- 3.Dean, C. H., J. B. Alarcon, A. M. Waterston, K. Draper, R. Early, F. Guirakhoo, T. P. Monath, and J. A. Mikszta. 2005. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax-JE) in non-human primates. Hum. Vaccines 1:106-111. [DOI] [PubMed] [Google Scholar]
- 4.Flick-Smith, H. C., J. E. Eyles, R. Hebdon, E. L. Waters, R. J. Beedham, T. J. Stagg, J. Miller, H. O. Alpar, L. W. Baillie, and E. D. Williamson. 2002. Mucosal or parenteral administration of microsphere-associated Bacillus anthracis protective antigen protects against anthrax infection in mice. Infect. Immun. 70:2022-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedlander, A. M., S. L. Welkos, M. L. Pitt, J. W. Ezzell, P. L. Worsham, K. J. Rose, B. E. Ivins, J. R. Lowe, G. B. Howe, P. Mikesell, and W. B. Lawrence. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239-1243. [DOI] [PubMed] [Google Scholar]
- 6.Gorse, G. J., W. Keitel, H. Keyserling, D. N. Taylor, M. Lock, K. Alves, J. Kenner, L. Deans, and M. Gurwith. 2006. Immunogenicity and tolerance of ascending doses of a recombinant protective antigen (rPA102) anthrax vaccine: a randomized, double-blinded, controlled, multicenter trial. Vaccine 24:5950-5959. [DOI] [PubMed] [Google Scholar]
- 7.Griffith, K. S., P. Mead, G. L. Armstrong, J. Painter, K. A. Kelley, A. R. Hoffmaster, D. Mayo, D. Barden, R. Ridzon, U. Parashar, E. H. Teshale, J. Williams, S. Noviello, J. F. Perz, E. E. Mast, D. L. Swerdlow, and J. L. Hadler. 2003. Bioterrorism-related inhalational anthrax in an elderly woman, Connecticut, 2001. Emerg. Infect. Dis. 9:681-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivins, B. E., M. L. M. Pitt, P. F. Fellows, J. W. Farchaus, G. E. Benner, D. M. Waag, S. F. Little, G. W. Anderson, P. H. Gibbs, and A. M. Friedlander. 1998. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 16:1141-1148. [DOI] [PubMed] [Google Scholar]
- 9.Jernigan, J. A., D. S. Stephens, D. A. Ashford, C. Omenaca, M. S. Topiel, M. Galbraith, M. Tapper, T. L. Fisk, S. Zaki, T. Popovic, R. F. Meyer, C. P. Quinn, S. A. Harper, S. K. Fridkin, J. J. Sejvar, C. W. Shepard, M. McConnell, J. Guarner, W. J. Shieh, J. M. Malecki, J. L. Gerberding, J. M. Hughes, and B. A. Perkins. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenney, R. T., S. A. Frech, L. R. Muenz, C. P. Villar, and G. M. Glenn. 2004. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 351:2295-2301. [DOI] [PubMed] [Google Scholar]
- 11.Kenney, R. T., J. M. Yu, M. Guebre-Xabier, S. A. Frech, A. Lambert, B. A. Heller, L. R. Ellingsworth, J. E. Eyles, E. D. Williamson, and G. M. Glenn. 2004. Induction of protective immunity against lethal anthrax challenge with a patch. J. Infect. Dis. 190:774-782. [DOI] [PubMed] [Google Scholar]
- 12.Kurugol, Z., S. Erensoy, S. Aksit, A. Egemen, and A. Bilgic. 2001. Low-dose intradermal administration of recombinant hepatitis B vaccine in children: 5-year follow-up study. Vaccine 19:3936-3939. [DOI] [PubMed] [Google Scholar]
- 13.Little, S. F., B. E. Ivins, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22:422-430. [DOI] [PubMed] [Google Scholar]
- 14.Little, S. F., B. E. Ivins, W. M. Webster, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2006. Duration of protection of rabbits after vaccination with Bacillus anthracis recombinant protective antigen vaccine. Vaccine 24:2530-2536. [DOI] [PubMed] [Google Scholar]
- 15.Little, S. F., S. H. Leppla, and A. M. Friedlander. 1990. Production and characterization of monoclonal antibodies against the lethal factor component of Bacillus anthracis lethal toxin. Infect. Immun. 58:1606-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikszta, J. A., J. B. Alarcon, J. M. Brittingham, D. E. Sutter, R. J. Pettis, and N. G. Harvey. 2002. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 8:415-419. [DOI] [PubMed] [Google Scholar]
- 17.Mikszta, J. A., M. I. Haider, and R. J. Pettis. 2006. Microneedles for drug and vaccine delivery. When will the dream become a reality? p. 309-325. In J. Wille (ed.), Skin delivery systems. Transdermals, dermatologicals, and cosmetic actives. Blackwell Press, Ames, Iowa.
- 18.Mikszta, J. A., V. J. Sullivan, C. Dean, A. M. Waterston, J. B. Alarcon, J. P. Dekker, J. M. Brittingham, J. Huang, C. R. Hwang, M. Ferriter, G. Jiang, K. Mar, K. U. Saikh, B. G. Stiles, C. J. Roy, R. G. Ulrich, and N. G. Harvey. 2005. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J. Infect. Dis. 191:278-288. [DOI] [PubMed] [Google Scholar]
- 19.Nagafuchi, S., S. Kashiwagi, S. Imayama, J. Hayashi, and Y. Niho. 1998. Intradermal administration of viral vaccines. Rev. Med. Virol. 8:97-111. [DOI] [PubMed] [Google Scholar]
- 20.Pancharoen, C., J. Mekmullica, U. Thisyakorn, S. Kasempimolporn, H. Wilde, and C. Herzog. 2005. Reduced-dose intradermal vaccination against hepatitis A with an aluminum-free vaccine is immunogenic and can lower costs. Clin. Infect. Dis. 41:1537-1540. [DOI] [PubMed] [Google Scholar]
- 21.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 22.Playford, E. G., P. G. Hogan, A. S. Bansal, K. Harrison, D. Drummond, D. F. M. Looke, and M. Whitby. 2002. Intradermal recombinant hepatitis B vaccine for healthcare workers who fail to respond to intramuscular vaccine. Infect. Control Hosp. Epidemiol. 23:87-90. [DOI] [PubMed] [Google Scholar]
- 23.Williamson, E. D., I. Hodgson, N. J. Walker, A. W. Topping, M. G. Duchars, J. M. Mott, J. Estep, C. LeButt, H. C. Flick-Smith, H. E. Jones, H. Li, and C. P. Quinn. 2005. Immunogenicity of recombinant protective antigen and efficacy against aerosol challenge with anthrax. Infect. Immun. 73:5978-5987. [DOI] [PMC free article] [PubMed] [Google Scholar]