Abstract
Infection with Helicobacter trogontum, a urease-positive helicobacter isolated from subclinically infected rats, was evaluated in B6.129P2-IL10tm1Cgn (interleukin-10−/− [IL-10−/−]) and C57BL/6 (B6) mice. In a first experiment, IL-10−/− mice naturally infected with Helicobacter rodentium had subclinical typhlocolitis but developed severe diarrhea and loss of body condition with erosive to ulcerative typhlocolitis within 1 to 3 weeks of experimental infection with H. trogontum. A second experiment demonstrated that helicobacter-free IL-10−/− mice dosed with H. trogontum also developed severe clinical signs and typhlocolitis within 2 to 4 weeks, whereas B6 mice colonized with H. trogontum were resistant to disease. In a third experiment, using helicobacter-free IL-10−/− mice, dosing with H. trogontum resulted in acute morbidity and typhlocolitis within 8 days. Acute typhlocolitis was accompanied by signs of sepsis supported by degenerative hemograms and recovery of Escherichia coli and Proteus spp. from the livers of infected mice. Quantitative PCR data revealed that H. rodentium and H. trogontum may compete for colonization of the lower bowel, as H. trogontum established higher colonization levels in the absence of H. rodentium (P < 0.003). H. trogontum-induced typhlocolitis was also associated with a significant decrease in the levels of colonization by five of eight anaerobes that comprise altered Schaedler's flora (P < 0.002). These results demonstrate for the first time that H. rodentium infection in IL-10−/− mice causes subclinical typhlocolitis and that infection with H. trogontum (with or without H. rodentium) induces a rapid-onset, erosive to ulcerative typhlocolitis which impacts the normal anaerobic flora of the colon and increases the risk of sepsis.
A variety of inbred mutant and genetically engineered strains of mice with immune function abnormalities develop spontaneous enterocolitis, which can be promoted by natural or experimental helicobacter infection (36). A notable example is the spontaneous typhlocolitis that progresses to neoplastic sequelae over 6 months in B6.129P2-IL-10tm1Cgn (interleukin-10−/− [IL-10−/−]) mice with undefined intestinal flora (4). The time course for lesions of typhlocolitis, epithelial hyperplasia, and dysplasia in IL-10−/− mice can be accelerated by experimental infection with Helicobacter hepaticus (24), Helicobacter bilis (8), or Helicobacter typhlonius (13). Notably, IL-10−/− mice develop Th1-mediated typhlocolitis whose severity depends on the genetic susceptibility of the background strain (4) and do not develop disease when maintained germfree (3). Thus, the proximal cause for the chronic typhlocolitis that develops under conventional husbandry conditions is presumed to be enteric flora, including natural or experimental infection with bacteria such as Helicobacter spp. (13) and enterococci (3).
During the past decade, the isolation and characterization of novel murine helicobacters have been an active area of interest (49). Enterohepatic helicobacter infections of mice can cause not only subclinical, intercurrent disease that may confound research but also overt disease, such as typhlocolitis in immunodeficient and immune-dysregulated mice, which has been studied as a model of human inflammatory bowel disease (IBD). Helicobacter trogontum is a urease-positive helicobacter first isolated from the colons of subclinically infected Wistar and Holtzman rats (28) and is closely related to H. hepaticus and members of “Helicobacter (Flexispira) rappini” taxa 1, 4, and 5 by 16S rRNA sequence analysis (10, 19). A taxon 1 isolate has been implicated in human gastroenteritis (2, 37), and taxon 4 contains an uncharacterized isolate from sheep (19). A taxon 5 isolate was associated with natural abortion in sheep (21) and experimental abortion in guinea pigs (7). H. trogontum also has morphology and growth characteristics similar to those of, and clusters phylogenetically with, a novel intestinal helicobacter associated with ulcerative colitis in cotton-top tamarins (39) that is in taxon 10 (10). Other than in vitro studies to demonstrate expression of urease and failure to express cytolethal distending toxin (22), the potential to model human disease with H. trogontum has been evaluated only in germfree mice that developed subclinical inflammatory lesions (31, 30, 32).
In an initial study of IL-10−/− mice on a C57BL/6 (B6) background that were naturally infected with multiple enterohepatic Helicobacter spp., experimental challenge with H. trogontum caused debilitating diarrhea associated with severe acute typhlocolitis (S. Danon, personal communication). An additional experiment using IL-10−/− mice from a separate colony naturally infected with Helicobacter rodentium replicated these observations; H. trogontum infection rapidly caused debilitating typhlocolitis. These experiments were expanded to determine if infection with H. trogontum alone would reproducibly cause severe typhlocolitis in helicobacter-free B6.129P2-IL-10tm1Cgn (IL-10−/−) mice similar to that observed in the IL-10−/− mice naturally infected with multiple enterohepatic helicobacters. Additionally, because both humans (45) and mouse models (5, 17) affected by IBD have been shown to develop differences in their colonic flora relative to control populations (45), we were interested in comparing the population dynamics of H. rodentium and H. trogontum and in the potential impact of inflammation on normal flora. We used real-time quantitative PCR (qPCR) to estimate levels of colonization in the proximal colon by both helicobacters and altered Schaedler's flora (ASF), which consists of eight anaerobes selected to provide gnotobiotic mice with a standardized intestinal flora (9, 17, 38).
MATERIALS AND METHODS
Animals.
Viral antibody- and parasite-free B6.129P2-IL-10tm1Cgn (IL-10−/−) mice naturally infected with H. rodentium were maintained at the Massachusetts Institute of Technology (Cambridge). Helicobacter-free IL-10−/− mice were rederived by embryo transfer from the H. rodentium-infected founder mice, and helicobacter-free C57BL/6 (B6) mice were purchased from Jackson Laboratories (Bar Harbor, ME). The helicobacter infection status of these separately housed colonies was confirmed by fecal PCR using genus- and species-specific primers (40, 41). Mice were provided pelleted rodent diet (ProLab 3000; Purina Mills, St. Louis, MO) and filtered water ad libitum and were maintained in AAALAC-accredited facilities in microisolator caging (Allentown Caging Equipment Inc., Allentown, NJ) under standard environmental conditions. All experiments were approved by the MIT Committee on Animal Care.
Natural infection with H. rodentium.
Naturally acquired infection with H. rodentium had been confirmed in a colony of barrier-maintained IL-10−/− mice that had been obtained from a commercial vendor prior to the recognition of H. rodentium as a novel helicobacter of mice (41). This colony was free of all other known Helicobacter species as well as pathogenic murine viruses, Salmonella spp., Citrobacter rodentium, and parasites.
Experimental infection with H. trogontum (ATCC 700114).
In a first experiment, 12 male and 13 female IL-10−/− mice from a colony naturally infected with H. rodentium were dosed at 6 to 8 weeks of age with 2 × 107 H. trogontum three times on alternate days by oral gavage or were sham-dosed as controls (11 males, 7 females). Mice were euthanized with carbon dioxide and necropsied at time points up to 6 weeks postinfection (PI) based on progressive evidence of bloody feces, dehydration, and weight loss in H. trogontum-infected mice. In a second experiment, five male and three female helicobacter-free IL-10−/− and five male and five female B6 mice were dosed at 6 to 8 weeks of age with 2 × 107 H. trogontum organisms twice over 5 days by oral gavage, and a similar number were sham-dosed as controls. Mice were necropsied 2 to 4 weeks PI based on the extent of morbidity. A third group of helicobacter-free male (n = 10) and female (n = 10) IL-10−/− mice were dosed at 6 weeks of age with 2 × 107 H. trogontum organisms twice over 5 days by oral gavage or were sham-dosed as controls (five males and six females). These mice were euthanized and necropsied 8 days PI due to rapid clinical deterioration.
Clinical assessment and necropsy.
Throughout the experiments, mice were assessed for evidence of diarrhea, dehydration, and deteriorating body condition (47). Although there were some deaths due to acute disease, mice were euthanized with CO2 and necropsied when they became moribund or when the PI interval of 6 weeks was reached.
Confirmation of helicobacter infection.
To demonstrate that mice were helicobacter free or colonized with H. rodentium or H. trogontum, fecal or cecal samples were tested by PCR using previously described methods and primer sets specific for the genus or each Helicobacter species. (12, 41).
Quantitative PCR for H. rodentium and H. trogontum.
After removal of gross cecal contents, approximately 0.5 cm of cecal tissue was sampled proximal to the cecal-colonic junction and stored at −20°C until DNA extraction. DNA was extracted using a Boehringer-Mannheim High Pure PCR template preparation kit (Roche Molecular Biochemicals, Indianapolis, IN) following the manufacturer's protocol for the isolation of nucleic acids from tissue. H. rodentium and H. trogontum organisms were quantified with species-specific primers in the samples, with host (mouse) DNA being measured using commercial 18S rRNA gene-based primers and probe (Applied Biosystems, Foster City, CA). The 16S rRNA gene-based primers for H. rodentium were previously reported (41), whereas the primers (forward, 5′-TATTGAGAGTAGTACTTATTGAA-3′; reverse, 5′-ATGCAAAGTTTTGATTTCAAGACCA-3′) for the H. trogontum genome were derived from the spacer region between the 16S and 23S rRNA genes of H. trogontum, which produce a 365-bp amplicon (J. Fox and Z. Shen, unpublished data). Real-time quantitative PCR was performed using the Applied Biosystems sequence detection system (model 7700). There was no cross-amplification between host DNA and H. rodentium- or H. trogontum-specific primers. For quantitation with Sybr green, duplicates of each sample contained the following in a 50-μl volume: 10 μl template DNA, 5 μl of 10× commercial buffer A, 7 μl of 25 mM MgCl2, 1 μl each of 10 μM dATP, dCTP, dGTP, and dUTP, 0.5 μl uracil-N-glycosylase, 2.5 μl AmpliTaq Gold, and balance DNase-free double-distilled H2O. Thermocycling was performed at 50°C for 2 min and 95°C for 10 min, followed by 40 repeats of 95°C for 15 s and 60°C for 60 s. To estimate copy numbers of H. trogontum and H. rodentium in samples, a standard curve was created using serial 10-fold dilutions (1 × 106 to 101) of copy number based on an estimate of the genome size of H. trogontum and H. rodentium. A mean mass value (1.71 Mb) was estimated by the average from two published H. pylori genomes (1, 46) and the H. hepaticus genome (44). The number of H. trogontum or H. rodentium genome copies was then normalized to μg of murine chromosomal DNA measured by qPCR using 18S rRNA gene-based primers and probe mixture (Applied Systems). Murine chromosomal DNA in each sample was compared to a standard curve based on 106, 105, 104, 103, and 102 pg of DNA obtained from Helicobacter-free mouse liver.
Quantitative PCR for ASF.
ASF consists of eight anaerobes selected to provide gnotobiotic mice with a standardized intestinal flora (9). The eight organisms include ASF356 and ASF502, which are Clostridium spp., ASF360 and ASF361, which are Lactobacillus spp., ASF500, an unclassified gram-positive rod, ASF492, a Eubacterium sp., ASF457, recently named Mucispirillum schaedleri (35), and ASF519, a Bacteroides sp. Quantitative PCR was performed on proximal colon samples obtained from four male and four female helicobacter-free IL-10−/− mice and from eight male and seven female IL-10−/− mice 8 days after infection with H. trogontum. DNA extraction was modified to maximize isolation of nucleic acids from gram-positive as well as gram-negative bacteria, and assay reagents and conditions were used as previously described (17, 38).
Liver culture.
To screen for translocation of bacteria from the gut in the third experiment, liver samples from four helicobacter-free and four H. trogontum-infected IL-10−/− mice were aseptically collected at necropsy 8 days PI. A 0.5-cm3 sample from the left liver lobe of each mouse was homogenized, plated on Trypticase soy agar (TSA) and MacConkey's medium (Remel, Inc., Lenexa, KA), and incubated under aerobic conditions at 37°C. For potential recovery of H. trogontum, tissue was inoculated onto TSA and incubated under microaerobic conditions (80:10:10 N2-H2-CO2) at 37°C.
Complete blood counts.
Whole blood was collected into EDTA at necropsy for evaluation of the hemogram from helicobacter-free and H. trogontum-infected IL-10−/− mice at 8 days PI. Most parameters were measured with a commercial hematology analyzer (CDC Technologies, Oxford, CT), while the differential was determined manually by a certified medical technologist blinded to the experimental status of the samples.
Histology.
Formalin-fixed tissues were routinely processed, embedded in paraffin, sectioned at 4 μm, stained with hematoxylin and eosin, and evaluated by a board-certified veterinary pathologist blinded to the sample identity. Inflammation, edema, epithelial defects, hyperplasia, and dysplasia of the cecal-colonic junction were scored on an ascending scale (0 [for normal] to 4) of severity and invasiveness of the lesion (Table 1). Scores for inflammation, edema, epithelial defects, hyperplasia, and dysplasia were totaled to generate a lesion index for comparison between groups of mice.
TABLE 1.
Lower bowel lesion scoring criteria
| Score | Inflammation | Edema | Epithelial defects | Hyperplasia | Dysplasia |
|---|---|---|---|---|---|
| 0 | None | None | None | Normal crypt length | None |
| 1 | Small multifocal lamina proprial and/or transepithelial leukocyte accumulations | Mild segmental expansion of submucosa | Decreased goblet cells, occasional dilated glands, mild surface “tattering” | ∼1.5× normal crypt length | Mild dysplasia characterized by epithelial cell pleomorphism, plump and attenuated forms, aberrant crypt foci, gland malformation with mild splitting, branching, and infolding, rare cystic dilation |
| 2 | Coalescing mucosal inflammation with or without early submucosal extension | Moderate diffuse submucosal with or without mucosal lamina propria expansion | Focally extensive surface epithelial tattering, many dilated glands with attenuated lining and luminal cell debris | ∼2×; with or without mitotic figures one-third way up to surface | Moderate dysplasia characterized by pleomorphism, early cellular and nuclear atypia, mild piling and infolding, occasional cystic dilation, bulging towards muscularis mucosae and projection into lumen, loss of normal glandular, mucous, or goblet cells, also known as atypical hyperplasia |
| 3 | Coalescing mucosal inflammation with prominent multifocal submucosal extension with or without follicle formation | Severe edema of mucosa and submucosa | Erosions | ∼3×; with or without mitotic figures half-way up to surface | Gastrointestinal intraepithelial neoplasia (6), severe dysplasia confined to mucosa, features as above but greater severity, frequent and sometimes bizarre mitoses |
| 4 | Severe diffuse inflammation of mucosa, submucosa, and deeper layers | Transmural; very severe | Ulceration | 4×+; with or without mitotic figures more than half-way up to surface | Invasive carcinoma: Submucosal invasion, or any demonstrated invasion into blood or lymphatic vessels, regional nodes, or other metastasis. |
Statistics.
Statistical comparisons of controls to helicobacter-infected mice for morbidity and lesion severity were performed using a software package (GraphPad Software, Inc., San Diego, CA.). Chi-square analysis and the nonparametric Mann-Whitney test were used for categorical data involving morbidity and lesion scores. Quantitative PCR data for levels of colonization by helicobacter and ASF species were first log transformed and then compared by Student's t test. Results were considered significant when P was <0.05.
RESULTS
H. rodentium infection was associated with subclinical typhlocolitis in IL-10−/− mice.
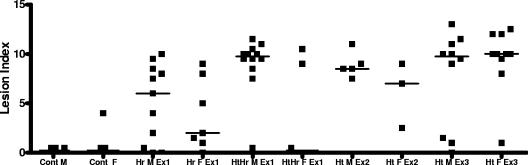
In the first experiment, IL-10−/− mice naturally infected with H. rodentium were clinically normal until necropsied at 10 to 12 weeks of age. H. rodentium infection was consistently associated with mild enlargement of mesenteric lymph nodes. Typhlocolitis associated with H. rodentium infection was mild to moderate (Table 2). Total lesion indices were significant in both male and female IL-10−/− mice infected with H. rodentium compared to helicobacter-free IL-10−/− mice used in the second and third experiments (P < 0.005 for males, P < 0.01 for females) (Table 2; Fig. 1 and 2). Reflected in the individual parameter scores for inflammation, epithelial defects, hyperplasia, and dysplasia as well as in the total lesion indices (Table 2), there was a trend for male IL-10−/− mice infected with H. rodentium to have moderate disease, compared to mild disease in female mice. In contrast to helicobacter-free IL-10−/− mice, IL-10−/− mice infected with H. rodentium had multifocal to coalescing areas of mucosal inflammation in the lamina propria of the cecal-colonic junction along with mild to moderate edema of the submucosa. Goblet cell numbers were decreased, and crypts were elongated, with features of moderate dysplasia.
TABLE 2.
Lesion indices observed at the cecal-colonic junction
| Expt | Infection status | Mouse | n | Severity score fora:
|
Lesion indexa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time point | Inflammation | Edema | Epithelial defect | Hyperplasia | Dysplasia | |||||
| 1 | H. rodentium | Male IL-10−/− | 11 | 3-6 wk | 2 (0-3) | 1 (0-1.5) | 0.5 (0-2.5) | 1 (0-3) | 0.5 (0-2) | 6 (0-10) |
| Female IL-10−/− | 7 | 4-6 wk | 1 (0-2.5) | 1 (0-1.5) | 0 (0-1.5) | 0.5 (0-2.5) | 0 (0-1.5) | 2 (0-9) | ||
| H. rodentium and H. trogontum | Male IL-10−/− | 12 | 1-3 wk | 2.5 (0-2.5) | 1.75 (0.5-3) | 2.5 (0-3) | 1.5 (0-2.5) | 1.25 (0-2) | 9.75 (0.5-11.5) | |
| Female IL-10−/− | 13 | 2-6 wk | 0 (0-2) | 0 (0-3) | 0 (0-2.5) | 0 (0-2) | 0 (0-1.5) | 0 (0-10.5) | ||
| 2 | Uninfected controls | Male B6 | 5 | 4 wk | 0 (0) | 0 (0-0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0-0.5) |
| Female B6 | 4 | 4 wk | 0 (0) | 0 (0-0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0-0.5) | ||
| H. trogontum | Male B6 | 4 | 4 wk | 0 (0-0.5) | 0 (0-0.5) | 0.5 (0-1) | 0 (0) | 0 (0) | 0 (0-1) | |
| Female B6 | 5 | 4 wk | 0 (0-0.5) | 0.5 (0-1) | 0 (0) | 0 (0) | 0 (0) | 0.5 (0-1.5) | ||
| Uninfected controls | Male IL-10−/− | 5 | 4 wk | 0 (0) | 0 (0-0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0-0.5) | |
| Female IL-10−/− | 5 | 4 wk | 0 (0-1) | 0 (0-2.5) | 0 (0-0.5) | 0 (0) | 0 (0) | 0 (0-4) | ||
| H. trogontum | Male IL-10−/− | 5 | 2-3 wk | 2.5 (2.5-3) | 2 (1.5-2) | 2.5 (2-3) | 1 (0.5-2) | 1 (0.5-1.5) | 8.5 (7.5-11) | |
| Female IL-10−/− | 3 | 3-4 wk | 2 (1-2.5) | 2 (1.5-2.5) | 1.5 (0-2) | 0.5 (0-1.5) | 0.5 (0-1) | 7 (2.5-9) | ||
| 3 | Uninfected controls | Male IL-10−/− | 5 | 8 days | 0 (0-0.5) | 0 (0-0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0-0.5) |
| Female IL-10−/− | 6 | 8 days | 0 (0) | 0 (0-0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0-0.5) | ||
| H. trogontum | Male IL-10−/− | 10 | 8 days | 2.5 (0-3) | 2.25 (0-3.5) | 2.5 (0-3.5) | 1.5 (0-2) | 0.5 (0-1) | 9.75 (0-13) | |
| Female IL-10−/− | 10 | 8 days | 3 (1-3.5) | 2 (0-2.5) | 3 (0-3.5) | 1.75 (0-2) | 1 (0-1) | 10 (1-12.5) | ||
Values are medians and ranges.
FIG. 1.
Total lesion index for individual IL-10−/− mice evaluated in the three experiments (Ex). Cont M and Cont F, helicobacter-free male and female IL-10−/− controls; Hr, H. rodentium; Ht, H. trogontum; HtHr, coinfection. Lines represent median values. See Table 2 for the numbers of mice evaluated.
FIG. 2.
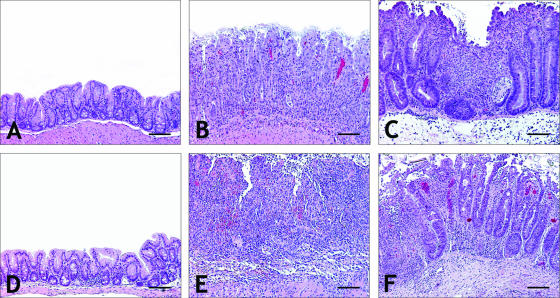
(A) Normal cecum from helicobacter-free IL-10−/− male mouse. (B) Moderate typhlitis from H. rodentium infection in an IL-10−/− male mouse. Note the moderate hyperplasia, loss of goblet cells, and inflammatory infiltrate. (C) Severe, diffuse typhlitis with focal erosion, glandular dysplasia and hyperplasia, loss of goblet cells, and crypt abscesses from H. rodentium and H. trogontum infection in an IL-10−/− male mouse. (D) Minimal typhlitis from H. trogontum infection in a male B6 mouse. (E) Severe erosive typhlitis with near-total crypt atrophy from H. trogontum infection in an IL-10−/− male mouse. (F) Lower magnification of the field adjacent to the lesion in panel E, showing severe inflammation, hyperplasia and dysplasia. Bar = 160 μm (A to E) and 250 μm (F).
H. trogontum infection was associated with rapid onset of ulcerative typhlocolitis in IL-10−/− mice.
In contrast to H. rodentium infection, clinical morbidity was severe in mice coinfected with H. rodentium and H. trogontum, particularly in male IL-10−/− mice (P < 0.002). Coinfected IL-10−/− male mice rapidly developed dehydration and weight loss and were either found dead of acute disease or euthanized when moribund at 1 or 3 weeks after infection with H. trogontum (n = 12). The extent of typhlocolitis and hyperplasia of the epithelium in males that developed acute morbidity by 1 week PI was similar to that in males that were necropsied at 3 weeks PI (P = 0.25 and 0.14, respectively), although dysplasia was more severe at 3 weeks PI (P = 0.05). Coinfected male IL-10−/− mice consistently developed gross thickening of the cecal-colonic junction, and cecal and colon contents were often grossly hemorrhagic. Mesenteric lymph nodes were markedly enlarged in the cecal-colonic mesentery. The cecal-colonic junctions of male IL-10−/− mice coinfected with H. rodentium and H. trogontum were extensively infiltrated with mononuclear cells that distorted crypt morphology. Crypt abscesses, depletion of goblet cells, and elongation of crypts to 2 to 3 times normal length with mild to moderate dysplasia were noted (Fig. 2C). Comparison of total lesion indices (Table 2) indicates that H. trogontum significantly exacerbated typhlocolitis in male IL-10−/− mice compared to natural infection with H. rodentium (P < 0.004).
In contrast to males, only 4 of 13 (31%) female IL-10−/− mice became clinically debilitated from concurrent H. rodentium and H. trogontum infection. Two female mice were euthanized for deteriorating clinical condition at 3 weeks, one female mouse was euthanized at 5 weeks, and one female mouse was euthanized at 6 weeks PI. Clinically normal female IL-10−/− mice infected with both H. rodentium and H. trogontum were necropsied for comparison of lesions to those of male mice and had gross and histologic changes that were mild in the majority of mice (Table 2; Fig. 1). Notably, experimental challenge with H. trogontum in females naturally infected with H. rodentium did not significantly exacerbate inflammation, hyperplasia, or dysplasia attributable to H. rodentium infection alone (P = 0.09).
A second experiment was then performed to determine if the gender differences were reproducible and to determine the effects of infection with H. trogontum in helicobacter-free IL-10−/− and wild-type B6 mice. One of 10 B6 mice was not colonized with H. trogontum and thus was not included in the analysis. Four male and five female helicobacter-free B6 mice experimentally colonized with H. trogontum remained clinically normal, with no gross abnormalities noted at necropsy. Histology assessment revealed that H. trogontum caused only mild typhlocolitis in B6 mice by 4 weeks PI, with no development of epithelial hyperplasia or dysplasia (Table 2; Fig. 2D). The body condition of helicobacter-free male and female IL-10−/− mice subsequently infected with H. trogontum progressively declined, necessitating euthanasia by 2 to 4 weeks PI. Similar to the first study, H. trogontum-infected IL-10−/− mice were clinically dehydrated and had enlarged mesenteric lymph nodes and grossly thickened ceca and colons. Lower bowel contents were fluid and sometimes hemorrhagic. Notably, there were no gender-associated differences in the extent of disease attributable to H. trogontum (P = 0.25), although there was a trend for females to resist clinical effects somewhat longer (3 to 4 weeks PI) than male IL-10−/− mice (2 to 3 weeks PI) (Table 2). Histologically, inflammation, edema, epithelial defects, hyperplasia, and dysplasia were moderate to severe in both male and female IL-10−/− mice, with similar total lesion indices (P = 0.07).
In a third experiment, 10 male and 10 female IL-10−/− mice infected with H. trogontum rapidly developed diarrhea with associated dehydration and weight loss that necessitated euthanasia at 8 days PI. Five uninfected control male and six control female IL-10−/− mice remained clinically normal. Within just 8 days, weight loss in H. trogontum-infected mice was severe, with an average loss of 32% in infected females and an average loss of 24% in infected males. Gross findings at necropsy in infected mice were consistent with observations from the first two experiments: dehydration, weight loss, enlarged mesenteric lymph nodes, and thickened cecal-colonic junction and distal colon, often with dark, liquid intestinal contents. Total lesion indices, incorporating inflammation, edema, epithelial defects, hyperplasia, and dysplasia at the cecal-colonic junction, were similar (P = 0.17) in male and female IL-10−/− mice infected with H. trogontum (Table 2; Fig. 1, 2E, and 2F). Coalescing, diffuse inflammation of the mucosa, submucosa, and deeper layers was observed in 9 of 10 female and 7 of 10 male IL-10−/− mice infected with H. trogontum and was accompanied by erosive to ulcerative epithelial lesions in seven mice of each gender (Fig. 2E). Crypt necrosis, crypt abscesses, erosion and ulceration of epithelium, submucosal edema, and extension of transmural inflammation progressed to serositis in some mice. Although lesions were most severe at the cecal-colonic junction in the H. trogontum-infected IL-10−/− mice, segmental areas of the middle and distal colon were similarly affected with moderate to severe inflammation, submucosal edema, epithelial defects, and mild to moderate hyperplasia and dysplasia of the epithelium (data not shown).
H. trogontum infection caused acute morbidity associated with leukocytosis and translocation of intestinal bacteria to the liver.
In the H. trogontum-infected IL-10−/− mice that became clinically ill within 8 days, a leukocytosis with a degenerative left shift in the hemogram suggested sepsis (data not shown), supporting the deteriorating clinical status of these mice. A subset of four liver samples from controls and four samples from H. trogontum-infected mice were collected aseptically at necropsy and cultured to determine if necrotic changes in the intestinal epithelia allowed translocation of intestinal bacteria. Although H. trogontum was not recovered by culture of liver samples, Escherichia coli and Proteus spp. were recovered by aerobic culture from the livers of three of the four selected H. trogontum-infected IL-10−/− mice, whereas liver samples from four selected control IL-10−/− mice were sterile. Mild portal hepatitis (not shown) was observed in the livers from the H. trogontum-infected mice but not in the control, sterile livers.
Natural infection with H. rodentium inhibited colonization of H. trogontum in the cecum.
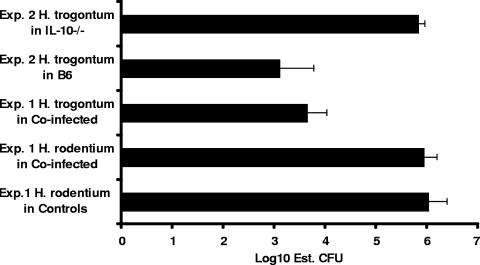
Conventional PCR was used to confirm the infection status of controls infected with H. rodentium and the helicobacter-free status of control mice used in the additional two studies. Real-time quantitative PCR was used for estimating the levels of colonization by H. rodentium and H. trogontum in the ceca of experimentally infected mice. In the first experiment, which evaluated male and female IL-10−/− mice naturally infected with H. rodentium and then experimentally challenged with H. trogontum, levels of colonization by H. rodentium and H. trogontum were similar in both genders of mice (P = 0.14 and higher; data by gender not shown). Levels of H. rodentium were approximately 2 logs higher than those of H. trogontum (P < 0.001) in coinfected mice (Fig. 3) and were not lowered by subsequent coinfection with H. trogontum (P = 0.43). In the second experiment, B6 mice were colonized with H. trogontum at levels approximately 3 logs lower than those in IL-10−/− mice (P < 0.004) (Fig. 3). Notably, in the absence of H. rodentium, H. trogontum levels were significantly higher in the monoinfected IL-10−/− mice in the second experiment than in coinfected IL-10−/− mice in the first experiment (P < 0.003).
FIG. 3.
Data from quantitative PCR indicated that H. rodentium levels in the cecum were approximately 2 logs higher than H. trogontum (P < 0.001) in coinfected mice and were not lowered by concurrent infection with H. trogontum (P = 0.43). B6 mice were colonized with H. trogontum at levels approximately 3 logs lower than IL-10−/− mice (P < 0.004). H. trogontum levels were significantly higher in monoinfected IL-10−/− mice (experiment 2) than in coinfected IL-10−/− mice (experiment 1) (P < 0.003). Data are medians and standard errors.
Experimental infection with H. trogontum lowered colonization of ASF.
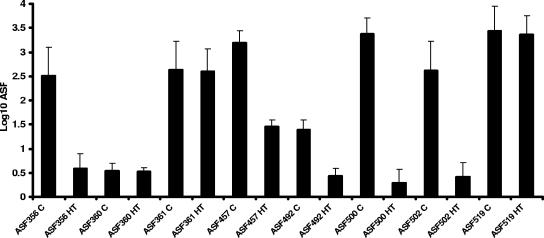
In the third experiment, samples from the proximal colon were obtained at necropsy to evaluate potential population shifts in resident ASF as the result of H. trogontum infection. Samples from 15 IL-10−/− mice infected with H. trogontum for 8 days were evaluated and showed significantly lower levels of colonization by five of the eight ASF species in the proximal colon than in eight helicobacter-free control IL-10−/− mice (Fig. 4). ASF356 (Clostridium sp.), ASF457 (M. schaedleri), ASF492 (Eubacterium sp.), ASF500 (unclassified gram-positive rod), and ASF502 (Clostridium sp.) all colonized at lower levels in H. trogontum-infected mice (P < 0.002). Levels of colonization by the other three ASF species (ASF360 and ASF361 [both Lactobacillus spp.] and ASF519 [Bacteroides sp.]) in the proximal colon were not impacted by H. trogontum infection (P = 0.43), nor were any of the changes in colonization by the eight species of ASF impacted by gender in the H. trogontum-infected mice (P = 0.2).
FIG. 4.
IL-10−/− mice infected with H. trogontum for 8 days had significantly lower levels of colonization by five of the eight ASF species in the proximal colon than helicobacter-free control IL-10−/− mice (P < 0.002). Levels of colonization by ASF360, ASF361, and ASF519 were not impacted by H. trogontum infection (P = 0.43). Data are medians and standard errors.
DISCUSSION
Infection with H. trogontum caused severe morbidity and typhlocolitis in IL-10−/− mice that were naturally infected with H. rodentium or were initially helicobacter free. Additionally, lesions attributable to H. rodentium infection in IL-10−/− mice have not been reported, but H. rodentium infection was associated with mild to moderate typhlocolitis in this study. Results from the first experiment suggested that male IL-10−/− mice may be predisposed to more severe disease from H. trogontum infection than females, but data from the two follow-up studies indicated that both genders were susceptible, albeit with a possible time course delay in female mice. Development of acute typhlocolitis associated with weight loss and diarrhea from H. trogontum infection was also associated with declines in ASF colonization, suggesting that H. trogontum infection impacts the levels of colonization by normal, potentially beneficial flora. Erosive to ulcerative epithelial changes from the acute inflammation may have increased mucosal permeability and permitted translocation of E. coli and Proteus spp. to the liver, suggesting that sepsis contributed to clinical deterioration. Compared to more chronic lesion development associated with other enterohepatic helicobacter infections in IL-10−/− mice (24, 13), H. trogontum-induced typhlocolitis is an acute model of bacterial inflammatory bowel disease.
Lack of disease attributable to H. trogontum infection in B6 mice and the acute typhlocolitis, epithelial hyperplasia, and dysplasia induced by H. trogontum in the IL-10−/− mice are consistent with previously reported models using H. hepaticus (24), H. bilis (8), and H. typhlonius (13); however, the clinical course of H. trogontum infection in IL-10−/− mice was faster and the lesions more severe. H. trogontum did not colonize B6 mice to the same level as IL-10−/− mice for unknown reasons, but lack of typhlocolitis in the B6 mice is likely more closely related to normal homeostatic IL-10 responses than to lower colonization. IL-10 is a Th2-associated cytokine with potent anti-inflammatory properties and has been shown to be critical to T-regulatory-cell function in a mouse model of H. hepaticus-mediated colon cancer (11). The acute nature of severe inflammation caused by H. trogontum infection in IL-10−/− mice provides a rapid-onset model for dysregulated intestinal epithelial cell responses to a bacterial pathogen.
The potential for H. trogontum to cause disease in mice with complex intestinal flora was previously unrecognized. However, a series of reports detailed how H. trogontum monoassociation in germfree outbred NIH mice caused vacuolation of ileal epithelial cells, loss of microvilli, and pronounced desquamation of cecal enterocytes (31). H. trogontum colonized the stomachs, ceca, and colons of these monoassociated mice and caused moderate, diffuse inflammation within at least one segment of the gastrointestinal tract in individual mice (30). Although H. trogontum was not recoverable from the liver, focal infiltration of inflammatory cells in the liver was suggestive of H. trogontum-associated hepatitis in monoassociated outbred NIH mice evaluated at 6, 12, and 18 months PI (32). Although consistent with H. hepaticus-associated (16, 14) and H. bilis-associated (15) hepatobiliary disease of selected inbred and outbred mice, infection with H. trogontum in the IL-10−/− and B6 mice did not cause liver lesions except for mild portal hepatitis (data not shown) in helicobacter-infected IL-10−/− mice. Helicobacter-associated hepatobiliary disease would not be expected in B6 mice (20, 48), particularly when they are infected for a short time. We believe that the mild portal infiltrates observed in the mice euthanized at day 8 after H. trogontum infection reflected antigenic stimulation from the lower bowel via the portal circulation.
Reports on H. rodentium indicate it may have a role in potentiating disease but probably causes minimal host tissue response in normal mice. Both H. rodentium (25, 34, 18) and H. trogontum (28) have been detected in laboratory rats by PCR or culture, with no reports of clinical disease or histologic bowel lesions. Of the formally named enterohepatic helicobacters, only H. bilis (43, 26), H. hepaticus (24), and H. typhlonius (13) have been shown to cause typhlocolitis in immunodeficient or immune-dysregulated mice, such as IL-10−/− mice. Prkdcscid/Tpr53tm1tyi mice on a B6.129/Sv × C.B-17 background developed severe diarrhea and typhlocolitis from coinfection with H. rodentium and H. bilis (42), although H. rodentium monoinfection was shown to be nonpathogenic in A/JCr and C.B-17/IcrCrl-scidBr mice (33). H. rodentium and H. hepaticus coinfection in C.B-17/IcrCrl-scidBr mice has also been shown to augment cecal gene expression and produce more severe clinical disease than can be attributed solely to H. hepaticus (33). H. rodentium was also recently shown to promote cholesterol gallstone formation in C57BL mice coinfected with H. hepaticus and fed a lithogenic diet (27).
Infection with H. trogontum in IL-10−/− mice in the second experiment quickly achieved higher levels of H. trogontum colonization than in coinfected mice from the first experiment, even though the mice received one less experimental dose of H. trogontum. Relatively higher H. trogontum colonization in the absence of H. rodentium may have been responsible for the acute morbidity and development of erosive typhlocolitis noted in the second and third experiments. The initial appearance of a gender bias for more severe disease in coinfected male IL-10−/− mice of the first experiment may have been related to preexisting H. rodentium infection. At necropsy, male and female IL-10−/− mice in the first experiment were colonized with H. trogontum at similar levels (data not shown), but H. rodentium infection may have primed an immune response against experimental challenge with H. trogontum or otherwise initially inhibited H. trogontum colonization in some mice, thereby allowing more time before colonization levels rose and morbidity developed.
The livers from the H. trogontum-infected mice in the third experiment were culture positive for E. coli and Proteus spp., and mild portal hepatitis was observed histologically. Mononuclear cells accumulating around the portal vessels may have responded to antigenic stimulation from the inflamed lower bowel. Recovery of the intestinal bacteria by culture from the liver may indicate true sepsis or, at a minimum, enhanced mucosal permeability from inflammation. Less probably, agonal events in concert with inflammatory damage to the epithelium may have allowed translocation of bacteria to the portal circulation (29).
Additionally, as observed in this study, it is unknown how H. trogontum acutely impacts the population balance of other resident flora. As a broad indicator of potential alterations in colon bacterial populations, many of which are unculturable, we used quantitative PCR to estimate levels of colonization by each of the eight ASF anaerobes that naturally colonize mice and have historically been used to colonize germfree mice to reestablish normal gut physiology. H. trogontum infection acutely lowered colonization density of five of the eight ASF species, which is consistent with observations that both murine enterohepatic Helicobacter spp. and the ASF anaerobes colonize mice in high numbers at the cecal-colonic junction and lower bowel (38). Others have also shown using terminal restriction fragment length polymorphisms that H. hepaticus infection decreased overall diversity of unculturable microbiota of the cecum in B6 mice within 14 days PI (23). Profiles of denaturing gradient gel electrophoresis of PCR products indicated that colon microbiota were altered by developing colitis in IL-10−/− mice (5); in particular, loss of lactobacilli and an increase in a Bacteroides sp. that has 99% identity by 16S rRNA gene sequence analysis to the Bacteroides organism ASF519, which in our study remained elevated despite H. trogontum-associated inflammation, were observed. H. trogontum may have inhibited ASF levels by direct competition for nutrients or other factors in the natural niche in the lower bowel, or, more likely, ASF inhibition was indirectly influenced by the inflammatory response elicited by H. trogontum. The latter possibility is supported by a previous report that H. hepaticus infection had limited impact on levels of colonization by ASF strains in outbred Swiss Webster mice that did not develop helicobacter-associated colitis (17). These results suggest that acute typhlocolitis impacted the normal resident intestinal flora and may have indirect consequences of inhibiting beneficial organisms or promoting overgrowth of opportunistic bacteria, such as E. coli and Proteus spp. cultured from the livers of three IL-10−/− mice.
Our results demonstrate that concurrent infection with H. trogontum and H. rodentium or infection with H. trogontum alone in IL-10−/− mice on the B6 background causes acute erosive typhlocolitis with the risk of sepsis. These models should prove useful in dissecting the pathogenesis of various clinical and pathological features noted in inflammatory bowel disease of humans. Further studies using germfree mice subsequently infected with one or more ASF species in combination with H. trogontum or other enterohepatic helicobacters are warranted to further establish the role of Helicobacter and ASF species and other resident flora in gut homeostasis and disease during pathogenic bacterial infections.
Acknowledgments
This work was supported in part by RO1-CA67529 and AI50952 from the National Institutes of Health (J.G.F.) and by Center for Environmental Health Sciences grants P30ES02109 and P01-CA26731 (J.G.F.).
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovia, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Archer, J. R., S. Romero, A. E. Ritchie, M. E. Hamacher, B. M. Steiner, J. H. Bryner, and R. F. Schell. 1988. Characterization of an unclassified microaerophilic bacterium associated with gastroenteritis. J. Clin. Microbiol. 26:101-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balish, E., and T. Warner. 2002. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am. J. Pathol. 160:2253-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, D. J., N. Davidson, R. Kuhn, W. Muller, S. Menon, G. Holland, L. Thompson-Snipes, M. W. Leach, and D. Rennick. 1996. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J. Clin. Investig. 98:1010-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibiloni, R., M. A. Simon, C. Albright, B. Sartor, and G. W. Tannock. 2005. Analysis of the large bowel microbiota of colitic mice using PCR/DGGE. Lett. Appl. Microbiol. 41:45-51. [DOI] [PubMed] [Google Scholar]
- 6.Boivin, G. P., K. Washington, K. Yang, J. M. Ward, T. P. Pretlow, R. Russell, D. G. Besselsen, V. L. Godfrey, T. Doetschman, W. F. Dove, H. C. Pitot, R. B. Halberg, S. H. Itzkowith, J. Groden, and R. J. Coffey. 2003. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 124:762-777. [DOI] [PubMed] [Google Scholar]
- 7.Bryner, J. H., A. E. Ritchie, L. Pollet, C. A. Kirkbride, and J. E. Collins. 1987. Experimental infection and abortion of pregnant guinea pigs with a unique spirillum-like bacterium isolated from aborted ovine fetuses. Am. J. Vet. Res. 48:91-95. [PubMed] [Google Scholar]
- 8.Burich, A., R. Hershberg, K. Waggie, W. Zeng, T. Brabb, G. Westrich, J. L. Viney, and L. Maggio-Price. 2001. Helicobacter-induced inflammatory bowel disease in IL-10- and T cell-deficient mice. Am. J. Physiol. Gastrointestinal Liver Physiol. 281:G764-G778. [DOI] [PubMed] [Google Scholar]
- 9.Dewhirst, F. E., C. C. Chien, B. J. Paster, R. L. Ericson, R. P. Orcutt, D. B. Schauer, and J. G. Fox. 1999. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl. Environ. Microbiol. 65:3287-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewhirst, F. E., J. G. Fox, E. N. Mendes, B. J. Paster, C. E. Gates, C. A. Kirkbride, and K. A. Eaton. 2000. ′Flexispira rappini' strains represent at least 10 Helicobacter taxa. Int. J. Syst. Evol. Microbiol. 50(Pt. 5):1781-1787. [DOI] [PubMed] [Google Scholar]
- 11.Erdman, S. E., V. P. Rao, T. Poutahidis, M. M. Ihrig, Z. Ge, Y. Feng, M. Tomczak, A. B. Rogers, B. H. Horwith, and J. G. Fox. 2003. CD4+ CD25+ regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 63:6042-6050. [PubMed] [Google Scholar]
- 12.Fox, J. G., F. E. Dewhirst, Z. Shen, N. S. Taylor, B. J. Paster, R. L. Ericson, C. N. Lau, P. Correa, J. C. Araya, and I. Roa. 1998. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology 114:755-763. [DOI] [PubMed] [Google Scholar]
- 13.Fox, J. G., P. L. Gorelick, M. C. Kullberg, Z. Ge, F. E. Dewhirst, and J. M. Ward. 1999. A novel urease-negative Helicobacter species associated with colitis and typhlitis in IL-10-deficient mice. Infect. Immun. 67:1757-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox, J. G., X. Li, L. Yan, R. J. Cahill, R. Hurley, R. Lewis, and J. C. Murphy. 1996. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect. Immun. 64:1548-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox, J. G., A. B. Rogers, M. T. Whary, N. S. Taylor, S. Xu, Y. Feng, and S. Keys. 2004. Helicobacter bilis-associated hepatitis in outbred mice. Comp. Med. 54:571-577. [PubMed] [Google Scholar]
- 16.Fox, J. G., L. Yan, B. Shames, J. Campbell, J. C. Murphy, and X. Li. 1996. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect. Immun. 64:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge, Z., Y. Feng, N. S. Taylor, M. Ohtani, M. F. Polz, D. B. Schauer, and J. G. Fox. 2006. Colonization dynamics of altered Schaedler flora is influenced by gender, aging, and Helicobacter hepaticus infection in the intestines of Swiss Webster mice. Appl. Environ. Microbiol. 72:5100-5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto, K., H. Ohashi, A. Takakura, and T. Itoh. 2000. Current status of Helicobacter contamination of laboratory mice, rats, gerbils, and house musk shrews in Japan. Curr. Microbiol. 41:161-166. [DOI] [PubMed] [Google Scholar]
- 19.Hänninen, M. L., M. Utriainen, I. Happonen, and F. E. Dewhirst. 2003. Helicobacter sp. flexispira 16S rDNA taxa 1, 4 and 5 and Finnish porcine Helicobacter isolates are members of the species Helicobacter trogontum (taxon 6). Int. J. Syst. Evol. Microbiol. 53:425-433. [DOI] [PubMed] [Google Scholar]
- 20.Ihrig, M., M. D. Schrenzel, and J. G. Fox. 1999. Differential susceptibility to hepatic inflammation and proliferation in AXB recombinant inbred mice chronically infected with Helicobacter hepaticus. Am. J. Pathol. 155:571-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkbride, C. A., C. E. Gates, J. E. Collins, and A. E. Ritchie. 1985. Ovine abortion associated with an anaerobic infection. JAVMA. 186:789-791. [PubMed] [Google Scholar]
- 22.Kostia, S., P. Veijalainen, U. Hirvi, and M. L. Hanninen. 2003. Cytolethal distending toxin B gene (cdtB) homologues in taxa 2, 3 and 8 and in six canine isolates of Helicobacter sp. flexispira. J. Med. Microbiol. 52:103-108. [DOI] [PubMed] [Google Scholar]
- 23.Kuehl, C. J., H. D. Wood, T. L. Marsh, T. M. Schmidt, and V. B. Young. 2005. Colonization of the cecal mucosa by Helicobacter hepaticus impacts the diversity of the indigenous microbiota. Infect. Immun. 73:6952-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kullberg, M. C., J. M. Ward, P. L. Gorelick, P. Caspar, S. Hieny, A. Cheever, D. Jankovic, and A. Sher. 1998. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect. Immun. 66:5157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingston, R. S., and L. K. Riley. 2003. Diagnostic testing of mouse and rat colonies for infectious agents. Lab. Anim. 32:44-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maggio-Price, L., H. Bielefeldt-Ohmann, P. Treuting, B. M. Iritani, W. Zeng, A. Nicks, M. Tsang, D. Shows, P. Morrissey, and J. L. Viney. 2005. Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a-/- mice results in colitis that progresses to dysplasia. Am. J. Pathol. 166:1793-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurer, K. J., M. M. Ihrig, A. B. Rogers, V. Ng, G. Bouchard, M. R. Leonard, M. C. Carey, and J. G. Fox. 2005. Identification of cholelithogenic enterohepatic helicobacter species and their role in murine cholesterol gallstone formation. Gastroenterology 128:1023-1033. [DOI] [PubMed] [Google Scholar]
- 28.Mendes, E. N., D. M. Queiroz, F. E. Dewhirst, B. J. Paster, S. B. Moura, and J. G. Fox. 1996. Helicobacter trogontum sp. nov., isolated from the rat intestine. Int. J. Syst. Bacteriol. 46:916-921. [DOI] [PubMed] [Google Scholar]
- 29.Morris, J. A., L. M. Harrison, and S. M. Partridge. 2006. Postmortem bacteriology: a re-evaluation. J. Clin. Pathol. 59:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moura, S. B., E. N. Mendes, D. M. Queiroz, J. R. Nicoli, M. M. Cabral, P. P. Magalhaes, G. A. Rocha, and E. C. Vieira. 1999. Microbiological and histological study of the gastrointestinal tract of germ-free mice infected with Helicobacter trogontum. Res. Microbiol. 150:205-212. [DOI] [PubMed] [Google Scholar]
- 31.Moura, S. B., E. N. Mendes, D. M. M. Queiroz, E. R. S. Camargos, M. E. F. Fonseca, G. A. Rocha, and J. R. Nicoli. 1998. Ultrastructure of Helicobacter trogontum in culture and in the gastrointestinal tract of gnotobiotic mice. J. Med. Microbiol. 47:513-520. [DOI] [PubMed] [Google Scholar]
- 32.Moura, S. B., D. M. Queiroz, G. A. Rocha, L. B. Comunian, and D. C. Cara. 2003. Hepatic changes in mice chronically infected with Helicobacter trogontum. Braz. J. Med. Biol. Res. 36:1209-1213. [DOI] [PubMed] [Google Scholar]
- 33.Myles, M. H., R. S. Livingston, and C. L. Franklin. 2004. Pathogenicity of Helicobacter rodentium in A/JCr and SCID mice. Comp. Med. 54:549-557. [PubMed] [Google Scholar]
- 34.Riley, L. K., C. L. Franklin, R. R. Hook, Jr., and C. Besch-Williford. 1996. Identification of murine helicobacters by PCR and restriction enzyme analyses. J. Clin. Microbiol. 34:942-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson, B. R., J. L. O'Rourke, B. A. Neilan, P. Vandamme, S. L. On, J. G. Fox, and A. Lee. 2005. Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. Int. J. Syst. Evol. Microbiol. 55:1199-1204. [DOI] [PubMed] [Google Scholar]
- 36.Rogers, A. B., and J. G. Fox. 2004. Inflammation and cancer. I. Rodent models of infectious gastrointestinal and liver cancer. Am. J. Physiol. Gastrointestinal Liver Physiol. 286:G361-G366. [DOI] [PubMed] [Google Scholar]
- 37.Romero, S., J. R. Archer, M. E. Hamacher, S. M. Bologna, and R. F. Schell. 1988. Case report of an unclassified microaerophilic bacterium associated with gastroenteritis. J. Clin. Microbiol. 26:142-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarma-Rupavtarm, R. B., Z. Ge, D. B. Schauer, J. G. Fox, and M. F. Polz. 2004. Spatial distribution and stability of the eight microbial species of the altered Schaedler flora in the mouse gastrointestinal tract. Appl. Environ. Microbiol. 70:2791-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saunders, K. E., Z. L. Shen, F. E. Dewhirst, B. J. Paster, C. A. Dangler, and J. G. Fox. 1999. Novel intestinal Helicobacter species isolated from cotton-top tamarins (Saguinus oedipus) with chronic colitis. J. Clin. Microbiol. 37:146-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shames, B., J. G. Fox, F. Dewhirst, L. L. Yan, Z. L. Shen, and N. S. Taylor. 1995. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J. Clin. Microbiol. 33:2968-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen, Z., J. G. Fox, F. E. Dewhirst, B. J. Paster, C. J. Foltz, L. Yan, B. Shames, and L. Perry. 1997. Helicobacter rodentium sp. nov., a urease-negative Helicobacter species isolated from laboratory mice. Int. J. Syst. Bacteriol. 47:627-634. [DOI] [PubMed] [Google Scholar]
- 42.Shomer, N. H., C. A. Dangler, R. P. Marini, and J. G. Fox. 1998. Helicobacter bilis/Helicobacter rodentium co-infection associated with diarrhea in a colony of scid mice. Lab. Anim. Sci. 48:455-459. [PubMed] [Google Scholar]
- 43.Shomer, N. H., C. A. Dangler, M. D. Schrenzel, and J. G. Fox. 1997. Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infect. Immun. 65:4858-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suerbaum, S., C. Josenhans, T. Sterzenbach, B. Drescher, P. Brandt, M. Bell, M. Droge, B. Fartmann, H. P. Fischer, Z. Ge, A. Horster, R. Holland, K. Klein, J. Konig, L. Macko, G. L. Mendz, G. Nyakatura, D. B. Schauer, Z. Shen, J. Weber, M. Frosch, and J. G. Fox. 2003. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc. Natl. Acad. Sci. USA 100:7901-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swidsinski, A., A. Ladhoff, A. Pernthaler, S. Swidsinski, V. Loening-Baucke, M. Ortner, J. Weber, U. Hoffmann, S. Schreiber, M. Dietel, and H. Lochs. 2002. Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44-54. [DOI] [PubMed] [Google Scholar]
- 46.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. Mckenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 47.Ullman-Culleré, M. H., and C. J. Foltz. 1999. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab. Anim. Sci. 49:319-323. [PubMed] [Google Scholar]
- 48.Whary, M. T., J. Cline, A. King, Z. Ge, Z. Shen, B. Sheppard, and J. G. Fox. 2001. Long-term colonization levels of Helicobacter hepaticus in the cecum of hepatitis-prone A/JCr mice are significantly lower than those in hepatitis-resistant C57BL/6 mice. Comp. Med. 51:413-417. [PubMed] [Google Scholar]
- 49.Whary, M. T., and J. G. Fox. 2006. Detection, eradication, and research implications of Helicobacter infections in laboratory rodents. Lab. Anim. (N.Y.) 35:25-36. [DOI] [PubMed] [Google Scholar]