Abstract
The hypothesis that tumor necrosis factor (TNF) aggravates malaria in children is supported by observations that TNF polymorphisms and high TNF levels have been associated with cerebral malaria. Nevertheless, severe malaria was not associated with polymorphisms located at positions −308A and −238A in the TNF alpha gene promoter or with a high TNF level in plasma in children from Bamako, Mali.
The sequestration of parasitized and nonparasitized erythrocytes in the microvasculature and imbalances in the inflammatory response are thought to be crucial in the development of cerebral malaria (CM) (13, 16). This view is further supported by the report of associations between polymorphisms in ICAM-1 and CD-36 and severe malaria (11, 15, 20).
Among inflammatory cytokines, tumor necrosis factor (TNF) could affect the outcome of Plasmodium falciparum infection in several ways. TNF promotes fever, that may suppress parasite growth (18), and it also induces the expression of adhesion molecules and proinflammatory molecules. Lethal CM has been associated with a high level of TNF in serum (9, 12). The TNF alpha (TNFA)-308A/A genotype has been associated with CM in Gambian children (14, 15) but not in a Thai population (7), and it is unclear whether the polymorphism located at position −308A in TNF alpha (TNFA-308G/A) has a functional effect (1, 4). Observations in TNF−/− mice are not conclusive, and it seems that linkage disequilibrium between the TNF gene and another gene may account for the association between the TNF gene and severe Plasmodium berghei infection (5).
The present study tested the association of TNFA alleles with severe malaria in children hospitalized for either cerebral malaria (CM) or severe anemia (SA) at the Gabriel Touré Hospital in Bamako, Mali. The study included 1,105 participants clustered in 348 families and 42 unrelated children with uncomplicated malaria (UM) for the cytokine assay. The criteria used to define the CM and UM phenotypes were as previously described (3). The SA children were subjects with a thick blood film positive for P. falciparum, a packed cell volume of ≤15%, and no impaired consciousness.
The study was approved by the Ethics Committee of the Faculty of Medicine of Bamako. Informed consent was obtained from the parents, and 2 to 5 ml of peripheral blood was taken from each child and from both parents (or siblings if only one parent was available) of the children with severe malaria. Plasma and DNA were obtained as described previously (3).
Lack of association between TNFA-308 alleles or TNFA-238 alleles and severe malaria.
We analyzed the TNFA-308G/A polymorphism in 208 families (136 CM and 72 SA) and the TNFA-238G/A polymorphism in 348 families (240 CM and 108 SA), with no parental inconsistencies (3). No significant deviation from the Hardy-Weinberg equilibrium was observed for both TNFA-308G/A and TNFA-238G/A in unrelated first-degree relatives of the CM groups (P = 0.308 and P = 0.192) and of the SA groups (P = 0.730 and P = 0.170) (Genepop program V3.4) (6). These alleles were not in linkage disequilibrium (LD) (Genepop program) (22).
TNFA-308G/A polymorphism in the promoter of the TNFA gene was determined as in the study by Wilson et al. (24) and Moukoko et al. (19). TNFA-308G was a common allele (frequency of 0.86). A total of 60 of the 136 children with CM and 31 of the 72 children with SA had at least one heterozygous parent (father and/or mother) and thus were informatives for statistical analysis. FBAT V1.4 (8, 23) analysis showed that neither of the TNFA-308 alleles was preferentially associated with disease (Table 1).
TABLE 1.
Lack of association between TNFA-308G/A and TNFA-238G/A and severe malariaa
| Phenotype | Allele | Allele frequencyb | No. of informative familiesc | No. of transmitted alleles
|
Zf | P | |
|---|---|---|---|---|---|---|---|
| Observedd | Expectede | ||||||
| CM | 308G | 0.86 | 89.0 | 86.0 | 0.74 | ||
| 308A | 0.14 | 60 | 31.0 | 34.0 | −0.74 | 0.46 | |
| SA | 308G | 0.86 | 44.0 | 43.5 | 0.174 | ||
| 308A | 0.14 | 31 | 18.0 | 18.5 | −0.174 | 0.86 | |
| CM | 238G | 0.96 | 48.0 | 46.3 | 0.56 | ||
| 238A | 0.04 | 32 | 16.0 | 17.7 | −0.56 | 0.57 | |
| SA | 238G | 0.97 | 19 | 16.5 | 1.51 | ||
| 238A | 0.03 | 11 | 3 | 5.5 | −1.51 | 0.13 | |
The analysis was carried out with FBAT using the additive model and a biallelic test.
Calculated from the genotype of the parents.
With at least one heterozygous parent.
That is, the number of alleles transmitted from heterozygous parents to their affected child.
That is, the number of alleles transmitted to an affected child on the hypothesis of no linkage disequilibrium.
Z = [S − E(S)]/Ã[overln]Var(S)[/overln], where S is the test statistic (i.e., observed), E(S) is the expected value according to the null hypothesis (H0), and Var(S) is the variance of the statistic test according to H0.
The TNFA-238G/A polymorphism was determined as described previously (19). TNFA-238G was the common allele (0.95), and TNFA-238A was rare. A total of 32 of 240 children with CM and 11 of 108 children with SA had at least one heterozygous parent. Neither of the TNFA-238 alleles was preferentially transmitted from a heterozygous parent to an affected (CM or SA) child (Table 1).
The TNFA-376G/A polymorphism in the promoter of the TNFA gene was determined as described previously (19). TNFA-376A was in total LD with TNFA-238A, as described by Knight et al. (10). However, the TNFA-376A allele was rare, and only four families were informative. Therefore, this allele could not be evaluated in the association test.
Since our results did not reach statistical significance, we performed power studies for the minor allele of the two SNPs, using PBAT v3.2, under the following assumptions: a type I error of 0.05, a population prevalence of the disease of 0.04 as derived previously (21), and an odds ratio of 3.6 as derived in an earlier study (10) for TNFA-308 and CM. PBAT yielded <0.50 power for the TNFA-238 polymorphism; TNFA-308A yielded 0.91 and 0.62 powers for CM and SA, respectively.
TNF levels were low and similar between severe malaria and UM.
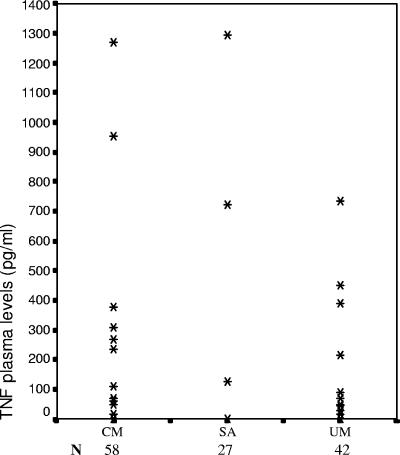
We measured the TNF levels by ELISA in the undiluted plasma (BD Pharmingen) of 42 children with UM, 58 children with CM, and 27 children with SA according to the manufacturer's instructions. Unmatched groups were compared by using the Mann-Whitney U test (SPSS 10.1 software), with P values of <0.05 being considered significant. The levels of TNF in the plasma of children with UM, CM, and SA were low (median 1pg/ml) and not significantly different (Fig. 1).
FIG. 1.
TNF levels in plasma of the studied children. Each column represents the plasma level observed for each phenotype; each point represents data from one or several children. The detection threshold was 10 pg/ml. The data are presented as arithmetic means of duplicate values.
In conclusion, we found no evidence of an association between the TNFA-308 or TNFA-238 alleles and either CM or SA in Malian children in spite of a large number of informative families for TNFA-308. Our analysis also failed to detect significant differences in TNF plasma levels between children with CM or SA and control children. We should stress that definitive conclusions cannot be drawn with respect to the associations between TNFA-308 and SA, as well as TNFA-238 and SA or CM, since our data yielded a relatively low power to detect them. In contrast, there is much stronger evidence that, in Malian children, the association between TNFA-308 and CM is either absent or, at least, present at a much lower level than has been described in other African populations (10, 14, 15). This discrepancy may have several explanations. First, the causative allele might not be TNFA-308A (and probably not TNFA-238A) but some other alleles in LD. This LD would be lost in certain populations such as the Malian and Thai populations (7). Second, children recruited in The Gambia (14, 15) may have been affected by a more severe disease than those recruited in Mali. This is suggested by the higher case fatality rate of the Gambian children with CM compared to our study participants. Thus, TNFA-308A may be specifically associated with a lethal outcome of CM. Histological observations on human brains have shown that TNF is produced in the small vessels of the brains of subjects who died from CM (2). Third, other polymorphisms located outside the TNFA gene may increase the risk of severe malaria in the Malian population, which is consistent with the low TNF levels in plasma in our patients. The lack of association between high TNF levels and CM might also be explained by the fact that the level of TNF measured by ELISA may not reflect the amount of active TNF in vivo. Indeed, it has been reported that high levels of soluble TNF receptors are also found in the plasma of patients with malaria and may neutralize bioactive TNF (17). Finally, the studies that have demonstrated an association of TNFA alleles with severe malaria were case-control studies, whose results are notoriously affected by population stratification bias. Thus, spurious associations cannot be ruled out. This limitation is precluded here by using a family-based study design.
Acknowledgments
We thank Laurent Argiro, Christophe Chevillard, Daouda Minta, and the staff of the pediatric wards of the Gabriel Toure Hospital in Bamako for their help.
This work was funded by the French Research Ministry (VIH-PAL grant) Action 2000 (INSERM reference HRA01G) and by EU grant IC18-CT98 0373 (INSERM reference RA036D). S.C. was supported by a fellowship from the VIH-PAL and FRM (Fondation pour la Recherche Médicale). We declare no conflict of interest.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Abraham, L. J., and K. M. Kroeger. 1999. Impact of the -308 TNF promoter polymorphism on the transcriptional regulation of the TNF gene: relevance to disease. J. Leukoc. Biol. 66:562-566. [DOI] [PubMed] [Google Scholar]
- 2.Brown, H., G. Turner, S. Rogerson, M. Tembo, J. Mwenechanya, M. Molyneux, and T. Taylor. 1999. Cytokine expression in the brain in human cerebral malaria. J. Infect. Dis. 180:1742-1746. [DOI] [PubMed] [Google Scholar]
- 3.Cabantous, S., B. Poudiougou, A. Traore, M. Keita, M. B. Cisse, O. Doumbo, A. J. Dessein, and S. Marquet. 2005. Evidence that interferon-gamma plays a protective role during cerebral malaria. J. Infect. Dis. 192:854-860. [DOI] [PubMed] [Google Scholar]
- 4.de Jong, B. A., R. G. Westendorp, A. M. Bakker, and T. W. Huizinga. 2002. Polymorphisms in or near tumour necrosis factor (TNF)-gene do not determine levels of endotoxin-induced TNF production. Genes Immun. 3:25-29. [DOI] [PubMed] [Google Scholar]
- 5.Engwerda, C. R., T. L. Mynott, S. Sawhney, J. B. De Souza, Q. D. Bickle, and P. M. Kaye. 2002. Locally up-regulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principle mediator of murine cerebral malaria. J. Exp. Med. 195:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo, S. W., and E. A. Thompson. 1992. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics 48:361-372. [PubMed] [Google Scholar]
- 7.Hananantachai, H., J. Patarapotikul, S. Looareesuwan, J. Ohashi, I. Naka, and K. Tokunaga. 2001. Lack of association of -308A/G TNFA promoter and 196R/M TNFR2 polymorphisms with disease severity in Thai adult malaria patients. Am. J. Med. Genet. 102:391-392. [DOI] [PubMed] [Google Scholar]
- 8.Horvath, S., X. Xu, and N. M. Laird. 2001. The family based association test method: strategies for studying general genotype-phenotype associations. Eur. J. Hum. Genet. 9:301-306. [DOI] [PubMed] [Google Scholar]
- 9.Kern, P., C. J. Hemmer, J. Van Damme, H. J. Gruss, and M. Dietrich. 1989. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am. J. Med. 87:139-143. [DOI] [PubMed] [Google Scholar]
- 10.Knight, J. C., I. Udalova, A. V. Hill, B. M. Greenwood, N. Peshu, K. Marsh, and D. Kwiatkowski. 1999. A polymorphism that affects OCT-1 binding to the TNF promoter region is associated with severe malaria. Nat. Genet. 22:145-150. [DOI] [PubMed] [Google Scholar]
- 11.Kun, J. F., J. Klabunde, B. Lell, D. Luckner, M. Alpers, J. May, C. Meyer, and P. G. Kremsner. 1999. Association of the ICAM-1Kilifi mutation with protection against severe malaria in Lambarene, Gabon. Am. J. Trop. Med. Hyg. 61:776-779. [DOI] [PubMed] [Google Scholar]
- 12.Kwiatkowski, D., A. V. Hill, I. Sambou, P. Twumasi, J. Castracane, K. R. Manogue, A. Cerami, D. R. Brewster, and B. M. Greenwood. 1990. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336:1201-1204. [DOI] [PubMed] [Google Scholar]
- 13.Malaguarnera, L., and S. Musumeci. 2002. The immune response to Plasmodium falciparum malaria. Lancet Infect. Dis. 2:472-478. [DOI] [PubMed] [Google Scholar]
- 14.McGuire, W., A. V. Hill, C. E. Allsopp, B. M. Greenwood, and D. Kwiatkowski. 1994. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature 371:508-510. [DOI] [PubMed] [Google Scholar]
- 15.McGuire, W., J. C. Knight, A. V. Hill, C. E. Allsopp, B. M. Greenwood, and D. Kwiatkowski. 1999. Severe malarial anemia and cerebral malaria are associated with different tumor necrosis factor promoter alleles. J. Infect. Dis. 179:287-290. [DOI] [PubMed] [Google Scholar]
- 16.Miller, L. H., D. I. Baruch, K. Marsh, and O. K. Doumbo. 2002. The pathogenic basis of malaria. Nature 415:673-679. [DOI] [PubMed] [Google Scholar]
- 17.Molyneux, M. E., H. Engelmann, T. E. Taylor, J. J. Wirima, D. Aderka, D. Wallach, and G. E. Grau. 1993. Circulating plasma receptors for tumour necrosis factor in Malawian children with severe falciparum malaria. Cytokine 5:604-609. [DOI] [PubMed] [Google Scholar]
- 18.Mordmuller, B. G., W. G. Metzger, P. Juillard, B. M. Brinkman, C. L. Verweij, G. E. Grau, and P. G. Kremsner. 1997. Tumor necrosis factor in Plasmodium falciparum malaria: high plasma level is associated with fever, but high production capacity is associated with rapid fever clearance. Eur. Cytokine Netw. 8:29-35. [PubMed] [Google Scholar]
- 19.Moukoko, C. E., N. El Wali, O. K. Saeed, Q. Mohamed-Ali, J. Gaudart, A. J. Dessein, and C. Chevillard. 2003. No evidence for a major effect of tumor necrosis factor alpha gene polymorphisms in periportal fibrosis caused by Schistosoma mansoni infection. Infect. Immun. 71:5456-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pain, A., B. C. Urban, O. Kai, C. Casals-Pascual, J. Shafi, K. Marsh, and D. J. Roberts. 2001. A non-sense mutation in Cd36 gene is associated with protection from severe malaria. Lancet 357:1502-1503. [DOI] [PubMed] [Google Scholar]
- 21.Ranque, S., I. Safeukui, B. Poudiougou, A. Traore, M. Keita, D. Traore, M. Diakite, M. B. Cisse, M. M. Keita, O. K. Doumbo, and A. J. Dessein. 2005. Familial aggregation of cerebral malaria and severe malarial anemia. J. Infect. Dis. 191:799-804. [DOI] [PubMed] [Google Scholar]
- 22.Raymond, M., and F. Rousset. 1995. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J. Heredity 86:248-249. [Google Scholar]
- 23.Wilk, J. B., J. S. Volcjak, R. H. Myers, N. E. Maher, B. A. Knowlton, N. L. Heard-Costa, S. Demissie, L. A. Cupples, and A. L. DeStefano. 2001. Family-based association tests for qualitative and quantitative traits using single-nucleotide polymorphism and microsatellite data. Genet. Epidemiol. 21:S364-9. [DOI] [PubMed] [Google Scholar]
- 24.Wilson, A. G., F. S. di Giovine, A. I. Blakemore, and G. W. Duff. 1992. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum. Mol. Genet. 1:353. [DOI] [PubMed] [Google Scholar]