Abstract
Four recombinant forms of the cell-invasive adenylate cyclase toxin (CyaA) of Bordetella pertussis were compared for the ability to enhance protection against B. pertussis in mice when coadministered with an acellular pertussis vaccine (ACV). The four forms were as follows: fully functional CyaA, a CyaA form lacking adenylate cyclase enzymatic activity (CyaA*), and the nonacylated forms of these toxins, i.e., proCyaA and proCyaA*, respectively. None of these forms alone conferred significant (P > 0.05) protection against B. pertussis in a murine intranasal challenge model. Mice immunized with ACV alone showed significant (P < 0.05) reductions in bacterial numbers in the lungs after intranasal challenge compared with those for control mice. When administered with ACV, both CyaA and CyaA* further reduced bacterial numbers in the lungs of mice after intranasal challenge compared with those for ACV-immunized mice, but the enhanced protection was only significant (P < 0.05) with CyaA*. Coadministration of CyaA* with ACV caused a significant (P < 0.05) increase in immunoglobulin G2a antibody levels against pertactin compared with those in mice immunized with ACV alone. Spleen cells from mice immunized with ACV plus CyaA* secreted larger amounts of interleukin-5 (IL-5), IL-6, gamma interferon (IFN-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF) than did cells from mice immunized with ACV plus CyaA or ACV alone after stimulation in vitro with a mixture of B. pertussis antigens. Spleen cells from mice immunized with ACV plus CyaA* also secreted larger amounts of IFN-γ and GM-CSF than did cells from mice immunized with CyaA* alone after stimulation in vitro with CyaA*. Macrophages from mice immunized with ACV plus CyaA* produced significantly (P < 0.05) higher levels of nitric oxide than did macrophages from mice immunized with CyaA* alone, ACV alone, or ACV plus CyaA after stimulation in vitro with a mixture of B. pertussis antigens or heat-killed B. pertussis cells. These data suggest that the enhancement of protection provided by CyaA* was due to an augmentation of both Th1 and Th2 immune responses to B. pertussis antigens.
Bordetella pertussis is a gram-negative bacterium that causes whooping cough in humans, and this disease may be especially severe in young infants. Several virulence-associated factors have been implicated in the disease process, including toxins such as lipopolysaccharide (LPS), pertussis toxin (PT), and the adenylate cyclase toxin (CyaA) and adhesins such as filamentous hemagglutinin (FHA), pertactin (PRN), and fimbriae (25). However, disease can be prevented by immunization with whole-cell pertussis vaccines (WCVs) and with newer acellular pertussis vaccines (ACVs) containing up to five purified B. pertussis antigens (PAgs), namely, detoxified pertussis toxin (dPT), FHA, PRN, and fimbriae (types 2 and 3). ACVs are generally less reactogenic than WCVs (12), which is presumed to be due to reduced PT activity and significantly reduced amounts of LPS (40). However, some ACVs may be less efficacious than WCVs (36, 45). For humans, it has been shown that WCVs may preferentially prime Th1 (type 1 CD4+ T-cell) responses that favor cell-mediated immunity, in contrast with ACVs, which promote more mixed Th1/Th2 (type 2 CD4+ T-cell) responses and favor humoral immunity (2, 42, 43). Evidence has indicated that humoral immunity alone may not be sufficient to confer long-term protection against B. pertussis infection in both mice and humans (34, 43).
CyaA, a 177-kDa protein endowed with adenylate cyclase (AC) and cell-invasive abilities, is synthesized as a protoxin (proCyaA) that is posttranslationally acylated by a separate protein, CyaC. CyaA has two functional domains, namely, the C-terminal domain (about 1,300 amino acids), which has membrane-targeting and pore-forming activities (21), and the 400-amino-acid N-terminal domain, which has AC enzymatic activity. Interaction with and invasion of mammalian target cells are facilitated by acylation of CyaA, and upon entry into the cell, the N-terminal AC enzymatic moiety is activated by host calmodulin to produce supraphysiological levels of cyclic AMP (cAMP) (11). In immune effector cells, this impairs their phagocytic and bactericidal capabilities and induces apoptosis, features that are assumed to assist survival of the bacterium in the initial stages of respiratory tract colonization (16). Anti-CyaA antibodies have been shown to enhance phagocytosis of B. pertussis through neutralization of CyaA, which normally inhibits phagocytosis by neutrophil polymorphonuclear leukocytes (35, 53). An immune response to this toxin may therefore be important in preventing colonization of the host by B. pertussis. The immunogenic properties of CyaA are indicated by reports of anti-CyaA antibodies in sera from convalescent patients and patients vaccinated with WCVs (1, 6, 10, 14). Immunization with CyaA, purified from B. pertussis or in recombinant form from Escherichia coli, protected mice against intranasal challenge with virulent B. pertussis (6, 18, 19). In addition, CyaA coadministered with ovalbumin (19), keyhole limpet hemocyanin (41), or other B. pertussis antigens (27) has been shown to enhance the serum immunoglobulin G (IgG) responses to these bystander antigens in the mouse.
In a previous report (27), mice that had been immunized with fully active recombinant toxin (CyaA) or an enzymically inactive form (CyaA*) in combination with a mixture of B. pertussis antigens (dPT, FHA, and PRN) did not show significantly enhanced protection against aerosol challenge with B. pertussis compared to mice immunized with the B. pertussis antigen mixture alone. However, CyaA* and, to a lesser extent, CyaA were shown to stimulate both humoral and cellular immune responses to PT, FHA, and PRN. In those experiments, only one immunizing dose was given and the CyaA preparations contained relatively high endotoxin levels, with an average value of ∼175 endotoxin units/dose. There was a possibility that LPS in the CyaA preparations may have acted synergistically with CyaA (41) or with PT, FHA, or PRN to modulate the immune responses. In the present study, four different forms of highly purified recombinant CyaA which were low in LPS were prepared. These were used in a two-dose immunization schedule in mice to assess the relative contributions of the enzymatic and pore-forming/invasive activities of the toxin to adjuvant activity with an ACV for both humoral and cell-mediated responses and against intranasal challenge with B. pertussis.
MATERIALS AND METHODS
Expression and purification of different CyaA forms.
E. coli BL21(DE3) (F− ompT rB− mB−) was used as the host strain for the production of recombinant proteins. The construction and expression of plasmids pGW44, pGW44/188, and pGW54 have been described previously (27, 55). Coexpression of pGW44 or pGW44/188 with pGW54 in E. coli BL21(DE3) generates fully active acylated CyaA or an enzymically inactive, acylated CyaA (CyaA*) carrying a Leu-Gln dipeptide insertion between codons 188 and 189, respectively. The latter form retains cell invasiveness but is unable to raise intracellular cAMP levels (27). Expression of pGW44 or pGW44/188 alone in E. coli BL21(DE3) produces a nonacylated protoxin with enzymatic activity (proCyaA) or without enzymatic activity (proCyaA*), respectively. The recombinant proteins were purified as described previously (27), with the following modification: the CyaA inclusion bodies were washed twice with 1% (wt/vol) 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS] (Sigma) in buffer A (50 mM Tris-HCl, 5 mM EDTA [pH 8.0]), three times with 2 M urea in buffer A, and once with pyrogen-free water before solubilization in 8 M urea-50 mM Tris-HCl (pH 8.0). The solubilized crude CyaA preparations were purified by DEAE-Sepharose and phenyl-Sepharose chromatography. The purified proteins contained very low levels of endotoxin, at <0.1 endotoxin unit/μg protein, as determined using a Kinetic-QCL (Biowhittaker) Limulus amoebocyte lysate assay, where 10 endotoxin units are equivalent to 1 ng of endotoxin (38). Only proCyaA and CyaA were enzymically active, with specific activities of 760 ± 110 and 854 ± 47 μmol cAMP/min/mg protein, respectively, determined in the presence of 1 μM calmodulin by using a rapid conductimetric procedure (22).
Mouse immunization.
Groups of 10 female NIH mice (Harlan, United Kingdom), aged 3 to 4 weeks, were injected intraperitoneally on days 0 and 28 with 0.5-ml volumes of antigen preparations. These consisted of different CyaA forms alone (25 μg/dose in Dulbecco's phosphate-buffered saline [PBS] without calcium and magnesium [Gibco]) with 10% (vol/vol) alum [2% (wt/vol) Al(OH)3, 1.3% (wt/vol) Al2O3] (JMF, Denmark) or CyaA forms without alum in combination with one-eighth of a single human dose of a commercially available diphtheria, tetanus, and acellular pertussis vaccine (DTaP/ACV; Infanrix, GSK). This vaccine contains 25 μg of dPT, 25 μg of FHA, 8 μg of PRN, 30 IU of diphtheria toxoid, and 40 IU of tetanus toxoid with 0.5 mg of Al(OH)3 per single human dose. This dose of ACV was chosen because preliminary data indicated that it gave partial protection against B. pertussis intranasal challenge. Any additional protective effects due to inclusion of CyaA would therefore be apparent. Controls included two groups of mice immunized with PBS in alum only and one group immunized with ACV alone. Two weeks after the second immunization (day 42), five mice from each group were challenged intranasally with B. pertussis (see below), and the other five mice were sampled for sera, spleens, and peritoneal macrophages. Sera were obtained from heart bleeds, and the preparation of spleen cells and peritoneal macrophages from pooled cells from five mice was done as described previously (27).
Respiratory challenge.
The preparation of a B. pertussis strain 18.323 challenge suspension was performed as previously described (27). For intranasal challenge with B. pertussis, anesthetized mice were inoculated with a total of 20 μl (10 μl/nostril) of bacterial suspension containing ∼4 × 108 CFU/ml. After the challenge, lungs and tracheas were removed from mice in one PBS control group at 2 h and from mice in all groups on day 7. The lungs and trachea from each mouse of each group were homogenized in 1 ml of Casamino Acids solution (1% [wt/vol] Casamino Acids [Difco], 0.6% [wt/vol] NaCl). Viable counts were made by plating serial dilutions of the homogenate on charcoal agar (Oxoid) containing 10% (vol/vol) defibrinated horse blood (E and O Labs, United Kingdom), and the numbers were expressed as CFU/lungs and trachea (detection limit of 100 CFU/mouse).
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was performed as described previously (27), with the following adjustments. A peroxidase substrate solution (1.25 ml of 1% tetramethylbenzidine [Sigma] in dimethyl sulfoxide [Sigma] in 124 ml of acetate buffer [1.25 mM NaCH3COOH · 3H2O, 0.0016% {vol/vol} glacial acetic acid, and 37 μl of H2O2]) was used to detect total IgG. For determinations of IgG1 and IgG2a antibody levels, biotin-conjugated rat anti-mouse IgG1 or IgG2a (BD Pharmingen), followed by streptavidin-horseradish peroxidase conjugate (BD Pharmingen), was used at a 1-in-1,000 dilution. IgG1 and IgG2a were detected using SigmaFast substrate (Sigma), the reaction was stopped with 50 μl of 3 M HCl in all wells, and the absorbance values were read as optical densities at 493 nm (OD493 values; Anthos ELISA plate reader [Life Science International, United Kingdom]). For determinations of total IgG, IgG1, and IgG2a antibody levels to FHA, PRN, and PT, antibody titers relative to the First International Reference anti-Bordetella pertussis mouse serum (97/642; NIBSC) (which had been assigned arbitrary values for FHA, PRN, and PT in ELISA units/ml) were obtained by parallel-line analysis of log10 sample ODs against log10 dilutions.
In vitro assays for cytokines and nitric oxide from immune cells.
Peritoneal macrophages and spleen cells were cultured in 24-well tissue culture plates at 4 × 106 cells/well, with or without 5 × 107 heat-killed B. pertussis cells (HKCs)/well, as described by Xing et al. (58); with a mixture of formalin-treated PT, FHA, and PRN (GSK, Belgium) (identical to the components in the ACV used for immunization) at final concentrations of 2 μg/ml, 2 μg/ml, and 5 μg/ml, respectively; with the antigen mixture plus CyaA*; or with CyaA* (final concentration, 1 μg/ml) alone. Cultures were incubated at 37°C in 5% CO2 and 90% humidity for 24 h for macrophages and 48 h for spleen cells. Cell viability was confirmed using trypan blue exclusion.
Nitrite determinations for assay of NO release were made in triplicate with 50-μl volumes of macrophage cell culture supernatants after stimulation with antigens in vitro. The sample was mixed with 50 μl of Greiss reagent (48), and the OD540 was measured after 5 min at room temperature in an Anthos ELISA plate reader. The concentration of NO in the macrophage supernatants was calculated by using a standard curve for sodium nitrite (Sigma).
Detection of cytokines in supernatants of stimulated spleen cells was done by multiplex fluorescence bead technology (Bio-Rad). A mouse cytokine 10-plex kit (which detects interleukin-1β [IL-1β], IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, tumor necrosis factor alpha [TNF-α], granulocyte-macrophage colony-stimulating factor [GM-CSF], and gamma interferon [IFN-γ]) (BioSource) was used according to the manufacturer's recommended procedure. The concentrations of the cytokines were determined by comparison with supplied standards, which served as positive controls. Cell culture medium alone served as a negative control. The sensitivities for the cytokines were as follows: IL-1β, 10 pg/ml; IL-2, 20 pg/ml; IL-4, 5 pg/ml; IL-5, 10 pg/ml; IL-6, 10 pg/ml; IL-10, 15 pg/ml; IL-12, 15 pg/ml; TNF-α, 5 pg/ml; GM-CSF, 10 pg/ml; and IFN-γ, 1 pg/ml.
Statistical analyses.
CFU of bacteria, antibody levels, and nitric oxide production by several groups were compared with those for one group, using Dunnet's one-way analysis of variance (ANOVA). For comparison of two groups, Student's t test was used. Statistical significance was defined as P values of <0.05.
RESULTS
Mouse protection with ACV plus CyaA forms or with CyaA forms alone.
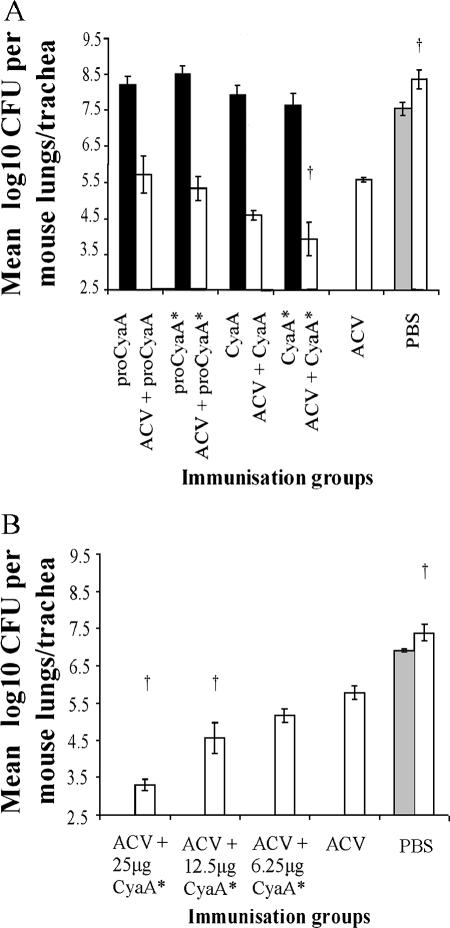
Groups of 10 mice were immunized on days 0 and 28 with four different CyaA forms (25 μg/dose), with and without ACV. On day 42, five mice from each group were challenged intranasally with B. pertussis 18.323, and the remaining five mice were sampled for sera, spleens, and peritoneal macrophages. A comparison of the bacterial counts from the lungs and tracheas of the two PBS control mouse groups, sampled at 2 h and 7 days postchallenge, respectively, showed an ∼1-log10 increase in bacterial counts at 7 days, confirming the growth and persistence of the challenge strain (Fig. 1A). Mice immunized with the different CyaA forms alone did not show significant protection against B. pertussis challenge (Fig. 1A). Mice immunized with ACV alone showed significant (P < 0.05) protection, as indicated by an ∼3-log10 reduction in counts compared with those for control mice (PBS group) (Fig. 1A). There were no significant differences by ANOVA in bacterial numbers in the lungs of mice immunized with ACV plus proCyaA, ACV plus proCyaA*, or ACV plus CyaA compared with those for mice immunized with ACV alone, although mice immunized with ACV plus CyaA showed a further ∼1-log10 reduction. However, administration of ACV plus CyaA* gave rise to a significant (P < 0.05) reduction in bacterial numbers (∼1.6 log10) compared with the administration of ACV alone (Fig. 1A).
FIG. 1.
Protection of mice against intranasal challenge with B. pertussis. (A) Mice were immunized intraperitoneally on days 0 and 28 with ACV alone, a CyaA form alone (25 μg/mouse), or ACV plus a CyaA form at 25 μg/mouse. Mice injected with PBS served as negative controls. Mice were challenged intranasally with B. pertussis 18.323 on day 42. Five mice from the PBS control group were sampled at 2 h postchallenge for enumeration of bacteria in lungs and tracheas (gray bar). All remaining mice immunized with the different CyaA forms alone (black bars) or with ACV plus the different CyaA forms (white bars) and mice from the PBS control group (white bars) were sampled at 7 days postchallenge. (B) Mice were immunized intraperitoneally on days 0 and 28 with ACV alone or with ACV plus graded doses (6.25, 12.5, and 25 μg per mouse) of CyaA*. Mice injected with PBS served as negative controls. Mice were challenged intranasally with B. pertussis 18.323 on day 42. Five mice from the PBS control group were sampled at 2 h postchallenge for enumeration of bacteria in lungs and tracheas (gray bar). All remaining mice immunized with ACV plus graded doses of CyaA* and mice from the PBS control group were sampled at 7 days postchallenge. Results represent the means of bacterial counts for five mice per group plus the standard errors of the means (SEM). †, P < 0.05 (group versus ACV-only group [ANOVA]).
A further study was performed to examine if the enhancement by CyaA* of the protection afforded by ACV was dose dependent (Fig. 1B). Again, there was no significant protection in mice immunized with any dose of CyaA* alone at 7 days postchallenge (data not shown). Mice immunized with ACV alone again showed significant (P < 0.05) protection compared with control mice (PBS group) (Fig. 1B), and coadministration of CyaA* gave further protection that was dose dependent (Fig. 1B). The enhanced protection was significant (P < 0.05) for the groups that had received ACV plus 25 μg or 12.5 μg of CyaA* in comparison to the group receiving ACV alone.
The ability of CyaA* to enhance the protective effect of ACV was tested in three independent experiments. Due to the nature of in vivo bioassays, the extents of reduction in bacterial colonization of the lungs and tracheas between mice that had received ACV alone and those that had received ACV plus CyaA* (25 μg/dose) appeared to be variable: log10 reductions of 1.65 (Fig. 1A), 2.49 (Fig. 1B), and 1.38 (data not shown) were obtained. Nevertheless, in each experiment, the reduction in bacteria in mice immunized with ACV plus CyaA* was significantly greater than that for the ACV control group (P < 0.05). The enhanced protective effect of CyaA* for ACV appeared to be more than the sum of the protective effects of CyaA* and ACV alone, as immunization with CyaA* alone afforded little protection against intranasal challenge (Fig. 1A).
Total IgG, IgG1, and IgG2a responses to FHA, PT, and PRN.
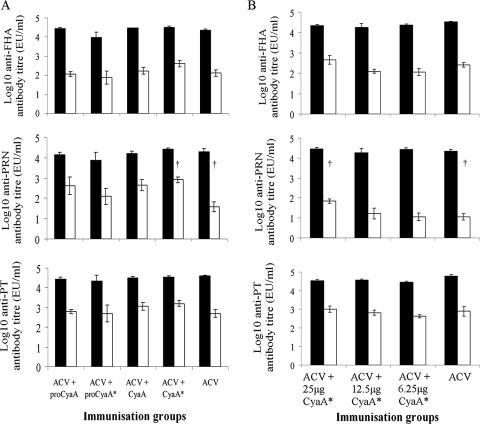
Immunization of mice with ACV plus the different CyaA forms did not significantly influence the total IgG antibody responses to PT, FHA, and PRN in serum on day 42 compared with those for mice immunized with ACV alone (P > 0.05) (data not shown). The IgG1 antibody levels to FHA, PRN, and PT were similar in mice immunized with ACV plus the different CyaA forms and in the ACV control group (Fig. 2A). Although there was an increase in IgG2a antibody levels to FHA, PRN, and PT in mice immunized with ACV plus CyaA or ACV plus CyaA* compared with those for the ACV-immunized group, these differences were not significant except for mice immunized with ACV plus CyaA*, which produced significantly (P < 0.05) greater levels of IgG2a to PRN than did mice immunized with ACV alone. These data were confirmed in a dose-response experiment (Fig. 2B), in which mice immunized with ACV plus the highest dose (25 μg) of CyaA* produced significantly (P < 0.05) greater levels of IgG2a towards PRN (Fig. 2B) than did mice immunized with ACV alone. Again, the total IgG levels (data not shown) and the levels of IgG1 antibodies to FHA, PRN, and PT were similar in all mice immunized with ACV, with or without different doses of CyaA* (Fig. 2B).
FIG. 2.
IgG1 (black bars) and IgG2a (white bars) antibody levels from mice immunized with (A) ACV, with or without different CyaA forms, or (B) ACV, with or without graded doses of CyaA*. A description of the immunization procedures is given in the legend to Fig. 1. Sera for antibody determinations were obtained on day 42. Results represent the mean antibody titers for five mice per group plus the SEM (error bars). †, P < 0.05 (IgG2a for ACV-plus-CyaA* group [25 μg] versus IgG2a for ACV group [Student's t test]).
Cytokine production by spleen cells after in vitro stimulation with B. pertussis antigens.
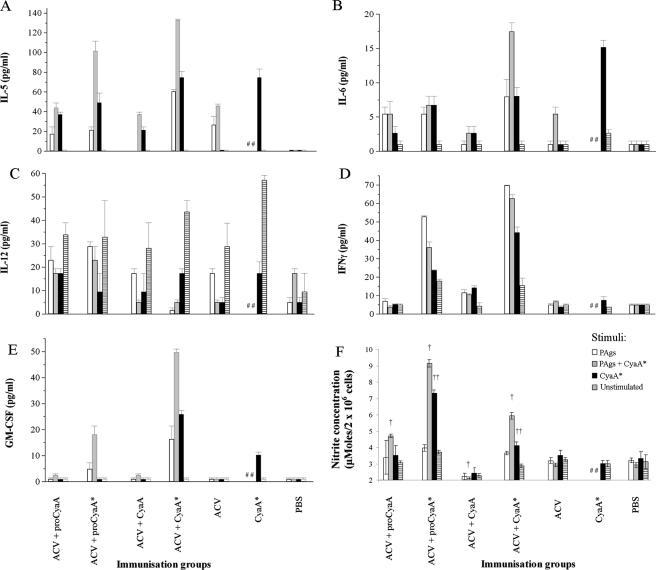
Figure 3 shows the results for five cytokines released from spleen cells collected from immunized mice on day 42 and then stimulated in vitro with a mixture of PAgs (consisting of formalin-treated PT, FHA, and PRN), with or without CyaA*, or with CyaA* alone. Antigen-stimulated spleen cells from mice given PBS served as controls. CyaA* was the form of CyaA chosen as the stimulant because it was less toxic than the native toxin and was nontoxic for spleen cells at 1 μg/ml.
FIG. 3.
Production of (A) IL-5, (B) IL-6, (C) IL-12, (D) IFN-γ, and (E) GM-CSF from spleen cells and of (F) nitric oxide from peritoneal macrophages from mice stimulated with different B. pertussis antigens for 48 h (spleen cells) or 24 h (macrophages). A description of the immunization procedure is given in the legend to Fig. 1A. Groups of mice were immunized with ACV alone, CyaA* alone, or ACV plus a CyaA form (25 μg per mouse) or were given PBS only. Spleen cells and macrophages were obtained on day 42, and the cells from five mice were pooled. PAgs (white bars), PAgs plus CyaA* (gray bars), and CyaA* alone (black bars) were used as stimuli. Other cells were not stimulated and served as controls (striped bars). PAgs consisted of a mixture of formalin-treated PT, FHA, and PRN, used at final concentrations of 2, 2, and 5 μg/ml, respectively. CyaA* was used at a final concentration of 1 μg/ml. Results represent the means for duplicate assays (for spleens) or triplicate assays (for macrophages) with SEM (error bars). †, P < 0.05 (PAg-plus-CyaA*-stimulated group versus PAg-plus-CyaA*-stimulated, ACV-immunized group [ANOVA]); ††, P < 0.05 (CyaA*-stimulated group versus CyaA*-stimulated, CyaA*-immunized group [ANOVA]); ##, stimulation with PAgs or PAgs plus CyaA* was not tested.
(i) Mice immunized with ACV.
Greater levels of IL-5 and IL-6 were produced by spleen cells from mice immunized with ACV than by spleen cells from the PBS control group, using PAgs plus CyaA* as a stimulant (Fig. 3A and B). As expected, the spleen cells from mice immunized with ACV alone did not respond to CyaA* as a stimulant. Spleen cells from the group receiving ACV alone did not produce any more IFN-γ or GM-CSF than did cells from PBS control mice (Fig. 3D and E). IL-12 production from unstimulated cells from the ACV-immunized group exceeded that of cells stimulated with specific antigens (Fig. 3C). This effect was not observed in the PBS control group.
(ii) Mice immunized with ACV plus CyaA or ACV plus proCyaA.
The cytokine profiles of spleen cells from mice immunized with ACV plus CyaA or ACV plus proCyaA were generally similar to that for the ACV control group upon antigen stimulation (Fig. 3A to E), although they responded well to CyaA* as a stimulant (Fig. 3A, B, D, and E). Again, IL-12 production from unstimulated cells exceeded that from antigen-stimulated cells (Fig. 3C).
(iii) Mice immunized with ACV plus CyaA*, ACV plus proCyaA*, or CyaA* alone.
Spleen cells from mice immunized with ACV plus CyaA* produced higher levels of IL-5 (∼130 pg/ml), IL-6 (∼20 pg/ml), IFN-γ (∼60 pg/ml), and GM-CSF (∼50 pg/ml) than did spleen cells from mice immunized with ACV (∼45 pg/ml for IL-5 and <5 pg/ml for IL-6, IFN-γ, and GM-CSF) when stimulated with PAgs plus CyaA*. Spleen cells from the ACV-plus-proCyaA* group also responded better with regard to IL-5, IFN-γ, and GM-CSF production than did cells from the group receiving ACV alone after stimulation by PAgs plus CyaA* (Fig. 3A, D, and E). Spleen cells from mice immunized with CyaA* alone produced lower levels of IFN-γ (<4 pg/ml) and GM-CSF (∼10 pg/ml) than did cells from mice immunized with ACV plus CyaA* when stimulated with CyaA*, although similar levels of IL-5 (∼70 pg/ml) and IL-6 (∼15 pg/ml) were recorded. It is noteworthy that only spleen cells from mice immunized with ACV plus CyaA*, ACV plus proCyaA*, or CyaA* alone produced larger amounts of GM-CSF than did cells from the PBS control group (Fig. 3E) in response to antigen stimulation. In addition, only spleen cells from mice immunized with ACV plus CyaA* or ACV plus proCyaA* produced large amounts of IFN-γ in response to specific antigen stimulation.
As expected, the spleen cells from mice immunized with ACV plus CyaA*, ACV plus proCyaA*, or CyaA* alone generally responded well to CyaA* as a stimulant (Fig. 3A, B, D, and E). This was not true, however, for IL-12, where again production by spleen cells from these groups was lower after PAg-plus-CyaA* or CyaA* stimulation than that in unstimulated cells (Fig. 3C).
(iv) Stimulation by HKCs.
No IL-5 was produced from any of the groups with HKCs as the stimulant (data not shown). HKCs stimulated high levels of IL-6 (40 to 120 pg/ml), IL-12 (180 to 250 pg/ml), and IFN-γ (150 to 600 pg/ml) from spleen cells from all mice, including the PBS control group. However, for GM-CSF, only cells from mice immunized with ACV plus proCyaA* (36 ± 13 pg/ml), ACV plus CyaA* (58 ± 3 pg/ml), or CyaA* alone (27 ± 7 pg/ml) produced GM-CSF in response to HKC stimulation.
(v) Other cytokines.
None of the antigens stimulated spleen cells to produce detectable IL-1β, IL-2, IL-4, or IL-10 (data not shown). However, HKC-stimulated spleen cells from all of the immunized groups and the PBS control group produced TNF-α at high levels (180 to 270 pg/ml), but only low-level responses (10 to 20 pg/ml) were seen upon stimulation with the other antigens.
Nitric oxide release by peritoneal macrophages after in vitro stimulation with B. pertussis antigens.
The levels of NO released from antigen-stimulated peritoneal macrophages from the ACV control group were similar to those for the PBS control group (Fig. 3F). Stimulation of peritoneal macrophages with PAgs plus CyaA* induced significantly greater (P < 0.05) production of NO from mice immunized with ACV plus proCyaA* or ACV plus CyaA* than that for the ACV or PBS control group (Fig. 3F). In addition, only macrophages from mice immunized with ACV plus proCyaA* or ACV plus CyaA* produced significantly (P < 0.05) greater levels of NO than those for the CyaA*-immunized group in response to CyaA* stimulation (Fig. 3F). Macrophages from the ACV-plus-CyaA group were the least responsive to antigen stimulation.
HKCs stimulated greater NO production from macrophages from all immunized mice than from macrophages from the PBS control group. Significantly higher levels (P < 0.05) of NO were produced in response to HKCs by macrophages from mice immunized with ACV plus proCyaA* (20 ± 0.2 μM/2 × 106 cells) or ACV plus CyaA* (15 ± 0.2 μM) than by those from mice immunized with ACV (12 ± 0.2 μM), ACV-plus-proCyaA (11.4 ± 0.2 μM), ACV-plus-CyaA (7.2 ± 0.1 μM), or CyaA* (10.6 ± 0.2 μM) or those from the PBS control group (2.5 ± 0.4 μM).
DISCUSSION
The objective of this study was to assess the relative contributions of the AC enzymatic activity and pore-forming/invasive features of CyaA to its protective and adjuvant properties when coadministered with an ACV. This was done by using four different purified recombinant forms of CyaA. All the CyaA forms in the present study contained very low levels of endotoxin (<0.01 endotoxin unit/μg protein). Thus, any potential synergistic effect of CyaA with LPS (41) would be minimized at the concentrations used.
Our data showed that posttranslational acylation of CyaA was essential for the enhanced effect of CyaA on protection afforded by the ACV against intranasal challenge with B. pertussis. Mice immunized twice with ACV plus CyaA or CyaA*, but not with one of the two nonacylated forms, proCyaA and proCyaA*, had reduced bacterial numbers in the lungs at 7 days postchallenge compared with the ACV-only group. The enhancement of protection by CyaA* was significant (P < 0.05), although that by CyaA was not, as determined by ANOVA. Although significant enhancements of protection by CyaA* for the ACV were obtained in three separate experiments, the comparison of the effects of different forms of CyaA on ACV was only done once. Thus, whether CyaA* could reproducibly enhance the protection afforded by the ACV to a greater extent than the other CyaA forms was not determined. The enhancement of protection by CyaA* for ACV was dose dependent.
Mice immunized with 25 μg of CyaA* alone were not significantly protected against intranasal challenge. This indicated that the enhanced protective effect of CyaA* with ACV was more than the sum of the protective effects of CyaA* and ACV alone. The lack of protection afforded by any of the CyaA forms is in contrast with previous reports, where recombinant CyaA was shown to act as a protective antigen in mouse models of B. pertussis infection (6, 18, 19). The reasons for this discrepancy are unclear but may be related to differences in antigen preparations (e.g., purity or LPS content), the route of immunization, the antigen dose, the aluminum adjuvant, or mouse strains compared with those used previously (6, 18, 19).
The enhanced protection against B. pertussis intranasal challenge in mice immunized with ACV plus CyaA* compared with that in mice immunized with ACV alone did not correlate with the total IgG antibody levels to FHA, PRN, and PT, as there were no statistically significant differences between these groups. This contrasts with previous work (19, 27, 41) which showed that CyaA or CyaA* could act as an adjuvant by enhancing the levels of total IgG antibodies to coadministered antigens. Again, differences in antigen preparations, the route of immunization, the antigen dose, the aluminum adjuvant, or mouse strains may explain this discrepancy. In particular, the two doses of ACV used here contained alum as an adjuvant, which would have resulted in increased antibody levels to FHA, PRN, and PT irrespective of the presence of the CyaA derivative.
ACVs have been shown to induce high levels of anti-pertussis IgG1 antibodies in mice (5), and this was also observed in this study, but the presence of the different CyaA forms did not alter the levels of anti-FHA, -PRN, or -PT IgG1 antibodies. However, mice immunized with ACV plus CyaA* generally promoted higher levels of IgG2a antibody production, and the levels against PRN were significantly higher than the levels in mice immunized with ACV alone. IgG2a has been implicated in opsonization and complement fixation of B. pertussis and is associated with superior protection (29, 34). PRN has been reported to play a role in the adhesion of B. pertussis to mammalian cells (13). Therefore, increased levels of anti-PRN IgG2a antibodies could presumably decrease the ability of B. pertussis to adhere to cells and could promote its clearance from the respiratory tract.
Humoral immunity alone may not be sufficient to confer long-term protection against B. pertussis infection, and the importance of cell-mediated immunity in the clearance of B. pertussis has been demonstrated (2, 4, 23, 32, 33, 39, 43). ACVs typically induce a Th2-associated T-cell response in mice, characterized by high levels of anti-pertussis IgG1 antibodies as well as Th2-associated cytokines, including IL-4 and IL-5, with little IFN-γ production (5, 32, 41). In humans, ACVs produce a more mixed Th1/Th2 response, including IL-4, IL-5, IFN-γ, and IgG2a production (2, 42, 43). Cytokines secreted by immune effector cells play a key role in determining IgG isotype production and the outcome of immune responses to infectious agents. In the present study, the cytokine profiles of spleen cells and NO production from peritoneal macrophages from immunized mice, after stimulation in vitro with various antigens, were rather complex, but some conclusions could be drawn from these data.
The spleen cells of mice immunized with ACV plus the nonenzymatic CyaA forms (CyaA* and proCyaA*) responded well to antigen stimulation by secreting higher levels of IL-5, IL-6, IFN-γ, and GM-CSF than those in cells from mice immunized with ACV alone. CyaA*-stimulated spleen cells from mice immunized with CyaA* alone were less able to produce IFN-γ and GM-CSF upon antigen stimulation than were spleen cells from mice immunized with ACV plus CyaA*, although they produced comparable levels of IL-5 and IL-6. Thus, the proCyaA* and CyaA* forms of CyaA appeared to promote a mixed Th1/Th2 response to B. pertussis antigens, which was most pronounced with the ACV-plus-CyaA* group. In particular, spleen cell production of IFN-γ and GM-CSF was greatly enhanced compared to that obtained from mice immunized with ACV or CyaA* alone, indicating that proCyaA*, and particularly CyaA*, had an adjuvant effect for production of these cytokines when administered with ACV. Spleen cells from mice immunized with ACV plus proCyaA or ACV plus CyaA responded to antigen stimulation in a similar way to that for cells from ACV-immunized mice, producing little or no IFN-γ or GM-CSF.
GM-CSF, which is produced by a range of cell types (24), activates immune effector cells (15, 51, 54), induces the maturation of dendritic cells for increased antigen presentation to promote both Th1 and Th2 immune responses (7, 26, 47, 49, 50), and induces the expression of CD11b/CD18 (CR3) receptors found on the surfaces of neutrophil polymorphonuclear leukocytes and monocytes (57). CR3 is a receptor for several B. pertussis virulence-associated factors, including FHA, PRN, and CyaA (13, 17, 20). Therefore, it is conceivable that increased CR3 expression, which might result from greater GM-CSF production, could enhance phagocytosis of B. pertussis. In addition, neutralizing anti-CyaA antibodies induced by mice immunized with ACV plus CyaA or ACV plus CyaA* (27) would block the inhibitory action of CyaA on neutrophil polymorphonuclear leukocytes (35, 52, 53) and also enhance clearance of the organism from the lungs. Macrophages from mice immunized with ACV plus CyaA* produced significantly more NO after antigen stimulation than did macrophages from mice immunized with CyaA* alone or ACV alone, suggesting that they are highly activated (9, 48, 58). Thus, increased levels of GM-CSF and NO in mice immunized with ACV plus CyaA* could also enhance the uptake and presentation of antigens through the activation of antigen-presenting cells, such as peritoneal macrophages (58) and dendritic cells (7, 49).
The HKC preparation was by far the best stimulant of cytokine production from spleen cells, except for that of IL-5, and of NO production from macrophages. In some cases, cytokine levels from the spleen cells of PBS control mice were similar to those in spleen cells from mice immunized with the antigen preparations when stimulated with HKCs. This is most likely due to the high level of LPS in the HKC preparation (28). LPS is a potent inducer of IL-12 in spleen cells. This cytokine was produced in large amounts by spleen cells from PBS control mice upon exposure to HKCs. On the other hand, less IL-12 was released from cells from immunized mice upon antigen stimulation than from unstimulated cells. This may have been due to the presence of FHA in the PAg antigen mixture, as FHA has been shown to suppress IL-12 production in mice (31). CyaA* treatment of spleen cells also appeared to suppress IL-12 production compared to that in unstimulated cells. It was clear that the level of LPS contamination in the PAg mixture or in the CyaA* preparation was insignificant, as little or no cytokine release was seen in the cells from the PBS control group upon stimulation with these antigens.
The data presented indicate that both CyaA and CyaA* have the ability to enhance the protective effects of an ACV, but they may act in different ways. AC enzymatic activity and binding of a receptor by the toxin may have distinct modulatory effects on cells of the immune system. Bagley et al. (3) reported that CyaA was a potent activator for the maturation of human monocyte-derived dendritic cells and that this activity was dependent on the ability of CyaA to raise intracellular cAMP concentrations. Also, CyaA and nonacylated CyaA were reported to induce antigen-specific CD4+ Th2 and Th1 regulatory cells, but an acylated, nonenzymically active form of CyaA, equivalent to the CyaA* used here, was unable to act in the same way as the enzymically active forms (8, 41), indicating that these effects are dependent on raised cAMP levels. The lack of adjuvanticity observed by Boyd et al. (8) for their CyaA* equivalent protein may be related to a different route of immunization or to the concentration of toxin that they used (1 μg/dose). Enzymically inactive mutants of the E. coli cAMP-elevating heat-labile toxin have no adjuvanticity at low doses, but their adjuvanticities are apparent at higher doses (37).
The AC enzymatic domain does not need to be active for efficient antigen presentation, as CyaA lacking AC activity and with T-cell epitopes inserted within the N-terminal AC domain can act as an efficient delivery vehicle to stimulate both humoral and cell-mediated immunity to these epitopes (46) as well as both CD8+ and CD4+ epitope-specific T cells, the latter of which are characterized as IFN-γ-producing Th1 cells (30, 44, 56). This indicates that the induction of these T-cell responses was by a mechanism not involving increased intracellular cAMP production. Thus, whereas CyaA may favor the induction of antigen-specific Th2-oriented responses via a mechanism dependent on increased intracellular cAMP, CyaA* appears to favor a more mixed Th1/Th2 response involving increased production of IL-5, IL-6, GM-CSF, and IFN-γ from spleen cells and of NO from peritoneal macrophages. The adjuvant and immunomodulatory properties of the CyaA* form also suggest that it has potential as a vaccine component through enhancement of Th1-oriented cell-mediated immune responses.
Acknowledgments
We thank Alastair Gracie, University of Glasgow, for providing assistance with the multiplex cytokine analysis; Peter Rigsby, NIBSC, for advice on statistical analyses; and Catpagavalli Asokanathan, Alex Douglas-Bardsley, and Penny Newland, NIBSC, for their technical assistance with the in vivo experiments.
G.Y.C.C. was supported by a studentship from the Biotechnology and Biological Sciences Research Council, United Kingdom.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Arciniega, J. L., E. L. Hewlett, F. D. Johnson, A. Deforest, S. G. Wassilak, I. M. Onorato, C. R. Manclark, and D. L. Burns. 1991. Human serologic response to envelope-associated proteins and adenylate cyclase toxin of Bordetella pertussis. J. Infect. Dis. 163:135-142. [DOI] [PubMed] [Google Scholar]
- 2.Ausiello, C. M., F. Urbani, A. la Sala, R. Lande, and A. Cassone. 1997. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect. Immun. 65:2168-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway. J. Leukoc. Biol. 72:962-969. [PubMed] [Google Scholar]
- 4.Barbic, J., M. F. Leef, D. L. Burns, and R. D. Shahin. 1997. Role of gamma interferon in natural clearance of Bordetella pertussis infection. Infect. Immun. 65:4904-4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnard, A., B. P. Mahon, J. Watkins, K. Redhead, and K. H. Mills. 1996. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0. Immunology 87:372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betsou, F., P. Sebo, and N. Guiso. 1993. CyaC-mediated activation is important not only for toxic but also for protective activities of Bordetella pertussis adenylate cyclase-hemolysin. Infect. Immun. 61:3583-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowne, W. B., J. D. Wolchok, W. G. Hawkins, R. Srinivasan, P. Gregor, N. E. Blachere, Y. Moroi, M. E. Engelhorn, A. N. Houghton, and J. J. Lewis. 1999. Injection of DNA encoding granulocyte-macrophage colony-stimulating factor recruits dendritic cells for immune adjuvant effects. Cytokines Cell Mol. Ther. 5:217-225. [PubMed] [Google Scholar]
- 8.Boyd, A. P., P. J. Ross, H. Conroy, N. Mahon, E. C. Lavelle, and K. H. Mills. 2005. Bordetella pertussis adenylate cyclase toxin modulates innate and adaptive immune responses: distinct roles for acylation and enzymatic activity in immunomodulation and cell death. J. Immunol. 175:730-738. [DOI] [PubMed] [Google Scholar]
- 9.Canthaboo, C., D. Xing, X. Q. Wei, and M. J. Corbel. 2002. Investigation of role of nitric oxide in protection from Bordetella pertussis respiratory challenge. Infect. Immun. 70:679-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherry, J. D., D. Xing, P. Newland, K. Patel, U. Heininger, and M. J. Corbel. 2004. Determination of serum antibody to Bordetella pertussis adenylate cyclase toxin in vaccinated and unvaccinated children and in children and adults with pertussis. Clin. Infect. Dis. 38:502-507. [DOI] [PubMed] [Google Scholar]
- 11.Confer, D. L., and J. W. Eaton. 1982. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science 217:948-950. [DOI] [PubMed] [Google Scholar]
- 12.Decker, M. D., and K. M. Edwards. 2000. Acellular pertussis vaccines. Pediatr. Clin. N. Am. 47:309-335. [DOI] [PubMed] [Google Scholar]
- 13.Everest, P., J. Li, G. Douce, I. Charles, J. De Azavedo, S. Chatfield, G. Dougan, and M. Roberts. 1996. Role of the Bordetella pertussis P.69/pertactin protein and the P.69/pertactin RGD motif in the adherence to and invasion of mammalian cells. Microbiology 142:3261-3268. [DOI] [PubMed] [Google Scholar]
- 14.Farfel, Z., S. Konen, E. Wiertz, R. Klapmuts, P. A. Addy, and E. Hanski. 1990. Antibodies to Bordetella pertussis adenylate cyclase are produced in man during pertussis infection and after vaccination. J. Med. Microbiol. 32:173-177. [DOI] [PubMed] [Google Scholar]
- 15.Fleischmann, J., D. W. Golde, R. H. Weisbart, and J. C. Gasson. 1986. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood 68:708-711. [PubMed] [Google Scholar]
- 16.Gueirard, P., A. Druilhe, M. Pretolani, and N. Guiso. 1998. Role of adenylate cyclase-hemolysin in alveolar macrophage apoptosis during Bordetella pertussis infection in vivo. Infect. Immun. 66:1718-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guermonprez, P., N. Khelef, E. Blouin, P. Rieu, P. Ricciardi-Castagnoli, N. Guiso, D. Ladant, and C. Leclerc. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J. Exp. Med. 193:1035-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guiso, N., M. Szatanik, and M. Rocancourt. 1991. Protective activity of Bordetella adenylate cyclase-hemolysin against bacterial colonization. Microb. Pathog. 11:423-431. [DOI] [PubMed] [Google Scholar]
- 19.Hormozi, K., R. Parton, and J. Coote. 1999. Adjuvant and protective properties of native and recombinant Bordetella pertussis adenylate cyclase toxin preparations in mice. FEMS Immunol. Med. Microbiol. 23:273-282. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi, Y., S. Claus, and D. A. Relman. 1994. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18). J. Exp. Med. 180:1225-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladant, D., and A. Ullmann. 1999. Bordetella pertussis adenylate cyclase: a toxin with multiple talents. Trends Microbiol. 7:172-176. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence, A. J., J. G. Coote, Y. F. Kazi, P. D. Lawrence, J. MacDonald-Fyall, B. M. Orr, R. Parton, M. Riehle, J. Sinclair, J. Young, and N. C. Price. 2002. A direct pyrophosphatase-coupled assay provides new insights into the activation of the secreted adenylate cyclase from Bordetella pertussis by calmodulin. J. Biol. Chem. 277:22289-22296. [DOI] [PubMed] [Google Scholar]
- 23.Leef, M., K. L. Elkins, J. Barbic, and R. D. Shahin. 2000. Protective immunity to Bordetella pertussis requires both B cells and CD4(+) T cells for key functions other than specific antibody production. J. Exp. Med. 191:1841-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liles, W. C., and W. C. Van Voorhis. 1995. Review: nomenclature and biologic significance of cytokines involved in inflammation and the host immune response. J. Infect. Dis. 172:1573-1580. [DOI] [PubMed] [Google Scholar]
- 25.Locht, C., R. Antoine, and F. Jacob-Dubuisson. 2001. Bordetella pertussis, molecular pathogenesis under multiple aspects. Curr. Opin. Microbiol. 4: 82-89. [DOI] [PubMed] [Google Scholar]
- 26.Lu, H., Z. Xing, and R. C. Brunham. 2002. GM-CSF transgene-based adjuvant allows the establishment of protective mucosal immunity following vaccination with inactivated Chlamydia trachomatis. J. Immunol. 169:6324-6331. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald-Fyall, J., D. Xing, M. J. Corbel, S. Baillie, R. Parton, and J. Coote. 2004. Adjuvanticity of native and detoxified adenylate cyclase toxin of Bordetella pertussis towards co-administered antigens. Vaccine 22:4270-4281. [DOI] [PubMed] [Google Scholar]
- 28.Mahon, B. P., M. S. Ryan, F. Griffin, and K. H. Mills. 1996. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect. Immun. 64:5295-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahon, B. P., B. J. Sheahan, F. Griffin, G. Murphy, and K. H. Mills. 1997. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J. Exp. Med. 186:1843-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mascarell, L., C. Fayolle, C. Bauche, D. Ladant, and C. Leclerc. 2005. Induction of neutralizing antibodies and Th1-polarized and CD4-independent CD8+ T-cell responses following delivery of human immunodeficiency virus type 1 Tat protein by recombinant adenylate cyclase of Bordetella pertussis. J. Virol. 79:9872-9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuirk, P., and K. H. Mills. 2000. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur. J. Immunol. 30:415-422. [DOI] [PubMed] [Google Scholar]
- 32.McGuirk, P., and K. H. Mills. 2000. A regulatory role for interleukin 4 in differential inflammatory responses in the lung following infection of mice primed with Th1- or Th2-inducing pertussis vaccines. Infect. Immun. 68:1383-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills, K. H., A. Barnard, J. Watkins, and K. Redhead. 1993. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 61:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills, K. H., M. Ryan, E. Ryan, and B. P. Mahon. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mobberley-Schuman, P. S., B. Connelly, and A. A. Weiss. 2003. Phagocytosis of Bordetella pertussis incubated with convalescent serum. J. Infect. Dis. 187:1646-1653. [DOI] [PubMed] [Google Scholar]
- 36.Olin, P., F. Rasmussen, L. Gustafsson, H. O. Hallander, and H. Heijbel. 1997. Randomised controlled trial of two-component, three-component, and five-component acellular pertussis vaccines compared with whole-cell pertussis vaccine. Lancet 350:1569-1577. [DOI] [PubMed] [Google Scholar]
- 37.Pizza, M., M. M. Giuliani, M. R. Fontana, E. Monaci, G. Douce, G. Dougan, K. H. Mills, R. Rappuoli, and G. Del Giudice. 2001. Mucosal vaccines: non toxic forms of LT and CT as mucosal adjuvants. Vaccine 19:2534-2541. [DOI] [PubMed] [Google Scholar]
- 38.Poole, S., D. Dawson, and R. E. Gaines Das. 1997. Second international standard for endotoxin: calibration in an international collaborative study. J. Endotoxin Res. 4:221-231. [Google Scholar]
- 39.Redhead, K., J. Watkins, A. Barnard, and K. H. Mills. 1993. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect. Immun. 61:3190-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson, A., L. I. Irons, and L. A. Ashworth. 1985. Pertussis vaccine: present status and future prospects. Vaccine 3:11-22. [DOI] [PubMed] [Google Scholar]
- 41.Ross, P. J., E. C. Lavelle, K. H. Mills, and A. P. Boyd. 2004. Adenylate cyclase toxin from Bordetella pertussis synergizes with lipopolysaccharide to promote innate interleukin-10 production and enhances the induction of Th2 and regulatory T cells. Infect. Immun. 72:1568-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan, M., G. Murphy, E. Ryan, L. Nilsson, F. Shackley, L. Gothefors, K. Oymar, E. Miller, J. Storsaeter, and K. H. Mills. 1998. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology 93:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan, M., L. Gothefors, J. Storsaeter, and K. H. Mills. 1997. Bordetella pertussis-specific Th1/Th2 cells generated following respiratory infection or immunization with an acellular vaccine: comparison of the T cell cytokine profiles in infants and mice. Dev. Biol. Stand. 89:297-305. [PubMed] [Google Scholar]
- 44.Schlecht, G., J. Loucka, H. Najar, P. Sebo, and C. Leclerc. 2004. Antigen targeting to CD11b allows efficient presentation of CD4+ and CD8+ T cell epitopes and in vivo Th1-polarized T cell priming. J. Immunol. 173:6089-6097. [DOI] [PubMed] [Google Scholar]
- 45.Simondon, F., M. P. Preziosi, A. Yam, C. T. Kane, L. Chabirand, I. Iteman, G. Sanden, S. Mboup, A. Hoffenbach, K. Knudsen, N. Guiso, S. Wassilak, and M. Cadoz. 1997. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 15:1606-1612. [DOI] [PubMed] [Google Scholar]
- 46.Simsova, M., P. Sebo, and C. Leclerc. 2004. The adenylate cyclase toxin from Bordetella pertussis—a novel promising vehicle for antigen delivery to dendritic cells. Int. J. Med. Microbiol. 293:571-576. [DOI] [PubMed] [Google Scholar]
- 47.Stampfli, M. R., R. E. Wiley, G. S. Neigh, B. U. Gajewska, X. F. Lei, D. P. Snider, Z. Xing, and M. Jordana. 1998. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J. Clin. Investig. 102:1704-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torre, D., G. Ferrario, G. Bonetta, L. Perversi, and F. Speranza. 1996. In vitro and in vivo induction of nitric oxide by murine macrophages stimulated with Bordetella pertussis. FEMS Immunol. Med. Microbiol. 13:95-99. [DOI] [PubMed] [Google Scholar]
- 49.Wang, J., D. P. Snider, B. R. Hewlett, N. W. Lukacs, J. Gauldie, H. Liang, and Z. Xing. 2000. Transgenic expression of granulocyte-macrophage colony-stimulating factor induces the differentiation and activation of a novel dendritic cell population in the lung. Blood 95:2337-2345. [PubMed] [Google Scholar]
- 50.Wang, J., A. Zganiacz, and Z. Xing. 2002. Enhanced immunogenicity of BCG vaccine by using a viral-based GM-CSF transgene adjuvant formulation. Vaccine 20:2887-2898. [DOI] [PubMed] [Google Scholar]
- 51.Wang, J. M., S. Colella, P. Allavena, and A. Mantovani. 1987. Chemotactic activity of human recombinant granulocyte-macrophage colony-stimulating factor. Immunology 60:439-444. [PMC free article] [PubMed] [Google Scholar]
- 52.Weingart, C. L., and A. A. Weiss. 2000. Bordetella pertussis virulence factors affect phagocytosis by human neutrophils. Infect. Immun. 68:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weingart, C. L., P. S. Mobberley-Schuman, E. L. Hewlett, M. C. Gray, and A. A. Weiss. 2000. Neutralizing antibodies to adenylate cyclase toxin promote phagocytosis of Bordetella pertussis by human neutrophils. Infect. Immun. 68:7152-7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weisbart, R. H., L. Kwan, D. W. Golde, and J. C. Gasson. 1987. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood 69:18-21. [PubMed] [Google Scholar]
- 55.Westrop, G. D., E. K. Hormozi, N. A. Da Costa, R. Parton, and J. G. Coote. 1996. Bordetella pertussis adenylate cyclase toxin: proCyaA and CyaC proteins synthesised separately in Escherichia coli produce active toxin in vitro. Gene 180:91-99. [DOI] [PubMed] [Google Scholar]
- 56.Wilkinson, K. A., M. Simsova, E. Scholvinck, P. Sebo, C. Leclerc, H. M. Vordermeier, S. J. Dickson, J. R. Brown, R. N. Davidson, G. Pasvol, M. Levin, and R. J. Wilkinson. 2005. Efficient ex vivo stimulation of Mycobacterium tuberculosis-specific T cells by genetically detoxified Bordetella pertussis adenylate cyclase antigen toxoids. Infect. Immun. 73:2991-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams, M. A., S. M. Kelsey, P. W. Collins, C. N. Gutteridge, and A. C. Newland. 1995. Administration of rHuGM-CSF activates monocyte reactive oxygen species secretion and adhesion molecule expression in vivo in patients following high-dose chemotherapy. Br. J. Haematol. 90:31-40. [DOI] [PubMed] [Google Scholar]
- 58.Xing, D. K., C. Canthaboo, and M. J. Corbel. 1998. Nitric oxide induction in murine macrophages and spleen cells by whole-cell Bordetella pertussis vaccine. Vaccine 16:16-23. [DOI] [PubMed] [Google Scholar]