Abstract
Immunologically active molecules such as cytokines and chemokines have been implicated in skeletal muscle weakness during sepsis as well as recovery from muscle injury. In sepsis, Toll-like receptors (TLRs) act as key sentinel molecules of the innate immune system. Here we determined skeletal muscle cell responses of two prototypical CC and CXC chemokine genes (monocyte chemoattractant protein 1 [MCP-1] and KC, respectively), to stimulation with specific TLR ligands. In addition, we examined whether NF-κB and calcineurin signaling are involved in these responses. Differentiated myotubes and intact whole muscles expressed TLR2, TLR4, TLR5, and TLR9. Stimulation with ligands for TLR2 (peptidoglycan) or TLR4 (LPS) elicited robust and equivalent levels of MCP-1 and KC mRNA expression, whereas stimulation of TLR5 (by flagellin) required gamma interferon priming to induce similar effects. Although both TLR2 and TLR4 ligands activated the NF-κB pathway, NF-κB reporter activity was approximately 20-fold greater after TLR4 stimulation than after TLR2 stimulation. Inhibitory effects of NF-κB blockade on TLR-mediated chemokine gene expression, by either pharmacological (pyrrolidine dithiocarbamate) or molecular (IKKβ dominant-negative transfection) methods, were also more pronounced during TLR4 stimulation. In contrast, inhibitory effects on TLR-mediated chemokine expression of calcineurin blockade (by FK506) were greater for TLR2 than for TLR4 stimulation. MCP-1 and KC mRNA levels also demonstrated differential responses to NF-κB and calcineurin blockade during stimulation with specific TLR ligands. We conclude that skeletal muscle cells differentially utilize the NF-κB and calcineurin pathways in a TLR-specific manner to enable complex regulation of CC and CXC chemokine gene expression.
Chemokines consist of a large family of low-molecular-weight cytokines which are involved in directing the recruitment and activation of leukocytes to sites of infection or inflammation (34). The chemokines have been broadly divided into CC, CXC, C, and CX3C subgroups, based upon the positioning of amino acids relative to the first two conserved cysteine residues (34). In general, CC chemokines act predominantly upon monocytes/macrophages, eosinophils, basophils, and lymphocytes, whereas CXC chemokines are able to attract neutrophils (34). Increased expression of multiple chemokines has been implicated in the infiltration of skeletal muscle by macrophages and lymphocytes in muscular dystrophies (11, 33) and inflammatory myopathies (10, 45). Along these same lines, we have reported increased chemokine expression in the murine diaphragm during acute sepsis (12) as well as in mice suffering from muscular dystrophy (11), two conditions which are associated with increased leukocyte trafficking to this vitally important muscle (5, 11, 29, 42). In addition, chemokines appear to play an important function in skeletal muscle repair (49, 50) and could thus play a key role in facilitating recovery from various forms of muscle fiber injury, including that induced by sepsis (14, 29).
The early host response to sepsis is mediated by the innate immune system, through identification of foreign pathogens and initiation of a proinflammatory cascade. The capacity to mount a vigorous host immune response without prior exposure to a particular pathogen is mediated by a family of Toll-like receptors (TLRs) that recognize unique microbial structures termed pathogen-associated molecular patterns (43, 48). The TLR family includes more than 10 members, with different ligand specificities and differential expression among cell types (32, 54). TLRs are type I transmembrane receptors containing an extracellular leucine-rich repeat domain linked to a cytoplasmic Toll/interleukin-1 receptor homology domain. Activation of TLRs generally leads to nuclear translocation of the transcription factor NF-κB, a critical component within many proinflammatory pathways (21), including those associated with chemokine gene expression (6, 15). Although TLRs share the ability to activate NF-κB (43), cross talk with other signaling pathways is one mechanism by which activation of specific TLRs may lead to different patterns of gene expression. Another key signaling molecule of the immune response, the calcium/calmodulin-dependent phosphatase known as calcineurin, has been reported to have a functional interaction with NF-κB (46). Interestingly, calcineurin also plays an important role in the growth, differentiation, and specialization of skeletal muscle (9, 31, 40).
The majority of previous studies investigating the immunological attributes of skeletal muscle have been directed at elucidating its ability to participate in the adaptive immune response. For example, muscle fibers of patients suffering from inflammatory myopathies and primary skeletal muscle cell cultures exposed to proinflammatory stimuli express human leukocyte antigen class I/II and costimulatory molecules (52). These are involved in the priming and activation of lymphocytes, and coculture experiments have confirmed that antigen-exposed skeletal muscle cells are capable of inducing lymphocyte activation (53). The chemokines interleukin-8 (IL-8) (CXCL8), RANTES (CCL5), and monocyte chemoattractant protein 1 (MCP-1) (CCL2) are produced by human myoblasts under proinflammatory conditions, and it has been speculated that these molecules may be important in autoimmune muscle disease (13). However, the extent to which skeletal muscle cells are capable of participating in the innate immune response is less well understood. It should be noted that innate immunity is not only involved in combating infection, but may also play a more general role in sensing “danger signals” related to other types of cellular injury or stress (48).
TLRs are the main sentinel molecules responsible for triggering the innate immune response and may therefore constitute an important pathway for inducing chemokine expression by skeletal muscle cells. Accordingly, the principal aim of our study was to evaluate the role of TLR-mediated signaling in the process of chemokine gene expression by skeletal muscle. We selected MCP-1 (CCL2) and KC (CXCL1) as prototypical members of the two largest chemokine families (CC and CXC, respectively). These two chemokine families have been implicated in different disease states and have also been reported to show differential regulation under various pathological conditions (10, 30). Our specific objectives in this study were as follows: (i) to ascertain the skeletal muscle cell expression pattern of different TLRs known to be active in bacterial or viral recognition, (ii) to determine whether there is differential regulation of MCP-1 and KC gene expression after stimulation of these TLRs by their respective ligands, and (iii) to evaluate the involvement of NF-κB and calcineurin in the regulation of MCP-1 and KC gene expression after specific TLR stimulation in skeletal muscle cells.
MATERIALS AND METHODS
Cell culture and functional stimulation with TLR ligands.
Myoblasts from the murine skeletal muscle cell line C2C12 (American Type Culture Collection, Manassas, VA) were grown on six-well plates coated with Matrigel (1 mg/ml in Dulbecco's modified Eagle's medium [DMEM]; Becton-Dickinson, Franklin Lakes, NJ). The cells (passage 3 or below) were expanded in growth medium consisting of DMEM with 10% fetal bovine serum. After reaching approximately 70% confluence, the cells were switched to differentiation medium composed of DMEM with 2% horse serum to induce myoblast fusion into differentiated myotubes. On day 5 in differentiation medium, myotubes were incubated with one of the following TLR ligands (43) diluted in DMEM with 5% heat-inactivated fetal bovine serum for a period of 4 h: 10 μg/ml Staphylococcus aureus peptidoglycan (PGN) (for TLR2), 25 μg/ml poly(I:C) (for TLR3), 1 μg/ml Escherichia coli O111:B4 ultrapure lipopolysaccharide (LPS) (for TLR4), 1 μg/ml Salmonella enterica serovar Typhimurium flagellin (for TLR5), 1 mM loxoribine (for TLR7), and 1 μM unmethylated CpG DNA consisting of phosphorothioated oligonucleotide with the sequence TCCATGACGTTCCTGACGTT (for TLR9) (37), where underlining indicates CpG sequences. TLR2, TLR3, TLR4, TLR5, and TLR7 ligands were purchased from Invivogen (San Diego, California); TLR9 ligand was obtained from Alpha DNA (Montreal, Quebec, Canada). Primary skeletal muscle cell cultures were derived from C57BL/6 mice and TLR2 null mutants on the same background strain (44), using single living muscle fibers isolated from the tibialis anterior limb muscle, according to procedures which we have previously described in detail (11). The primary cultures were stimulated on the fifth day after induction of differentiation, with either PGN or LPS at the same doses mentioned above.
Pharmacological and dominant-negative inhibition studies.
Treatment with pyrrolidine dithiocarbamate (PDTC; Fisher Scientific, Nepean, Ontario, Canada), which acts an inhibitor of NF-κB by blocking the E3 ligase responsible for IκB degradation (20), was initiated 24 h before and maintained during the 4-h TLR stimulation period at a dose of 100 μM (27). Treatment with FK506 (Eton Bioscience, Inc., San Diego, California), an inhibitor of calcineurin, was similarly applied for 24 h before and during TLR stimulation, at a dose of 100 ng/ml (40). To evaluate the role of free radical species, cells were treated with one of two antioxidants, N-acetylcysteine (10 mM) or catalase (2,000 U/ml) (Fisher Scientific), initiated 1 h before and maintained during TLR stimulation; these doses have previously been shown to block reactive oxygen species-mediated IL-6 upregulation in skeletal muscle cells (25). All of the above studies were performed on C2C12 myotubes after 5 days in differentiation medium as described above.
A dominant-negative mutant form of the IκB kinase, IKKβ (provided by P. Barker, McGill University, Montreal, Canada), was employed to inhibit NF-κB activation as previously described (3). Myoblasts transfected with a vector containing the same plasmid backbone (pAdtrack) served as controls. C2C12 cells (5 × 105) were seeded onto 60-mm plates, incubated overnight in growth medium, and transfected the following day at approximately 50% confluence. To achieve transfection, Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and 8 μg of plasmid DNA were combined according to the manufacturer's protocol and placed onto the monolayer of cells in the presence of Optimum medium (Invitrogen) for 4 h. The transfection mixture was subsequently replaced with growth medium for 24 h. The cells were then replated in Matrigel-coated six-well plates and switched to differentiation medium. Stimulation experiments with TLR ligands were performed 5 days later as described earlier.
Evaluation of NF-κB pathway activation.
To assess IκBα phosphorylation and degradation after TLR stimulation, myotubes were harvested from six-well plates and placed in cell lysis buffer (Cell Signaling, Danvers, MA) containing aprotinin (10 μg/ml), leupeptin (10 μg/ml), and the phosphatase inhibitor sodium orthovanadate (10 mM). Total protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA). Protein samples were size separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto polyvinylidene difluoride membranes. Immunodetection was performed with primary antibodies directed against total IκBα and phosphorylated IκBα (both from Cell Signaling), which were reacted with membranes blotted with 20 μg and 60 μg protein per lane, respectively. The immunoblot signals were revealed using appropriate horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies, in conjunction with the ECL detection system (Amersham, Piscataway, NJ).
NF-κB transcriptional activity was evaluated using a NF-κB response element-driven firefly luciferase reporter plasmid (pNF-κB-Luc; Clontech, Mountain View, CA), which was transfected into C2C12 cells prior to TLR stimulation. The pNF-κB-Luc vector was cotransfected into C2C12 myoblasts together with a thymidine kinase promoter-driven Renilla luciferase vector (pRL-TK; Promega, Madison, WI), which is constitutively active at a low level. For each 60-mm plate, 7.7 μg of pNF-κB-Luc and 0.3 μg of pRL-TK were used in conjunction with Lipofectamine as described earlier. The Dual-Luciferase reporter assay system (Promega) was used to quantify the activity of both luciferase reporters within the same samples, according to the manufacturer's instructions. Light emission was measured in an Lmax 384 luminometer (Molecular Devices, Downingtown, PA). All results were expressed as the ratio of NF-κB firefly luciferase to Renilla luciferase activity in relative light units, using the constitutively active Renilla as an internal control to adjust for differences in transfection efficiency.
Analysis of MCP-1 and KC gene expression.
RNase protection assays were employed to quantify MCP-1 and KC mRNA levels. Myotubes were harvested at 4 h after stimulation with the various TLR ligands, in the presence or absence of NF-κB/calcineurin inhibition as described above, and total RNA was extracted. 32P-labeled riboprobes were synthesized using a custom-made mouse multiprobe kit (BD Biosciences). The riboprobes were hybridized with each RNA sample (10 μg) overnight at 56°C according to the manufacturer's instructions. The protected RNA fragments were separated using a 5% polyacrylamide gel and detected by autoradiography. Bands representing the individual mRNA species were then quantified using an image analysis system (FluorChem 8000; Alpha Innotech Corp, San Leandro, CA), and the signals were normalized to the L32 housekeeping gene to control for any loading differences across lanes as previously described (16, 17).
Reverse transcription (RT)-PCR detection of TLR expression.
Intact diaphragm and tibialis anterior muscles of C57BL/10 mice (8 to 10 weeks of age) were snap-frozen in liquid nitrogen immediately after euthanasia. Primary myotubes from the diaphragm and tibialis anterior were derived from dissociated single fibers as previously described in detail (11). Myoblasts and myotubes from C2C12 cells were prepared as described above. Total RNA from muscle tissues and cell cultures was extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. The RNA (1 μg) was treated with DNase I (Invitrogen) and reverse transcribed using Moloney murine leukemia virus reverse transcriptase and random primers. Negative controls lacking reverse transcriptase were included in all cases to exclude genomic DNA contamination. PCR amplification was performed for 35 cycles at 94°C for 45 s, 55°C for 45 s, and 72°C for 45 s. The following primers were used (5′ to 3′): TLR2 Forward, CACCATTTCCACGGACTGTGGTACCTG; TLR2 Reverse, CTGGCCTTATCCAAGGGCCACTCCAG; TLR3 Forward, GACTGGGTCTGGGAACATTTCTCC; TLR3 Reverse, AGCTCCATGTGCTACTTGCAATTTGT; TLR4 Forward, ATCTACTCGAGTCAGAATGAGGACTGG; TLR4 Reverse, CTCTGCTGTTTGCTCAGGATTCGAGGC; TLR5 Forward, GGAATCTGTTTCCTGTGTGCTATAAGACC; TLR5 Reverse, ATGGTTGCTATGGTTCGCAACTGGATGG; TLR7 Forward, TCTATTCAGAGGCTCCTGGATGAC; TLR7 Reverse, CTTCAGGTACCAAGGGATGTCCTA; TLR9 Forward, CTAGACGTGAGAAGCAACCCTCTG; TLR9 Reverse, CAGCTCGTTATACACCCAGTCGGC; HPRT Forward, GTGGATACAGGCCAGACTTTGTTG; HPRT Reverse, GAGGGTAGGCTGGCCTATTGGCT. All PCR products were electrophoresed on 1% agarose gels containing ethidium bromide and visualized under UV light.
Statistical analysis.
All data are expressed as means ± standard errors (SE). For each experimental condition and time point, four independent replicate analyses were performed, unless otherwise noted. Groups were compared using the Mann-Whitney rank sum test. The analyses were performed using SigmaStat software (SPSS, Chicago, IL), and statistical significance was set at a P value of <0.05.
RESULTS
TLR expression patterns in cultured muscle cells and whole muscles.
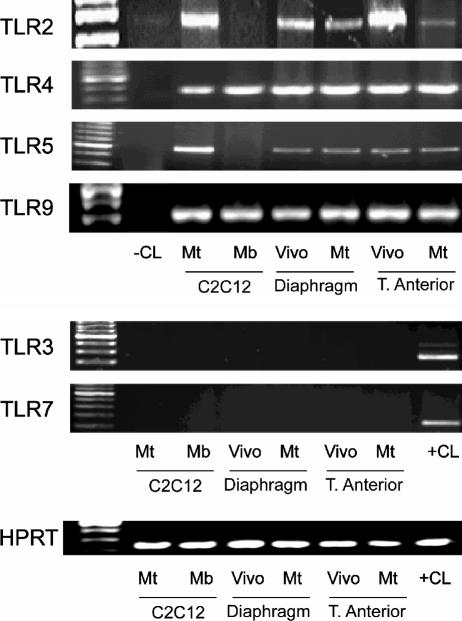
TLR2, TLR4, and TLR5 are primarily involved in the recognition of bacterial surface structures, whereas TLR3, TLR7, and TLR9 recognize nucleic acids (43). RT-PCR was employed to determine whether these TLRs are expressed in skeletal muscle cells, using C2C12 cells and primary (from diaphragm and tibialis anterior) skeletal muscle cell cultures. In addition, whole-tissue samples from diaphragm and tibialis anterior muscles obtained in vivo were examined in the same manner. As shown in Fig. 1 (upper panel), TLR2, TLR4, TLR5, and TLR9 were expressed in whole muscles obtained in vivo as well as in differentiated myotubes from C2C12 or primary cultures. On the other hand, we were unable to detect TLR3 or TLR7 under the same experimental conditions (Fig. 1, lower panel). Interestingly, TLR2 and TLR5 were expressed in C2C12 myotubes but not myoblasts, indicating that the expression pattern of these TLRs is regulated by the state of skeletal muscle cell differentiation.
FIG. 1.
Expression of TLRs by skeletal muscle. RT-PCR of RNA extracted from the C2C12 muscle cell line at the myoblast (Mb) and myotube (Mt) stages, as well as C57BL/10 mouse diaphragm, limb muscle (tibialis anterior), and corresponding primary muscle cell cultures (myotube stage). PCRs were also performed without addition of reverse transcriptase (−CL) and in murine monocytes (+CL).
Functional responses to stimulation of individual TLRs.
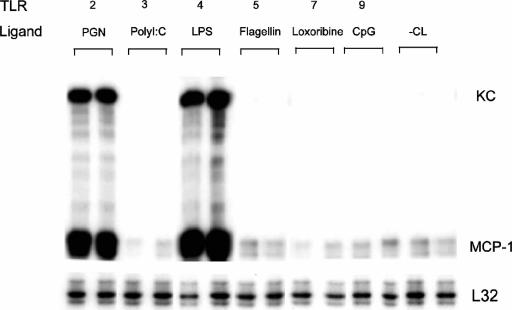
As shown in Fig. 2, an RNase protection assay was used to evaluate MCP-1 and KC gene expression at the mRNA level in C2C12 myotubes stimulated with specific TLR ligands. Consistent with their lack of detectable expression by RT-PCR, there was no response to TLR3 ligand [poly(I:C)] or TLR7 ligand (loxoribine) stimulation. In contrast, there was marked induction of MCP-1 and KC expression after 4 h of incubation with TLR2 ligand (PGN) or TLR4 ligand (LPS). Interestingly, although TLR5 and TLR9 expression were observed by RT-PCR as shown in Fig. 1, stimulation with their respective ligands failed to elicit detectable expression of MCP-1 or KC mRNA (Fig. 2).
FIG. 2.
TLR-mediated chemokine expression by skeletal muscle cells. RNase protection assays performed 4 h after incubation of C2C12 myotubes with S. aureus peptidoglycan (10 μg/ml), poly(I:C) (25 μg/ml), E. coli LPS (1 μg/ml), S. enterica serovar Typhimurium flagellin (1 μg/ml), loxoribine (1 mM), or unmethylated CpG motif oligonucleotide (1 μM), representing TLR 2, 3, 4, 5, 7, and 9 ligands, respectively. −CL, unstimulated C2C12 myotubes.
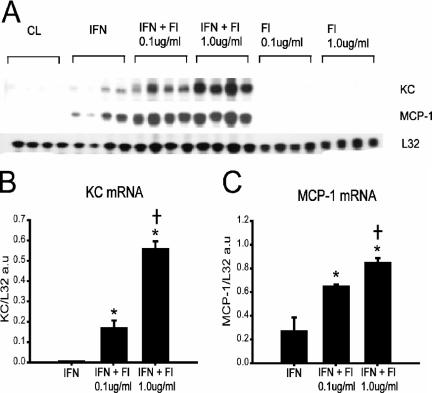
We next examined whether TLR5 or TLR9 responses could be induced by initially priming myotubes with gamma interferon (IFN-γ), since this has been shown to increase TLR expression as well as responsiveness in other systems (19, 47). IFN-γ priming for 24 h in the absence of TLR ligand stimulation resulted in a small but detectable upregulation of KC and MCP-1 mRNA levels (Fig. 3A). The addition of TLR5 ligand (flagellin) further increased MCP-1 and KC mRNA levels under these conditions, and this occurred in a flagellin dose-dependent manner (Fig. 3B and C). On the other hand, chemokine gene expression responses to TLR9 ligand (CpG DNA) were not enhanced by prior exposure to IFN-γ, although stimulation of murine monocytes with the same CpG DNA resulted in robust upregulation of MCP-1 and KC, thus confirming its efficacy (data not shown). Overall, these findings indicate that TLR2 and TLR4 ligands, as well as TLR5 ligand in the setting of IFN-γ priming, are able to signal both CC and CXC chemokine gene expression in differentiated skeletal muscle cells.
FIG. 3.
IFN-γ priming of skeletal muscle cells augments chemokine expression in response to TLR5 stimulation. (A) RNase protection assay demonstrating chemokine expression by C2C12 myotubes under the following conditions: unstimulated cells (CL), cells primed for 24 h with IFN-γ (IFN; 200 U/ml), cells stimulated for 4 h with flagellin (Fl) alone, and IFN-primed cells stimulated for 4 h with flagellin (IFN + Fl). (B and C) Quantification of chemokine mRNA levels by densitometry, expressed in arbitrary units (a.u.) and normalized to the L32 housekeeping gene (n = 4 per group). *, P < 0.05 versus IFN alone; †, P < 0.05 for comparisons between IFN + Fl 0.01 μg/ml and IFN + Fl 1.0 μg/ml.
Role of NF-κB in TLR-mediated chemokine expression by skeletal muscle cells.
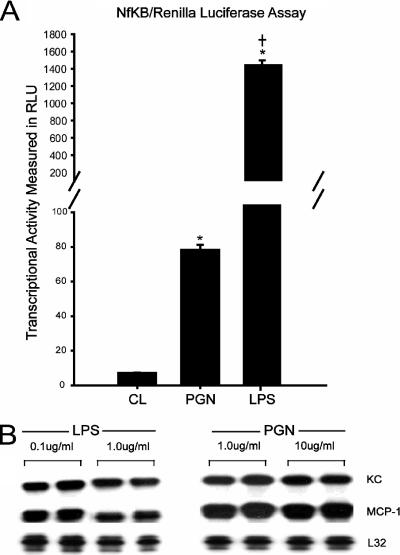
Because TLR2 and TLR4 stimulation produced the most prominent effects on MCP-1 and KC expression, subsequent experiments were focused upon these two receptors. To compare the relative abilities of TLR2 and TLR4 to induce NF-κB transcriptional activity in cultured myotubes, an expression plasmid containing an NF-κB luciferase reporter was transfected into muscle cells prior to TLR stimulation. Figure 4A indicates that NF-κB reporter values were significantly increased above basal values after engagement of either TLR2 or TLR4 with their respective ligands. However, TLR4 stimulation resulted in luciferase activity that was an order of magnitude larger than that achieved by TLR2 stimulation. It should be noted that the ligand concentrations employed (10 μg/ml PGN for TLR2; 1 μg/ml LPS for TLR4) represented maximal doses with respect to chemokine mRNA induction. This is demonstrated in Fig. 4B, which demonstrates equivalent MCP-1 and KC mRNA expression levels in response to either ligand at a 10-fold lower dose.
FIG. 4.
TLR2 and TLR4 stimulation induce different levels of NF-κB luciferase reporter activity. (A) C2C12 cells were cotransfected with a NF-κB firefly luciferase reporter plasmid and a constitutively active Renilla luciferase plasmid to control for transfection efficiency. Myotubes were stimulated for 4 h with LPS (1 μg/ml) or PGN (10 μg/ml) (n = 4 per group). *, P < 0.05 versus control (CL); †, P < 0.05 for comparisons between PGN and LPS. (B) RNase protection assay demonstrating that equivalent levels of KC and MCP-1 mRNA expression were observed in response to both ligands over a 10-fold dose range.
We next sought to determine whether inhibition of NF-κB signaling would attenuate chemokine gene expression induced by either TLR2 or TLR4 stimulation. Therefore, the effects of a pharmacological inhibitor of NF-κB (PDTC) on MCP-1 and KC mRNA levels were examined, as shown in Fig. 5A. Treatment of the cells with PDTC significantly reduced chemokine mRNA levels in C2C12 myotubes exposed to PGN or LPS. This did not appear to be mediated through effects on free radical species, since treatment of the cells with two different antioxidants, N-acetylcysteine (NAC) and catalase, did not have any significant effects upon chemokine gene expression under the same conditions. Interestingly, the effects of PDTC treatment on KC mRNA levels were more pronounced for TLR4 than TLR2 stimulation (Fig. 5B and C). In addition, while PDTC treatment in the setting of TLR4 stimulation led to a dramatic decrease in MCP-1 mRNA, the MCP-1 expression level during TLR2 stimulation was not significantly affected by PDTC treatment.
FIG. 5.
Chemical inhibition of NF-κB signaling decreases TLR-mediated chemokine expression in a differential manner. (A) RNase protection assay demonstrating chemokine expression by C2C12 myotubes after LPS or PGN stimulation for 4 h in the presence or absence of the NF-κB inhibitor PDTC (100 μM) and the antioxidant NAC (10 mM) or catalase (2,000 units/ml). (B and C) Quantification of chemokine mRNA levels by densitometry, expressed in arbitrary units (a.u.) and normalized to the L32 housekeeping gene (n = 4 per group). *, P < 0.05 versus LPS or PGN alone.
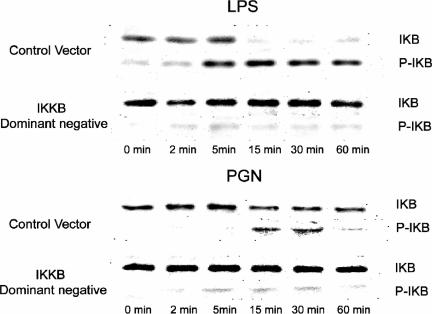
To further evaluate the role of NF-κB in TLR2- and TLR4-mediated chemokine gene expression, muscle cells were stimulated with TLR ligands in the presence or absence of a dominant-negative form of IKKβ. In response to TLR activation, IKKβ normally phosphorylates cytoplasmic IκB, thereby targeting the protein for ubiquitination and degradation, which then permits the release and nuclear translocation of NF-κB. Immunoblotting was used to monitor changes in total and phospho-IκBα protein levels after TLR2 and TLR4 stimulation as well as to determine the potential of the dominant-negative IKKβ construct to interfere with this process. As shown in Fig. 6, both PGN and LPS stimulation induced rapid IκBα phosphorylation in muscle cells transfected with a control plasmid. This was associated with a decrease in total IκBα, which persisted for at least 60 min after stimulation with either PGN or LPS. Notably, these responses were more rapid as well as increased in magnitude after LPS compared to PGN exposure, again suggesting stronger activation of the NF-κB pathway with TLR4 than with TLR2 stimulation.
FIG. 6.
TLR2 and TLR4 ligands induce IκBα phosphorylation and degradation in skeletal muscle cells. Immunoblot analysis of phosphorylated IκBα (P-IκB) and total IκBα (IκB) in C2C12 myotubes transfected with a dominant-negative IKKβ plasmid vector or an empty control vector containing the same plasmid backbone and stimulated with LPS (1 μg/ml) or PGN (10 μg/ml).
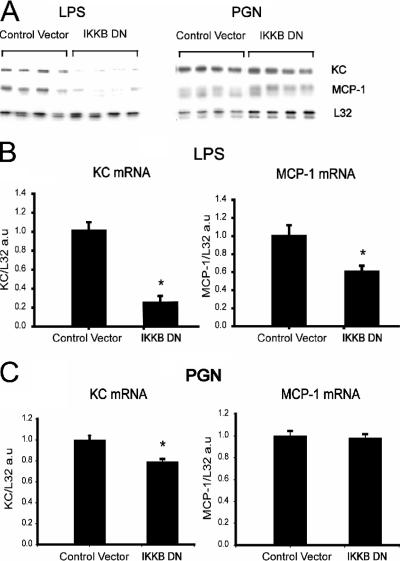
Transfection with the dominant-negative form of IKKβ was able to abrogate IκB phosphorylation/degradation to a very large extent in both PGN- and LPS-stimulated muscle cells (Fig. 6). Furthermore, Fig. 7 shows that transfection with the dominant-negative IKKβ construct significantly reduced both MCP-1 and KC mRNA in myotubes exposed to LPS. However, during PGN exposure, dominant-negative inhibition of IKKβ had lesser effects on KC expression, and MCP-1 expression was entirely unaffected, in a manner that closely resembled the results obtained during PGN stimulation in the presence of PDTC. Overall, these observations are consistent with a greater role for IKKβ and the NF-κB signaling pathway in TLR4- than in TLR2-mediated MCP-1 and KC gene expression by skeletal muscle cells.
FIG. 7.
Dominant-negative molecular inhibition of NF-κB signaling decreases TLR-mediated chemokine expression in a differential manner. (A) RNase protection assay demonstrating chemokine mRNA responses to LPS or PGN stimulation after transfection with either dominant-negative IKKβ (IKKB DN) or control plasmid vector as described in the legend to Fig. 6. (B and C) Quantification of chemokine mRNA levels by densitometry, expressed in arbitrary units (a.u.) and normalized to the L32 housekeeping gene (n = 4 per group). *, P < 0.05 versus control vector.
TLR-mediated chemokine expression by skeletal muscle cells involves calcineurin.
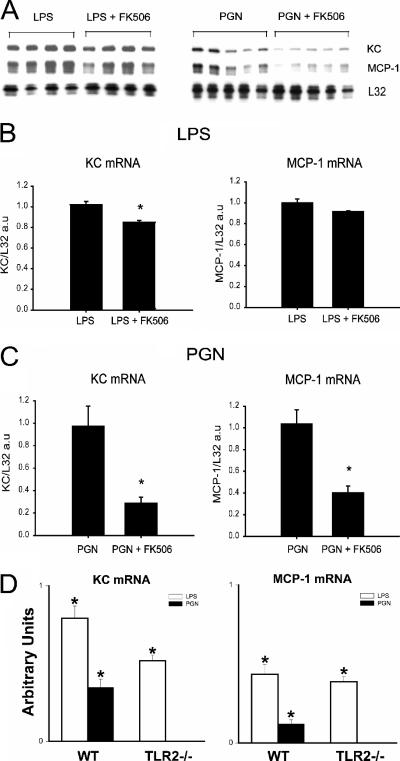
We next employed FK506 to probe the role of calcineurin in the observed MCP-1 and KC responses to TLR2 and TLR4 stimulation. Treatment of myotubes with FK506, a calcineurin inhibitor, resulted in a small but statistically significant reduction in LPS-induced KC mRNA levels, whereas no significant effects on LPS-induced MCP-1 expression were observed (Fig. 8A and B). In contrast, FK506 greatly reduced the magnitude of both MCP-1 and KC mRNA levels induced by TLR2 stimulation with PGN (Fig. 8A and C). Therefore, these data suggest a relatively greater role for calcineurin signaling in the process of TLR2-mediated chemokine gene expression by skeletal muscle. In addition, to verify the specificity of TLR2 in mediating PGN effects on skeletal muscle cell chemokine expression, primary myotubes derived from TLR2 null mutant mice were also stimulated with either PGN or LPS. As shown in Fig. 8D, PGN-mediated chemokine responses were abolished in myotubes lacking TLR2, whereas responsiveness to LPS was preserved.
FIG. 8.
The calcineurin inhibitor FK506 differentially affects MCP-1 and KC gene expression in a TLR-specific manner. (A) RNase protection assay demonstrating the effects of FK506 (100 ng/ml) on chemokine expression by C2C12 myotubes after LPS or PGN stimulation for 4 h (together with the vehicle used for FK506, 0.1 μl ethanol/ml of media). (B and C) Corresponding quantification of chemokine mRNA levels by densitometry; *, P < 0.05 versus LPS or PGN alone. (D) Quantification of increases in chemokine mRNA levels in primary myotubes derived from wild-type (WT, C57BL/6) and TLR2 null mutant mice following stimulation with either LPS or PGN for 4 h (n = 4 per group). *, P < 0.05 versus unstimulated control.
DISCUSSION
Skeletal muscle weakness occurs during severe infections and may even result in the development of respiratory failure and death due to weakness of the diaphragm and other respiratory muscles (5, 14, 24, 29). The CC chemokines such as MCP-1 are classically considered to be chemotactic factors for mononuclear cells, whereas KC belongs to the ELR motif (glutamate-leucine-arginine)-positive group of CXC chemokines involved in neutrophil recruitment (34). We have recently found that both CC and CXC chemokines are highly upregulated in the septic mouse diaphragm in vivo (12), and infiltration of the diaphragm by macrophages and neutrophils during sepsis has been previously implicated in the pathogenesis of sepsis-induced diaphragmatic weakness (5, 29, 42). Increased chemokine expression, leukocyte infiltration, and weakness are also found in the diaphragm and other muscles of mice suffering from muscular dystrophy (11). Therefore, it is reasonable to speculate that chemokine expression by skeletal muscles is likely to play a significant role in the pathogenesis of these conditions.
In the present investigation, we observed that differentiated myotubes (from C2C12 cells or primary cells) and intact whole muscles all expressed TLR2, TLR4, TLR5, and TLR9. Our TLR expression pattern findings are generally consistent with those of other investigators, although there are some differences. Frost and colleagues have reported that TLRs 1 to 7 (but not TLR8 or TLR9) are expressed in C2C12 myotubes (16, 17). This differs from our own findings with respect to TLRs 3 and 7 (not detected in our study) as well as TLR9 (detected in our study). However, these authors also reported a failure to achieve functional responses (IL-6 expression) upon stimulation with TLR3 and TLR9 ligands (17), which is in agreement with the results of our study. Two other groups have recently reported TLR9 mRNA expression in human skeletal muscle tissue (32) and human myoblasts (38). The latter study also found TLR3 expression in cultured human myoblasts and in diseased muscles from myopathic patients but not in normal healthy muscle fibers (38). It is likely that the slightly divergent findings among these investigations is related at least in part to differences in tissue culture conditions (e.g., duration and stage of differentiation, etc.) as well as possible species differences.
To our knowledge, the present study is the first to systematically evaluate the ability of the different TLRs to mediate CC and CXC chemokine expression by skeletal muscle cells. We assessed a broad range of different TLR-ligand interactions and noted a complex pattern in which certain TLRs expressed by the cells responded to stimulation in a straightforward manner (TLRs 2 and 4), while others required immune modulation by another molecule to respond (IFN-γ for TLR5) or did not respond at all (TLR9). The ability of IFN-γ to potentiate TLR responses (as shown here for TLR5) is well described in other cell types (19, 47). Therefore, one possible reason for the lack of responsiveness to TLR9 ligand in our study is a similar dependence upon another as yet unidentified immunostimulatory molecule. In addition, while many cell types respond when CpG DNA is applied at the cell surface (22), the subcellular location of TLR9 is largely endosomal (26, 28). It is thus possible that skeletal muscle cells require additional measures or a more prolonged period of exposure to achieve intracellular uptake of CpG DNA and effective TLR9 engagement.
Not surprisingly, we observed that the NF-κB pathway is activated after both TLR2 and TLR4 stimulation of skeletal muscle cells. However, a novel finding in our study was the documentation of major differences in the degree of NF-κB activation induced by TLR2 versus TLR4 stimulation of skeletal muscle cells. Hence, at TLR ligand doses which were supramaximal with respect to chemokine gene induction, TLR4 stimulation induced more rapid and complete IκB degradation than was observed after TLR2 stimulation. In keeping with these findings, TLR4 stimulation was also associated with dramatically higher NF-κB luciferase reporter activity. In addition, interference with NF-κB activation during TLR4 stimulation had greater inhibitory effects upon chemokine gene expression. The compound PDTC, which blocks the E3 ubiquitin ligase involved in IκBα degradation (20), greatly reduced the levels of both KC and MCP-1 expression triggered by TLR4 stimulation but had no effect upon MCP-1 expression induced by TLR2 stimulation. Because PDTC also has antioxidant properties, and chemokine expression can be increased by mechanisms which are dependent upon reactive oxygen species (27), we also evaluated the effects of free radical scavengers. We employed two potent antioxidants, NAC and catalase, with documented abilities to inhibit reactive oxygen species-mediated cytokine upregulation in muscle cells at the doses employed in this study (25). In contrast to PDTC, these antioxidants had no significant effects upon TLR2- or TLR4-mediated chemokine gene expression, suggesting that free radicals were not responsible for chemokine induction in our experiments. Furthermore, as in the case of PDTC, dominant-negative IKKβ significantly inhibited KC and MCP-1 expression induced by TLR4 stimulation, whereas for TLR2 stimulation, it had substantially smaller effects upon KC and no impact at all upon MCP-1 expression. Therefore, it appears that NF-κB activation is greater in magnitude during TLR4 stimulation and also plays a more important role in TLR4- than in TLR2-mediated chemokine expression by skeletal muscle cells.
It is important to note that, despite a significantly lower level of NF-κB activation in the case of TLR2 stimulation, the magnitude of MCP-1 and KC mRNA expression was equally robust when cells were exposed to supramaximal doses of either PGN or LPS. This finding indicates that TLR2 and TLR4 must recruit different signaling pathways to regulate the same chemokine genes in skeletal muscle. A plausible candidate for another signaling molecule is the calcium-dependent serine/threonine phosphatase calcineurin, which is known to be expressed by skeletal muscle cells, where its actions in favoring the nuclear translocation of NFAT have been implicated in the regulation of skeletal muscle growth, differentiation, and specialization (9, 31, 40). In activated T lymphocytes, calcineurin inhibition by FK506 has been found to have differential effects on chemokines, with several CC chemokines being repressed to variable degrees (MIP-1α, MIP-1β, RANTES), whereas a CXC chemokine (IP-10) actually demonstrated upregulation (41). In addition, calcineurin has been reported to upregulate MCP-1 expression in vascular smooth muscle cells by augmenting mRNA stability (36).
Here we show for the first time that calcineurin plays a major role in the regulation of chemokine expression by skeletal muscle cells. In this regard, we found that TLR2-mediated induction of both MCP-1 and KC was markedly attenuated by the calcineurin inhibitor FK506. On the other hand, during TLR4 stimulation, FK506 had a less pronounced inhibitory influence on KC expression, and no significant effect on MCP-1 expression could be demonstrated. Therefore, the impact of calcineurin inhibition on chemokine expression was substantially greater for TLR2- than TLR4-mediated responses, which is in direct contradistinction to the results obtained during NF-κB inhibition as discussed earlier. For MCP-1 in particular, there was a high degree of TLR-dependent differential regulation by NF-κB and calcineurin. Our findings are reminiscent of recent observations in human airway epithelial cells, in which it was reported that another calcineurin inhibitor, cyclosporine, achieved almost complete abrogation of TLR2-mediated induction of IL-8 (CXCL8) expression, whereas responses to heat-killed gram-negative bacteria (presumably TLR4 mediated to a large extent) could be inhibited by about 25% only (51). This suggests that calcineurin may play a predominant role in the mediation of TLR2-stimulated chemokine responses not only within skeletal muscle but within other cell types as well.
It is of interest to consider the potential effects of calcineurin on several different transcription factors which could be involved in MCP-1 or KC gene regulation. One possibility would be an effect of calcineurin on NF-κB itself (46), particularly since forced overexpression of activated calcineurin in C2C12 cells has been reported to increase NF-κB activity (2), and mitochondrial stress can also lead to NF-κB activation in C2C12 cells through a calcineurin-dependent mechanism (4). However, FK506 produced substantially less inhibition of chemokine expression during stimulation of TLR4 relative to TLR2, despite the fact that NF-κB activity during TLR4 stimulation was an order of magnitude higher than for TLR2. Therefore, it seems unlikely that the major effects of calcineurin inhibition observed during TLR2 stimulation were mediated through an effect on NF-κB. Calcineurin also has the potential to modulate several other proinflammatory signaling pathways which could be involved in TLR-mediated chemokine gene expression by skeletal muscle cells, such as NFAT, CREB, and C/EBP (18, 31, 39). The latter, in particular, has been strongly implicated in transcriptional regulation of both MCP-1 (1, 39) and KC (7) gene expression.
Recently, it has become apparent that chemokines have important biological functions in skeletal muscle which extend well beyond their classical roles as leukocyte chemoattractants. In particular, there is accumulating evidence for a significant role in muscle regeneration following injury (49, 50). In animal models of acute and subacute sepsis, skeletal muscle fiber injury has been demonstrated (14, 29). Recovery from injury involves myoblast precursors (called satellite cells) that are normally quiescent in adult skeletal muscle but which become activated to proliferate and migrate to form new muscle fibers (regeneration) when skeletal muscle is damaged (23). It has recently been demonstrated that CCR2 (the major receptor for MCP-1) is expressed by skeletal muscle cells in vivo, and both CCR2 and MCP-1 are required for optimal functional recovery from injury (49, 50). In addition, RANTES (CCL5) has been shown to be a chemotactic factor for myoblasts (8), and the CXC chemokine LIX (LPS-induced CXC chemokine) is expressed in satellite cells shortly after induced muscle injury (35). Therefore, although the blocking of chemokine expression in muscle during sepsis could mitigate leukocyte-mediated adverse effects, there is also the potential for interference with muscle repair mechanisms. Accordingly, the rational design of therapeutic interventions in this area will require a detailed understanding of the roles played by specific chemokines in skeletal muscle during different stages of sepsis and recovery from injury. Finally, in view of the possible role of TLRs in sensing other types of cellular injury or stress beyond those associated with infectious insults (48), it will be of considerable interest in future studies to determine whether TLR-mediated signaling plays a role in the augmented skeletal muscle chemokine gene expression found in noninfectious pathological conditions such as the muscular dystrophies and inflammatory myopathies.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research, the Respiratory Health Network of the Fonds de la Recherche en Sante du Quebec, and the Burroughs Wellcome Fund.
We thank J. Marshall and S. Akira for providing the use of TLR2 null mutant mice.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Abraham, S., T. Sweet, B. E. Sawaya, J. Rappaport, K. Khalili, and S. Amini. 2005. Cooperative interaction of C/EBP beta and Tat modulates MCP-1 gene transcription in astrocytes. J. Neuroimmunol. 160:219-227. [DOI] [PubMed] [Google Scholar]
- 2.Alzuherri, H., and K. C. Chang. 2003. Calcineurin activates NF-kappaB in skeletal muscle C2C12 cells. Cell. Signal. 15:471-478. [DOI] [PubMed] [Google Scholar]
- 3.Bhakar, A. L., L. L. Tannis, C. Zeindler, M. P. Russo, C. Jobin, D. S. Park, S. MacPherson, and P. A. Barker. 2002. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J. Neurosci. 22:8466-8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas, G., H. K. Anandatheerthavarada, M. Zaidi, and N. G. Avadhani. 2003. Mitochondria to nucleus stress signaling: a distinctive mechanism of NFkappaB/Rel activation through calcineurin-mediated inactivation of IkappaBbeta. J. Cell Biol. 161:507-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boczkowski, J., S. Lanone, D. Ungureanu-Longrois, G. Danialou, T. Fournier, and M. Aubier. 1996. Induction of diaphragmatic nitric oxide synthase after endotoxin administration in rats. J. Clin. Investig. 98:1550-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boekhoudt, G. H., Z. Guo, G. W. Beresford, and J. M. Boss. 2003. Communication between NF-kappa B and Sp1 controls histone acetylation within the proximal promoter of the monocyte chemoattractant protein 1 gene. J. Immunol. 170:4139-4147. [DOI] [PubMed] [Google Scholar]
- 7.Cortes-Canteli, M., M. Wagner, W. Ansorge, and A. Perez-Castillo. 2004. Microarray analysis supports a role for ccaat/enhancer-binding protein-beta in brain injury. J. Biol. Chem. 279:14409-14417. [DOI] [PubMed] [Google Scholar]
- 8.Corti, S., S. Salani, R. Del Bo, M. Sironi, S. Strazzer, M. G. D'Angelo, G. P. Comi, N. Bresolin, and G. Scarlato. 2001. Chemotactic factors enhance myogenic cell migration across an endothelial monolayer. Exp. Cell Res. 268:36-44. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree, G. R., and E. N. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell 109(Suppl.):S67-S79. [DOI] [PubMed] [Google Scholar]
- 10.De Bleecker, J. L., B. De Paepe, I. E. Vanwalleghem, and J. M. Schroder. 2002. Differential expression of chemokines in inflammatory myopathies. Neurology 58:1779-1785. [DOI] [PubMed] [Google Scholar]
- 11.Demoule, A., M. Divangahi, G. Danialou, D. Gvozdic, G. Larkin, W. Bao, and B. J. Petrof. 2005. Expression and regulation of CC class chemokines in the dystrophic (mdx) diaphragm. Am. J. Respir. Cell Mol. Biol. 33:178-185. [DOI] [PubMed] [Google Scholar]
- 12.Demoule, A., L. Yahiaoui, M. Divangahi, G. Danialou, D. Gvozdic, W. Bao, and B. J. Petrof. 2005. Expression and regulation of CC class chemokine receptors and ligands in the septic mouse diaphragm. Proc. Am. Thorac. Soc. 2:A884. [Google Scholar]
- 13.De Rossi, M., P. Bernasconi, F. Baggi, M. R. de Waal, and R. Mantegazza. 2000. Cytokines and chemokines are both expressed by human myoblasts: possible relevance for the immune pathogenesis of muscle inflammation. Int. Immunol. 12:1329-1335. [DOI] [PubMed] [Google Scholar]
- 14.Ebihara, S., S. N. A. Hussain, G. Danialou, W. K. Cho, S. B. Gottfried, and B. J. Petrof. 2002. Mechanical ventilation protects against diaphragm injury in sepsis. Interaction of oxidative and mechanical stresses. Am. J. Respir. Crit. Care Med. 165:221-228. [DOI] [PubMed] [Google Scholar]
- 15.Feng, G., Y. Ohmori, and P. L. Chang. 2006. Production of chemokine CXCL1/KC by okadaic acid through the nuclear factor-kappaB pathway. Carcinogenesis 27:43-52. [DOI] [PubMed] [Google Scholar]
- 16.Frost, R. A., G. J. Nystrom, and C. H. Lang. 2002. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283:R698-R709. [DOI] [PubMed] [Google Scholar]
- 17.Frost, R. A., G. J. Nystrom, and C. H. Lang. 2006. Multiple toll-like receptor ligands induce an IL-6 transcriptional response in skeletal myoctyes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290:R773-R784. [DOI] [PubMed] [Google Scholar]
- 18.Gupta, D., Q. Wang, C. Vinson, and R. Dziarski. 1999. Bacterial peptidoglycan induces CD14-dependent activation of transcription factors CREB/ATF and AP-1. J. Biol. Chem. 274:14012-14020. [DOI] [PubMed] [Google Scholar]
- 19.Harada, K., K. Isse, and Y. Nakanuma. 2006. Interferon gamma accelerates NF-kappa B activation of biliary epithelial cells induced by Toll-like receptor and ligand interaction. J. Clin. Pathol. 59:184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayakawa, M., H. Miyashita, I. Sakamoto, M. Kitagawa, H. Tanaka, H. Yasuda, M. Karin, and K. Kikugawa. 2003. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 22:3356-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden, M. S., and S. Ghosh. 2004. Signaling to NF-kappaB. Genes Dev. 18:2195-2224. [DOI] [PubMed] [Google Scholar]
- 22.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 23.Holterman, C. E., and M. A. Rudnicki. 2005. Molecular regulation of satellite cell function. Semin. Cell Dev. Biol. 16:575-584. [DOI] [PubMed] [Google Scholar]
- 24.Hussain, S. N., G. Simkus, and C. Roussos. 1985. Respiratory muscle fatigue: a cause of ventilatory failure in septic shock. J. Appl. Physiol. 58:2033-2040. [DOI] [PubMed] [Google Scholar]
- 25.Kosmidou, I., T. Vassilakopoulos, A. Xagorari, S. Zakynthinos, A. Papapetropoulos, and C. Roussos. 2002. Production of interleukin-6 by skeletal myotubes: role of reactive oxygen species. Am. J. Respir. Cell Mol. Biol. 26:587-593. [DOI] [PubMed] [Google Scholar]
- 26.Latz, E., A. Schoenemeyer, A. Visintin, K. A. Fitzgerald, B. G. Monks, C. F. Knetter, E. Lien, N. J. Nilsen, T. Espevik, and D. T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5:190-198. [DOI] [PubMed] [Google Scholar]
- 27.Lee, Y. W., B. Hennig, and M. Toborek. 2003. Redox-regulated mechanisms of IL-4-induced MCP-1 expression in human vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 284:H185-H192. [DOI] [PubMed] [Google Scholar]
- 28.Leifer, C. A., M. N. Kennedy, A. Mazzoni, C. Lee, M. J. Kruhlak, and D. M. Segal. 2004. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J. Immunol. 173:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, M. C., S. Ebihara, Q. el-Dwairi, S. N. A. Hussain, L. Yang, S. B. Gottfried, A. Comtois, and B. J. Petrof. 1998. Diaphragm sarcolemmal injury is induced by sepsis and alleviated by nitric oxide synthase inhibition. Am. J. Respir. Crit. Care Med. 158:1656-1663. [DOI] [PubMed] [Google Scholar]
- 30.Lutgens, E., B. Faber, K. Schapira, C. T. Evelo, R. van Haaften, S. Heeneman, K. B. Cleutjens, A. P. Bijnens, L. Beckers, J. G. Porter, C. R. Mackay, P. Rennert, V. Bailly, M. Jarpe, B. Dolinski, V. Koteliansky, T. de Fougerolles, and M. J. Daemen. 2005. Gene profiling in atherosclerosis reveals a key role for small inducible cytokines: validation using a novel monocyte chemoattractant protein monoclonal antibody. Circulation 111:3443-3452. [DOI] [PubMed] [Google Scholar]
- 31.McCullagh, K. J., E. Calabria, G. Pallafacchina, S. Ciciliot, A. L. Serrano, C. Argentini, J. M. Kalhovde, T. Lomo, and S. Schiaffino. 2004. NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc. Natl. Acad. Sci. USA 101:10590-10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura, M., and S. Naito. 2005. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol. Pharm. Bull. 28:886-892. [DOI] [PubMed] [Google Scholar]
- 33.Porter, J. D., W. Guo, A. P. Merriam, S. Khanna, G. Cheng, X. Zhou, F. H. Andrade, C. Richmonds, and H. J. Kaminski. 2003. Persistent over-expression of specific CC class chemokines correlates with macrophage and T-cell recruitment in mdx skeletal muscle. Neuromuscul. Disord. 13:223-235. [DOI] [PubMed] [Google Scholar]
- 34.Rossi, D., and A. Zlotnik. 2000. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18:217-242. [DOI] [PubMed] [Google Scholar]
- 35.Sachidanandan, C., R. Sambasivan, and J. Dhawan. 2002. Tristetraprolin and LPS-inducible CXC chemokine are rapidly induced in presumptive satellite cells in response to skeletal muscle injury. J. Cell Sci. 115:2701-2712. [DOI] [PubMed] [Google Scholar]
- 36.Satonaka, H., E. Suzuki, H. Nishimatsu, S. Oba, R. Takeda, A. Goto, M. Omata, T. Fujita, R. Nagai, and Y. Hirata. 2004. Calcineurin promotes the expression of monocyte chemoattractant protein-1 in vascular myocytes and mediates vascular inflammation. Circ. Res. 94:693-700. [DOI] [PubMed] [Google Scholar]
- 37.Schnare, M., A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2000. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr. Biol. 10:1139-1142. [DOI] [PubMed] [Google Scholar]
- 38.Schreiner, B., J. Voss, J. Wischhusen, Y. Dombrowski, A. Steinle, H. Lochmuller, M. Dalakas, A. Melms, and H. Wiendl. 2006. Expression of toll-like receptors by human muscle cells in vitro and in vivo: TLR3 is highly expressed in inflammatory and HIV myopathies, mediates IL-8 release and up-regulation of NKG2D-ligands. FASEB J. 20:118-120. [DOI] [PubMed] [Google Scholar]
- 39.Sekine, O., Y. Nishio, K. Egawa, T. Nakamura, H. Maegawa, and A. Kashiwagi. 2002. Insulin activates CCAAT/enhancer binding proteins and proinflammatory gene expression through the phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. J. Biol. Chem. 277:36631-36639. [DOI] [PubMed] [Google Scholar]
- 40.Semsarian, C., M. J. Wu, Y. K. Ju, T. Marciniec, T. Yeoh, D. G. Allen, R. P. Harvey, and R. M. Graham. 1999. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 400:576-581. [DOI] [PubMed] [Google Scholar]
- 41.Staruch, M. J., R. Camacho, and F. J. Dumont. 1998. Distinctive calcineurin-dependent (FK506-sensitive) mechanisms regulate the production of the CC chemokines macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, and RANTES vs IL-2 and TNF-alpha by activated human T cells. Cell. Immunol. 190:121-131. [DOI] [PubMed] [Google Scholar]
- 42.Supinski, G., D. Stofan, D. Nethery, L. Szweda, and A. DiMarco. 1999. Apocynin improves diaphragmatic function after endotoxin administration. J. Appl. Physiol. 87:776-782. [DOI] [PubMed] [Google Scholar]
- 43.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 44.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 45.Tidball, J. G., and M. Wehling-Henricks. 2005. Damage and inflammation in muscular dystrophy: potential implications and relationships with autoimmune myositis. Curr. Opin. Rheumatol. 17:707-713. [DOI] [PubMed] [Google Scholar]
- 46.Trushin, S. A., K. N. Pennington, A. Algeciras-Schimnich, and C. V. Paya. 1999. Protein kinase C and calcineurin synergize to activate IkappaB kinase and NF-kappaB in T lymphocytes. J. Biol. Chem. 274:22923-22931. [DOI] [PubMed] [Google Scholar]
- 47.Uchijima, M., T. Nagata, T. Aoshi, and Y. Koide. 2005. IFN-gamma overcomes low responsiveness of myeloid dendritic cells to CpG DNA. Immunol. Cell Biol. 83:92-95. [DOI] [PubMed] [Google Scholar]
- 48.Ulevitch, R. J. 2004. Therapeutics targeting the innate immune system. Nat. Rev. Immunol. 4:512-520. [DOI] [PubMed] [Google Scholar]
- 49.Warren, G. L., T. Hulderman, D. Mishra, X. Gao, L. Millecchia, L. O'Farrell, W. A. Kuziel, and P. P. Simeonova. 2005. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 19:413-415. [DOI] [PubMed] [Google Scholar]
- 50.Warren, G. L., L. O'Farrell, M. Summan, T. Hulderman, D. Mishra, M. I. Luster, W. A. Kuziel, and P. P. Simeonova. 2004. Role of CC chemokines in skeletal muscle functional restoration after injury. Am. J. Physiol. Cell Physiol. 286:C1031-C1036. [DOI] [PubMed] [Google Scholar]
- 51.Waters, V., S. Sokol, B. Reddy, G. Soong, J. Chun, and A. Prince. 2005. The effect of cyclosporin A on airway cell proinflammatory signaling and pneumonia. Am. J. Respir. Cell Mol. Biol. 33:138-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiendl, H., R. Hohlfeld, and B. C. Kieseier. 2005. Immunobiology of muscle: advances in understanding an immunological microenvironment. Trends Immunol. 26:373-380. [DOI] [PubMed] [Google Scholar]
- 53.Wiendl, H., M. Mitsdoerffer, D. Schneider, A. Melms, H. Lochmuller, R. Hohlfeld, and M. Weller. 2003. Muscle fibres and cultured muscle cells express the B7.1/2-related inducible costimulatory molecule, ICOSL: implications for the pathogenesis of inflammatory myopathies. Brain 126:1026-1035. [DOI] [PubMed] [Google Scholar]
- 54.Zarember, K. A., and P. J. Godowski. 2002. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 168:554-561. [DOI] [PubMed] [Google Scholar]