Abstract
The ability of Plasmodium falciparum-infected erythrocytes to adhere to host endothelial cells via receptor molecules such as ICAM-1 and CD36 is considered a hallmark for the development of severe malaria syndromes. These molecules are also expressed on leukocytes such as dendritic cells. Dendritic cells are antigen-presenting cells that are crucial for the initiation of adaptive immune responses. In many human diseases, their frequency and function is perturbed. We analyzed the frequency of peripheral blood dendritic cell subsets and the plasma concentrations of interleukin-10 (IL-10) and IL-12 in Kenyan children with severe malaria and during convalescence and related these parameters to the adhesion phenotype of the acute parasite isolates. The frequency of CD1c+ dendritic cells in children with acute malaria was comparable to that in healthy controls, but the frequency of BDCA3+ dendritic cells was significantly increased. Analysis of the adhesion phenotypes of parasite isolates revealed that adhesion to ICAM-1 was associated with the frequency of peripheral blood CD1c+ dendritic cells, whereas the adhesion of infected erythrocytes to CD36 correlated with high concentrations of IL-10 and low concentrations of IL-12 in plasma.
In areas of endemicity, infection with asexual blood stages of Plasmodium falciparum can result in asymptomatic infection, mild clinical symptoms, or severe, life-threatening disease (1). Clinical immunity against asexual blood-stage infection is never sterile but is built up with repeated exposure, eventually preventing clinical disease and controlling parasitemia (11). Antigenic variation of surface proteins such as the P. falciparum erythrocyte membrane protein 1 (PfEMP-1) contributes to the slow acquisition of immunity to falciparum malaria and promotes chronic infection (4). Importantly, PfEMP-1 also mediates adhesion of mature forms of infected red blood cells (iRBCs) to host proteins expressed on endothelial cells and leukocytes. Although almost all parasite isolates adhere to CD36, some also binds to other receptors such as CD54 (ICAM-1 [intercellular adhesion molecule 1]), CD31 (PECAM-1 [platelet endothelial cell adhesion molecule 1]) or CD35 (CR1 [complement receptor 1]) (18). Cytoadhesion of iRBCs is thought to play an important role in malaria pathology because it allows mature forms to sequester in the vascular bed, leading to the obstruction of capillary vessels. In particular, adhesion to ICAM-1 has been associated with cerebral malaria, while high binding to CD36 has been associated with mild disease in some studies (22, 30, 31). However, the host receptors for PfEMP-1 are also expressed on leukocytes, including T cells, monocytes, and dendritic cells (DCs), all of which are involved in the immune response to P. falciparum infection.
Both monocytes and DCs ingest pathogens and can present pathogen-derived peptides to T cells. Although activated monocytes may be able to activate primed T cells, only DCs can activate naive T cells and thus DCs are crucial for the initiation of immune responses (2). In peripheral blood, two major DC subsets can be detected that have distinct but overlapping functions. Myeloid DCs (mDCs) express HLA DR, CD11c, and CD1c and are the main producers of interleukin-12 (IL-12), whereas plasmacytoid DCs (pDCs) express HLA DR, CD123, and BDCA2 (blood dendritic cell antigen 2) and are the main producers of IFN-α. A third, minor population of CD11c+BDCA3+ mDCs in peripheral blood has been described but is not well characterized (9). In vitro studies on monocyte-derived DCs suggested that adhesion of iRBCs to surface-expressed CD36 modulated both their maturation and function (32). In these studies, parasite-modulated DCs failed to secrete IL-12 or to induce proliferation in naive or primed T cells, although they secreted IL-10 and tumor necrosis factor alpha (TNF-α).
We have previously reported that the frequency of total peripheral blood DCs remained constant during acute falciparum malaria, whereas HLA DR expression was reduced, suggesting that modulation of DCs may occur in vivo (33). Furthermore, a recent study by Pichyangkul et al. showed that the frequency of pDCs in peripheral blood was reduced in adult Thai patients with acute malaria (26). We now wanted to establish whether changes in DC numbers and the expression of HLA DR were similar for all subsets in Kenyan children with severe malaria or whether these phenomena are different for each subset. Therefore, we investigated changes in the frequency of specific DC subsets in Kenyan children with severe malaria in acute and convalescent samples compared to healthy community controls. In addition, we analyzed whether there is any relationship between the frequency of peripheral blood DC subsets, the concentration of key cytokines in plasma, and the adhesion phenotype of the acute parasite isolate.
MATERIALS AND METHODS
Study population.
Blood samples were collected from children presenting to Kilifi District Hospital on the coast of Kenya with severe P. falciparum malaria. Severe malaria was characterized by the presence of one or more of the following features: signs of deep breathing, coma (Blantyre coma score of ≤2), prostration, or severe anemia (hemoglobin [Hb] < 5 g/dl) in the presence of hyperparasitemia (iRBC > 10%). Children were excluded if they showed any sign of bacterial or viral meningitis, including positive blood or cerebrospinal fluid cultures or white blood cells in the cerebrospinal fluid. Children were invited for convalescent sampling 14 days after discharge from hospital, at which time they were examined clinically and treated if necessary. Children who were still slide positive for parasites were excluded from the analysis. Control blood samples were collected from children living in the Ngerenya area of Kilifi District, who were part of a cohort under active surveillance for malaria as described in detail elsewhere (23). These children were sampled during a cross-sectional survey conducted during a period of low transmission in October 2004. Children who were slide positive for parasites or had a temperature above 37°C were excluded from analysis. Thirty-three children from the control group were matched for age (±4 months) with children suffering from severe malaria. Individual written informed consent was obtained from the children's parents or their representative prior to sampling. This study received ethical approval from both the Kenya Medical Research Institute National Ethical Review and the Oxford Tropical Medicine Research Ethics committees.
Antibodies.
The following antibodies were used: phycoerythrin-Texas Red-x-conjugated anti-CD3, anti-CD14, and anti-CD19; PC5-conjugated anti-HLA DR; fluorescein isothiocyanate (FITC)-conjugated anti-human immunoglobulin G1, phycoerythrin (PE)-conjugated anti-human immunoglobulin G1 (all from Beckman Coulter, United Kingdom); FITC-conjugated anti-CD11c (Serotec, United Kingdom); PE-conjugated anti-CD123 (Becton Dickinson, United Kingdom); FITC-conjugated anti-BDCA2; PE-conjugated anti-BDCA3 and PE-conjugated anti-CD1c (Miltenyi Biotec, Germany); and FITC-conjugated anti-HLA DR and R-phycoerythrin-conjugated anti-CD14 (Dako, United Kingdom).
Flow cytometry.
Venous blood samples (500 μl) were drawn into EDTA blood tubes (TekLab, United Kingdom), and 50-μl aliquots were incubated for 30 min at 4°C with a cocktail containing the lineage markers antibodies (anti-CD3, anti-CD14, and anti-CD19) and anti-HLA DR antibody. In addition, aliquots were stained with the isotype control antibodies anti-CD11c and anti-CD1c, anti-CD11c and anti-BDCA3, or anti-BDCA2 and anti-CD123 in order to identify background staining, CD11c+ CD1c+ mDCs, CD11c+ BDCA3+ mDCs, and CD123+ BDCA2+ pDCs, respectively (9). To identify monocytes, 50 μl of whole blood was incubated for 30 min at 4°C with anti-CD14 and anti-HLA DR. After erythrocyte lysis with Optilyse C solution (Beckman Coulter, United Kingdom), white blood cells were washed in phosphate-buffered saline and analyzed by flow cytometry (Epics II; Beckman Coulter, United Kingdom). For each sample, we acquired at least 1,000 lineage marker-negative, HLA DR-positive events. This gate was set to include HLA DRdim cells. Lineage marker-negative, HLA DR+ cells were gated within the PBMC gate, and CD11c+ CD1c+ mDCs, CD11c+ BDCA3+ mDCs, or CD123+ BDCA2+ pDCs were quantified as a percentage of the total peripheral blood mononuclear cells (PBMC). Absolute numbers of CD1c+ or BDCA3+ mDC, pDC, and monocytes were calculated by using whole-blood counts. All flow cytometry data were analyzed by using FlowJo software (TriStar).
Enzyme-linked immunosorbent assay.
Plasma and PBMC were separated by density centrifugation using Lymphoprep according to standard methods, and plasma was frozen immediately at −80°C. The concentrations of IL-10, TNF-α, and IL-12 in plasma were analyzed by using proprietary ELISA kits (Pharmingen) according to the manufacturer's instructions.
Parasite culture and binding assay.
RBCs from acute blood samples were taken into culture and parasites grown to maturity in the presence of aphidicolin (Sigma), a reversible DNA synthesis inhibitor which arrests parasite development during the trophozoite stages. Presence of aphidicolin does not affect agglutination, rosetting or adhesion to ICAM-1 and CD36 (3, 13). Parasitemia and hematocrit was determined by flow cytometry after staining iRBCs with ethidium bromide. Cultures with a parasitemia above 0.3% were used for binding assays as previously described (22). Briefly, 2 μl each of 100, 20, 4, or 0.8 μg of ICAM-Fc (8) or purified human immunoglobulins/ml and 50, 10, 2, or 0.4 μg of CD36-Fc (R&D Systems, United Kingdom) or CD31-Fc (10)/ml were absorbed onto plastic bacteriological dishes. Parasite cultures were adjusted to 1% hematocrit in binding medium and then added to the plastic dish. Parasites were incubated for 1 h at 37°C with gentle rotation every 10 min. Nonadherent cells were washed away, and adherent cells were fixed with 1% glutaraldehyde (Sigma, United Kingdom) and then stained with Giemsa (Sigma, United Kingdom). The number of adherent parasites in three different sections of each protein spot were counted by using light microscopy (magnification, ×400) and corrected to the number of cells bound per square millimeter of protein at 2% parasitemia and 1% hematocrit. At the highest protein concentration, the average standard deviation for binding to ICAM-1 was ±7 iRBC/mm2, and the average standard deviation for binding to CD36 was ±11 iRBC/mm2.
Proliferation Assay.
Responder T cells (100,000 cells/well) from one blood donor were labeled with 0.5 μM carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, The Netherlands) and cocultured with 50,000 or 100,000 PBMC from acute, convalescent, or control samples; 2 μg of phytohemagglutinin/ml; or medium alone (13). On day 5, the proportion of nondividing, CFSE+ responder T cells was analyzed by flow cytometry. To determine the proportion of proliferating CFSE+ T cells, the proportion of nondividing T cells was corrected for background proliferation in medium alone and then subtracted from 100. The proliferation induced by phytohemagglutinin ranged from 80 to 90% (standard deviation of 5%) in all assays.
Statistical analysis.
All data were analyzed in SPSS (SPSS, Inc.). We compared continuous data between groups by using Mann-Whitney U tests, the Kruskal-Wallis test for trends, or the Wilcoxon signed-rank test for paired acute and convalescence samples. The concentrations of IL-10, TNF-α, and IL-12 in plasma, the number of iRBCs binding to immobilized protein, and the number of peripheral blood DC subsets and monocytes were normally distributed after logarithmic transformation. Logarithmic transformed values were used to obtain correlation coefficients between these parameters within one group and for linear regression analysis. All P values are two sided.
RESULTS
Frequency of peripheral blood DC subsets.
Of the 65 children with severe malaria recruited into the study, 33 returned to give a convalescent blood sample on day 14. As expected, children with severe malaria had significantly lower RBC counts and hemoglobin concentrations compared to controls. While these parameters had improved in convalescence, they had not yet returned to normal (Table 1).
TABLE 1.
Basic characteristics of study population
| Parametera | Median (25th-75th percentiles)b
|
||
|---|---|---|---|
| Acute sample (n = 33) | Convalescent sample (n = 33) | Control sample (n = 33) | |
| Subject age (mo) | 35 (26-42) | 35 (26-42) | 40 (31-45) |
| Parasitemia (no./μl) | 315,000 (12,321-548,240) | 0 | 0 |
| WBCb count (106/ml) | 11.1 (7.8-19.8) | 10.4 (8.0-13.4) | 10.8 (8.2-12.1) |
| RBC count (109/ml) | 3.3* (2.3-4.1) | 3.7* (3.3-4.3) | 4.7 (4.2-4.9) |
| Hb concn (g/dl) | 8.1* (6-9.5) | 9.2† (8.8-10.1) | 10.6 (9.9-11.3) |
WBC, white blood cell; Hb, hemoglobin.
*, P < 0.001 compared to control; †, P < 0.05 compared to control (Mann-Whitney U test). n, number of samples.
We analyzed the absolute number of peripheral blood DC subsets and monocytes in paired samples from children with severe malaria and during convalescence and in the control population by whole-blood staining (Fig. 1). The absolute number of myeloid CD1c+ mDCs and pDCs remained relatively constant during severe malaria and convalescence (Table 2). In contrast, the absolute number of BDCA3+ mDCs and monocytes was significantly increased in children with severe malaria versus controls (Table 2).
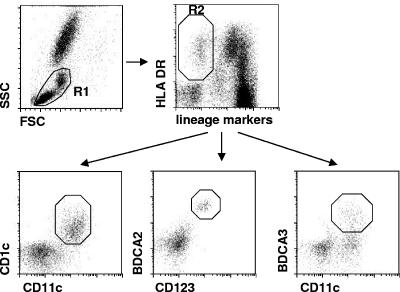
FIG. 1.
Flow cytometric analysis of peripheral blood DC subsets. Within the PBMC gate (R1), lineage marker-negative, HLA DR+ cells were gated (R2), and CD11c+ CD1c+ mDCs, CD123+ BDCA2+ pDCs, or CD11c+ BDCA3+ mDCs were determined as a percentage of the total PBMC.
TABLE 2.
DC numbers and cytokine concentrations in cases and controls
| Parametera | Median (25th-75th percentile)b
|
||
|---|---|---|---|
| Acute sample (n = 33) | Convalescent sample (n = 33) | Control sample (n = 33) | |
| CD1c+ mDCs (103/ml) | 9.5 (5.8-18.9) | 15 (8.8-26.5) | 13 (9.3-18.5) |
| CD123+ pDCs (103/ml) | 4.9 (2.3-13.1) | 9.4 (5.3-20.8) | 9.3 (4.6-13.8) |
| BDCA3+ mDCs (103/ml) | 5.0* (2.1-9.0) | 6.6* (2.1-30.0) | 1.2 (0.6-2.0) |
| CD14+ monocytes (106/ml) | 0.39* (0.19-0.73) | 0.51* (0.26-0.93) | 0.10 (0.08-0.14) |
| CD1c+ mDCs (GMFI HLA DR) | 24.3* (17.0-34.2) | 41.4 (28.3-49.6) | 36.5 (30.3-44.3) |
| CD123+ pDCs (GMFI HLA DR) | 12.6 (6.6-21.1) | 15.1 (10.8-19.3) | 16.9 (11.5-20.1) |
| BDCA3+ mDCs (GMFI HLA DR) | 9.2* (5.1-15.0) | 24.1 (15.8-37.5) | 19.7 (13.4-28.8) |
| CD14+ monocytes (GMFI HLA DR) | 13.6* (10.1-24.1) | 27.5 (17.8-52.0) | 31.5 (28.3-36.5) |
| IL-10 (pg/ml) | 1,731* (538-2,533) | 69 (21-140) | 44 (17-82) |
| TNF-α (pg/ml) | 35* (11-82) | 0 | 0 |
| IL-12 (pg/ml) | 183* (91-274) | 0 | 0 |
GMFI HLA DR, Geometric mean fluorescence intensity of HLA DR expressed on monocytes or DC subsets. Of note, the fluorescence intensity scale on an Epics II flow cytometer from Beckman Coulter begins with 0.1 rather than 1.
*, P < 0.001 compared to control (Mann-Whitney U test).
We observed previously that the expression of HLA DR was reduced on peripheral blood DCs, suggesting functional impairment of DCs, although in that study we were not able to distinguish mDCs and pDCs. We confirm here that expression of HLA DR was reduced on monocytes and CD1c+ and BDCA3+ mDC but not on pDC when acute and convalescent samples (Wilcoxon signed-rank test: monocyte z score −3.85, P < 0.01; CD1c+ mDC z score −3.385, P < 0.001; BDCA3+ mDC z score −3.74, P < 0.01) and acute and control samples were compared (Table 2).
Parasitemia is an important parameter of disease severity and could influence any direct effect of iRBC or their products on monocytes or peripheral blood DCs. We observed in children with severe malaria that only the number of monocytes was inversely correlated with parasitemia (Pearson correlation coefficient r = −0.433, P < 0.05). Parasitemia had no effect on the expression level of HLA DR on monocytes or mDCs.
Plasma cytokine concentrations.
We determined the plasma concentrations of TNF-α, IL-12, and IL-10, all of which are produced by parasite-modulated DCs in vitro, to investigate whether any of these were associated with the frequency of DC subsets. All cytokines were significantly increased during acute disease compared to convalescent and control samples (Table 2), whereas IL-10 was also detectable in plasma during convalescence and in some healthy children. In children with acute disease, the concentrations of TNF-α, IL-12, and IL-10 in plasma did not correlate with each other. The plasma concentration of IL-10 was positively correlated with parasitemia (14) and the number of BDCA3+ mDCs in children with acute malaria (Pearson correlation coefficient: parasitemia, r = 0.794, P < 0.001; BDCA3+ mDC, r = 0.393, P < 0.05). The concentration of IL-12 or TNF-α in plasma did not correlate with the absolute number of or HLA DR expression on any of the DC subsets or monocytes.
Antigen-presenting function of PBMC.
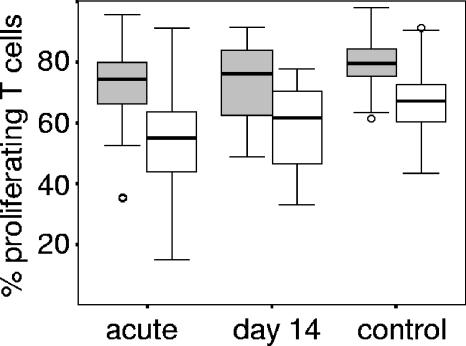
Chehimi et al. had reported an association between DC frequencies in PBMC and their ability to stimulate proliferation of allogeneic T cells (6). We used this test to investigate whether the frequency of DC subsets or the expression level of HLA DR in PBMC from children with severe disease, from children during convalescence, and from controls was related to their ability to induce proliferation in allogeneic T cells. PBMC from children with severe malaria induced less T-cell proliferation than PBMC from healthy children irrespective of the PBMC/T-cell ratio used (Fig. 2, P < 0.01 [Mann-Whitney U test] at a PBMC/T-cell ratio of 1:1 and 1:2). The extent of T-cell proliferation was negatively correlated with the number of BDCA3+ mDC (Spearman rho −0.363 [P = 0.035] and rho −0.352 [P = 0.046] for PBMC/T-cell ratios of 1:2 and 1:1, respectively) during acute disease, and a similar trend was observed for the convalescence group. Unlike the observation by Chehimi et al., we found no correlation among the control population between the DC frequency in PBMC and the induction of proliferation in T cells.
FIG. 2.
PBMC from children with severe malaria are less efficient in inducing allogeneic T-cell proliferation. Boxplots of the percentages of proliferating allogeneic T cells induced by PBMC from children with severe malaria, during convalescence (day 14), and healthy controls. PBMC and CFSE-labeled allogeneic T cells were incubated for 5 days at ratios of 1:1 (gray) and 1:2 (white). Boxplots represent the median and the 25th and 75th percentiles. Outliers are marked by circles.
Adhesion phenotypes of homologous parasite isolates.
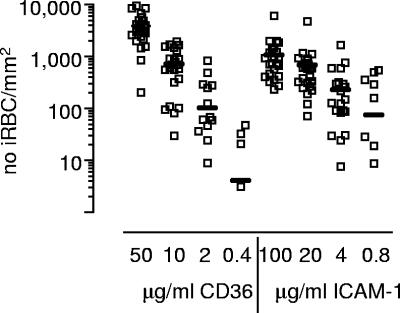
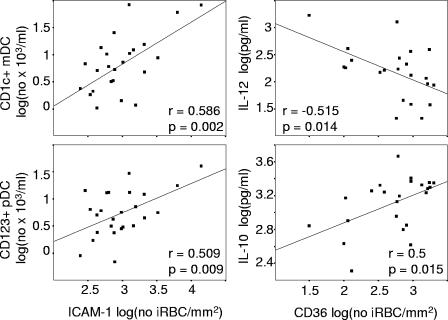
We were interested to see whether the adhesion phenotype of the acute parasite isolate correlated with the frequency of peripheral blood DCs or the plasma concentrations of IL-10, IL-12, or TNF-α. We therefore determined the binding to plate-bound CD36, ICAM-1, CD31, and human immunoglobulin for all parasite isolates, which grew to maturity (n = 26), whether or not the child returned for a follow-up appointment. All isolates bound to CD36 and to ICAM-1 and, in general, more trophozoites bound to CD36 at the highest concentration tested than to ICAM-1; the median (25th to 75th percentile) values for 50 μg of CD36/ml and 100 μg of ICAM-1/ml were 3,412 iRBC/mm2 (2,650 to 5,156) and 759 iRBC/mm2 (428 to 1,429), respectively (Fig. 3) . Binding to CD31 was weak and above background levels only for two isolates, as has been observed in one study but not another study on Kenyan parasite isolates (7, 22). We did not observe any binding to human immunoglobulin, although we cannot exclude that immunoglobulin carried over from culture medium was bound to the surface of iRBC and blocked binding to plate-bound immunoglobulin (7). At concentrations of 100 μg of ICAM-1/ml and of 10 μg of CD36/ml, the numbers of iRBCs bound to ICAM-1 and to CD36 were comparable. At this concentration, the binding of iRBCs to CD36 was correlated with high plasma concentrations of IL-10 and low plasma concentrations of IL-12 (Fig. 4) but not with the plasma concentration of TNF-α or the absolute number of any DC subset. Similar relationships were observed when we analyzed the binding of iRBC to 50 or 2 μg of plate-bound CD36/ml (Pearson correlation coefficients: for 50 μg of CD36/ml, r = 0.575, P = 0.04 [IL-10], and r = −0.557, P = 0.031 [IL-12]; for 2 μg of CD36/ml, r = 0.862, P = 0.013 [IL-10] and r = −0.416 P = 0.035 [IL-12]), although the binding of iRBCs to plate-bound CD36 at a concentration of 50 μg/ml was outside the linear range. As described above, the concentration of IL-10 in plasma was also correlated with parasitemia. We therefore used multiple regression to determine the individual contributions of parasitemia and adhesion to CD36. In this model, both parameters were independently associated with the concentration of IL-10 in plasma (overall model r = 0.717, P < 0.001; standardized beta coefficient of 0.42, P = 0.019 for parasitemia and 0.496, P = 0.007 for adhesion to CD36). In contrast, the binding of iRBCs to 50 μg of plate-bound ICAM-1/ml showed a positive correlation with the number of CD1c+ mDCs and pDCs (Fig. 4) but not with the concentration of IL-10, IL-12, or TNF-α in plasma. Again, these correlations were also observed at the lower concentration of 10 μg of plate-bound ICAM-1/ml (Pearson correlation coefficient for CD1c+ DC: r = 0.572, P = 0.008; Pearson correlation coefficient for BDCA2+ DC: r = 0.466, P = 0.038).
FIG. 3.
Cytoadhesion of iRBCs to CD36 and ICAM-1. The mean number of mature iRBC from individual field isolates that bound to various concentrations of CD36 and ICAM-1 in a static binding assay is shown. The line represents the median of the number of iRBC bound to a given protein concentration.
FIG. 4.
The adhesion of iRBCs is correlated with the concentrations of IL-10 and IL-12 and the number of DC subsets. The correlation between the number of iRBCs binding to ICAM-1 or CD36 with the absolute number of CD1c+ mDCs and pDCs and the plasma concentrations of IL-10 and IL-12, respectively, is shown. Logarithmic transformed values were compared by using the Pearson correlation.
DISCUSSION
Here we have shown that severe infection with P. falciparum malaria induces profound changes in the number of BDCA3+ mDCs in the peripheral circulation, whereas the overall number of CD1c+ mDCs and pDCs was not significantly altered during acute disease compared to convalescence samples or controls.
To our knowledge, this is the first report showing increased frequency of BDCA3+ mDCs in a human infection. The function of this rather small population in healthy individuals is not known. However, BDCA3, together with other inhibitory receptors, is upregulated on IL-10-treated monocyte-derived DCs. IL-10-treated monocyte-derived DCs express intermediate levels of HLA DR and costimulatory molecules and fail to activate T cells (34), a phenotype similar to that of parasite-modulated monocyte-derived DCs. An immunomodulatory function of this DC subset is in agreement with our observation that reduced allogeneic T-cell proliferation induced by PBMC from children with acute malaria was associated with an increased frequency of BDCA3+ mDCs. It has recently been suggested that CD1c+ and BDCA3+ mDC subsets represent different stages of mDCs rather than different lineages because their transcription profile is very similar (21). We observed an increase in the expression of BDCA3 on CD1c+ mDCs cocultured with iRBC in vitro (our unpublished observation), suggesting that the increased number of BDCA3+ mDCs may be due in part to the induction of BDCA3 expression on a proportion of CD1c+ DCs in children with acute malaria. We are now investigating the function of CD1c+ and BDCA3+ mDCs cocultured with iRBCs in vitro.
In addition to BDCA3+ mDCs, the number of monocytes was increased in the peripheral circulation, whereas the number of CD1c+ mDCs and pDCs remained constant. In a previous study, we reported that the total number of HLA DR+, lineage marker-negative DCs, comprising all three subsets, was apparently reduced due to the lower expression of HLA DR on DCs in children with acute malaria. In the present study, we therefore gated on all HLA DR+ cells whether or not expression of HLA DR was high or low to avoid underestimation of the number of DCs in the peripheral circulation. In agreement with the previous study (33), we show here that the expression of HLA DR was reduced on monocytes and CD1c+ and BDCA3+ mDCs, whereas pDCs showed normal expression of HLA DR. In contrast to the study by Pichyangkul et al., who reported decreased frequencies of pDCs in Thai patients with acute malaria, we did not observe any significant change but a trend toward lower numbers in this DC population (26). The reason for the differences in pDC frequency in these two studies are not clear but could be due to the difference in ethnicity, age, and previous exposure to falciparum malaria, resulting in different kinetics of pDC activation in the two study populations. pDCs are activated by a parasite protein found in schizont lysate or by hemozoin in vitro and show a fundamentally different response to mDCs. In contrast, mDCs are modulated in vitro by the adhesion of iRBC to CD36, as well as by the phagocytosis of hemozoin. In vivo, all of these different mechanisms could act on DC subsets and induce different types of immune response over the course of an infection depending on the dose of the modulating/activating substance, localization of the responding cell type, and the kinetics of the cellular response. Some evidence for such a scenario comes from studies on the DC function in rodent models of malaria. Although some of these studies provide apparently conflicting results, a consensus seems to emerge whereby DC may be activated and induce T-cell responses early during infection but show a modulated phenotype and fail to initiate T-cell responses later during infection (12, 20, 24-27, 29).
We have shown previously in vitro that when monocyte-derived DCs are cocultured with CD36-binding iRBCs they remain phenotypically immature and fail to secrete IL-12, but they do secrete IL-10 and TNF-α. In the present study, we began to investigate whether we could observe a relationship between the cytoadhesion phenotype of iRBC and DC frequency or plasma cytokine concentration during acute disease. We therefore cultured homologous parasites to maturity and analyzed binding to ICAM-1, CD36, CD31, and immunoglobulins in a static binding assay. These assays can only provide results for the average binding of all parasites that have grown to maturity and do not take into account differences in binding between different strains of parasites if a child is infected with more than one strain. Bearing this confounding factor in mind, we report here that the overall concentration of IL-12 in plasma was inversely correlated with the adhesion of iRBCs to CD36, whereas the concentration of IL-10 in plasma showed a positive correlation independent of the effect of parasitemia. Myeloid DCs are the main producers of IL-12, although it is conceivable that monocytes differentiating into DCs after activation via TLRs may be an additional source of IL-12 (17). IL-10 is produced by DCs and monocytes (which express CD36), as well as T cells and B cells (which do not express CD36). In future immunoepidemiological studies, we will establish the cellular source(s) of IL-10 and IL-12 by intracellular cytokine staining. Although our observations do not prove a causal relationship, they allow for the possibility that the adhesion of iRBCs to CD36 expressed on mDCs may occur in vivo and result in reduced secretion of IL-12 but enhanced secretion of IL-10. This hypothesis demands further investigations into the relationship between parasite adhesion phenotypes and the type and duration of the immune response using methods that allow analysis of the parasite adhesion phenotype on the single cell level by flow cytometry. To our surprise, we also observed a positive relationship between the adhesion of iRBCs to ICAM-1 and the frequency of CD1c+ mDCs and pDCs. The effect of ICAM-1-binding iRBCs on the number of DC subsets was independent of adhesion to CD36 or the plasma concentration of IL-10, IL-12, or TNF-α. ICAM-1 is expressed on lymphocytes including T cells, NK cells, and NKT cells, as well as on monocytes and DCs. Binding of iRBCs to ICAM-1 expressed on these cells could induce the release of cytokines or chemokines acting on DCs. Whether the effect of adhesion of iRBCs to ICAM-1 is direct or indirect, it does deserve further investigation to establish its biological significance.
Previous studies have shown that the binding of iRBCs to CD36 is higher in children with uncomplicated malaria than in children with severe disease (22, 28, 30). It is important to establish whether a relationship between the adhesion of iRBCs to CD36 and the concentration of IL-10 and IL-12 in plasma we observed here in children with severe malaria also exists in children with uncomplicated malaria with the lower parasitemias commonly found in this group. Furthermore, at least in the genome of the laboratory line 3D7, a subgroup of var genes encoding PfEMP-1 has been identified that do not bind to CD36 (16). Importantly, this subgroup appears to be predominantly expressed in nonimmune volunteers experimentally infected with 3D7 iRBCs during vaccine trials and in laboratory lines selected with antibodies from children with severe disease (15, 19). In addition, children with severe malaria and heterozygotic for a null mutation in CD36 are more likely to be infected with parasites expressing PfEMP-1 with a high frequency of recognition by heterologous immune serum, indicating that adhesion to CD36 is associated with parasites with a low frequency of recognition (5). So, what could be the relationship between adhesion to CD36, the immune response to PfEMP-1 and the immune selection of the expressed PfEMP-1 in nonimmune and semi-immune individuals? One explanation would be that parasites with no or low binding to CD36 are more immunogenic because they induce the production of IL-12 rather than IL-10 by DCs and possibly monocytes, resulting in better T-cell activation and helper function. A high or low frequency of recognition of a parasite isolate by heterologous immune serum could then be due to differences in the immunogenicity of the expressed PfEMP-1. Longitudinal studies addressing the interaction between the phenotype and the duration of immune responses to P. falciparum infection and the parasites genetic and phenotypic make-up may be able to answer these questions.
Acknowledgments
This article is published with the permission of the Director of the Kenya Medical Research Institute. This study was supported by the Wellcome Trust. B.C.U. holds a Wellcome Trust Career Development Fellowship, and T.N.W. holds a Senior Clinical Fellowship. P.B., C.I.N., and K.M. are supported by the Wellcome Trust. None of the authors reported any conflicting interests.
We thank the children and their parents or guardians for participating in the study. We also greatly appreciate the help of clinical teams at the KEMRI ward and the Outpatients Clinic and the field workers of the mild malaria study.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 25 September 2006.
REFERENCES
- 1.Anonymous. 1997. World malaria situation in 1994. Part I. Population at risk. Wkly. Epidemiol. Rec. 72:269-274. [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.-J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Bull, P. C., B. S. Lowe, M. Kortok, and K. Marsh. 1999. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect. Immun. 67:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull, P. C., B. S. Lowe, M. Kortok, C. S. Molyneux, C. I. Newbold, and K. Marsh. 1998. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 4:358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bull, P. C., A. Pain, F. M. Ndungu, S. M. Kinyanjui, D. J. Roberts, C. I. Newbold, and K. Marsh. 2005. Plasmodium falciparum antigenic variation: relationships between in vivo selection, acquired antibody response, and disease severity. J. Infect. Dis. 192:1119-1126. [DOI] [PubMed] [Google Scholar]
- 6.Chehimi, J., D. E. Campbell, L. Azzoni, D. Bacheller, E. Papasavvas, G. Jerandi, K. Mounzer, J. Kostman, G. Trinchieri, and L. J. Montaner. 2002. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 168:4796-4801. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Q., A. Heddini, A. Barragan, V. Fernandez, S. F. Pearce, and M. Wahlgren. 2000. The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J. Exp. Med. 192:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig, A., D. Fernandez-Reyes, M. Mesri, A. McDowall, D. C. Altieri, N. Hogg, and C. Newbold. 2000. A functional analysis of a natural variant of intercellular adhesion molecule-1 (ICAM-1Kilifi). Hum. Mol. Genet. 9:525-530. [DOI] [PubMed] [Google Scholar]
- 9.Dzionek, A., A. Fuchs, P. Schmidt, S. Cremer, M. Zysk, S. Miltenyi, D. W. Buck, and J. Schmitz. 2000. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165:6037-6046. [DOI] [PubMed] [Google Scholar]
- 10.Fawcett, J., C. Buckley, C. L. Holness, I. N. Bird, J. H. Spragg, J. Saunders, A. Harris, and D. L. Simmons. 1995. Mapping the homotypic binding sites in CD31 and the role of CD31 adhesion in the formation of interendothelial cell contacts. J. Cell Biol. 128:1229-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta, S., R. W. Snow, C. A. Donnelly, K. Marsh, and C. Newbold. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat. Med. 5:340-343. [DOI] [PubMed] [Google Scholar]
- 12.Ing, R., M. Segura, N. Thawani, M. Tam, and M. M. Stevenson. 2006. Interaction of mouse dendritic cells and malaria-infected erythrocytes: uptake, maturation, and antigen presentation. J. Immunol. 176:441-450. [DOI] [PubMed] [Google Scholar]
- 13.Inselburg, J., and H. S. Banyal. 1984. Plasmodium falciparum: synchronization of asexual development with aphidicolin, a DNA synthesis inhibitor. Exp. Parasitol. 57:48-54. [DOI] [PubMed] [Google Scholar]
- 14.Jakobsen, P. H., V. McKay, R. N′Jie, B. O. Olaleye, U. D'Alessandro, K. Bendtzen, I. Schousboe, and B. M. Greenwood. 1996. Soluble products of inflammatory reactions are not induced in children with asymptomatic Plasmodium falciparum infections. Clin. Exp. Immunol. 105:69-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen, A. T., P. Magistrado, S. Sharp, L. Joergensen, T. Lavstsen, A. Chiucchiuini, A. Salanti, L. S. Vestergaard, J. P. Lusingu, R. Hermsen, R. Sauerwein, J. Christensen, M. A. Nielsen, L. Hviid, C. Sutherland, T. Staalsoe, and T. G. Theander. 2004. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 199:1179-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraemer, S. M., and J. D. Smith. 2003. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol. Microbiol. 50:1527-1538. [DOI] [PubMed] [Google Scholar]
- 17.Krutzik, S. R., B. Tan, H. Li, M. T. Ochoa, P. T. Liu, S. E. Sharfstein, T. G. Graeber, P. A. Sieling, Y. J. Liu, T. H. Rea, B. R. Bloom, and R. L. Modlin. 2005. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 11:653-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyes, S., P. Horrocks, and C. Newbold. 2001. Antigenic variation at the infected red cell surface in malaria. Annu. Rev. Microbiol. 55:673-707. [DOI] [PubMed] [Google Scholar]
- 19.Lavstsen, T., P. Magistrado, C. C. Hermsen, A. Salanti, A. T. Jensen, R. Sauerwein, L. Hviid, T. G. Theander, and T. Staalsoe. 2005. Expression of Plasmodium falciparum erythrocyte membrane protein 1 in experimentally infected humans. Malar. J. 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leisewitz, A. L., K. A. Rockett, B. Gumede, M. Jones, B. Urban, and D. P. Kwiatkowski. 2004. Response of the splenic dendritic cell population to malaria infection. Infect. Immun. 72:4233-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindstedt, M., K. Lundberg, and C. A. Borrebaeck. 2005. Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J. Immunol. 175:4839-4846. [DOI] [PubMed] [Google Scholar]
- 22.Newbold, C., P. Warn, G. Black, A. Berendt, A. Craig, B. Snow, M. Msobo, N. Peshu, and K. Marsh. 1997. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 57:389-398. [DOI] [PubMed] [Google Scholar]
- 23.Nyakeriga, A. M., M. Troye-Blomberg, A. K. Chemtai, K. Marsh, and T. N. Williams. 2004. Malaria and nutritional status in children living on the coast of Kenya. Am. J. Clin. Nutr. 80:1604-1610. [DOI] [PubMed] [Google Scholar]
- 24.Ocana-Morgner, C., M. M. Mota, and A. Rodriguez. 2003. Malaria blood stage suppression of liver stage immunity by dendritic cells. J. Exp. Med. 197:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry, J. A., A. Rush, R. J. Wilson, C. S. Olver, and A. C. Avery. 2004. Dendritic cells from malaria-infected mice are fully functional APC. J. Immunol. 172:475-482. [DOI] [PubMed] [Google Scholar]
- 26.Pichyangkul, S., K. Yongvanitchit, U. Kum-arb, H. Hemmi, S. Akira, A. M. Krieg, D. G. Heppner, V. A. Stewart, H. Hasegawa, S. Looareesuwan, G. D. Shanks, and R. S. Miller. 2004. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J. Immunol. 172:4926-4933. [DOI] [PubMed] [Google Scholar]
- 27.Pouniotis, D. S., O. Proudfoot, V. Bogdanoska, V. Apostolopoulos, T. Fifis, and M. Plebanski. 2004. Dendritic cells induce immunity and long-lasting protection against blood-stage malaria despite an in vitro parasite-induced maturation defect. Infect. Immun. 72:5331-5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogerson, S. J., R. Tembenu, C. Dobano, S. Plitt, T. E. Taylor, and M. E. Molyneux. 1999. Cytoadherence characteristics of Plasmodium falciparum-infected erythrocytes from Malawian children with severe and uncomplicated malaria. Am. J. Trop. Med. Hyg. 61:467-472. [DOI] [PubMed] [Google Scholar]
- 29.Seixas, E., C. Cross, S. Quin, and J. Langhorne. 2001. Direct activation of dendritic cells by the malaria parasite, Plasmodium chabaudi chabaudi. Eur. J. Immunol. 31:2970-2978. [DOI] [PubMed] [Google Scholar]
- 30.Traore, B., K. Muanza, S. Looareesuwan, S. Supavej, S. Khusmith, M. Danis, P. Viriyavejakul, and F. Gay. 2000. Cytoadherence characteristics of Plasmodium falciparum isolates in Thailand using an in vitro human lung endothelial cells model. Am. J. Trop. Med. Hyg. 62:38-44. [DOI] [PubMed] [Google Scholar]
- 31.Turner, G. D., H. Morrison, M. Jones, T. M. Davis, S. Looareesuwan, I. D. Buley, K. C. Gatter, C. I. Newbold, S. Pukritayakamee, B. Nagachinta, et al. 1994. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am. J. Pathol. 145:1057-1069. [PMC free article] [PubMed] [Google Scholar]
- 32.Urban, B. C., D. J. Ferguson, A. Pain, N. Willcox, M. Plebanski, J. M. Austyn, and D. J. Roberts. 1999. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400:73-77. [DOI] [PubMed] [Google Scholar]
- 33.Urban, B. C., T. Mwangi, A. Ross, S. Kinyanjui, M. Mosobo, O. Kai, B. Lowe, K. Marsh, and D. J. Roberts. 2001. Peripheral blood dendritic cells in children with acute Plasmodium falciparum malaria. Blood 98:2859-2861. [DOI] [PubMed] [Google Scholar]
- 34.Velten, F. W., K. Duperrier, J. Bohlender, P. Metharom, and S. Goerdt. 2004. A gene signature of inhibitory MHC receptors identifies a BDCA3+ subset of IL-10-induced dendritic cells with reduced allostimulatory capacity in vitro. Eur. J. Immunol. 34:2800-2811. [DOI] [PubMed] [Google Scholar]