Abstract
Campylobacter jejuni is a leading cause of human enterocolitis and is associated with postinfectious complications, including irritable bowel syndrome and Guillain-Barré syndrome. However, the pathogenesis of C. jejuni infection remains poorly understood. Paracellular pathways in intestinal epithelial cells are gated by intercellular junctions (tight junctions and adherens junctions), providing a functional barrier between luminal microbes and host immune cells in the lamina propria. Here we describe alterations in tight junctions in intestinal epithelial monolayers following C. jejuni infection. Apical infection of polarized T84 monolayers caused a time-dependent decrease in transepithelial electrical resistance (TER). Immunofluorescence microscopy revealed a redistribution of the tight junctional transmembrane protein occludin from an intercellular to an intracellular location. Subcellular fractionation using equilibrium sucrose density gradients demonstrated decreased hyperphosphorylated occludin in lipid rafts, Triton X-100-soluble fractions, and the Triton X-100-insoluble pellet following apical infection. Apical infection with C. jejuni also caused rapid activation of NF-κB and AP-1, phosphorylation of extracellular signal-regulated kinase, Jun N-terminal protein kinase, and p38 mitogen-activated protein kinases, and basolateral secretion of the CXC chemokine interleukin-8 (IL-8). Basolateral infection with C. jejuni caused a more rapid decrease in TER, comparable redistribution of tight-junction proteins, and secretion of more IL-8 than that seen with apical infection. These results suggest that compromised barrier function and increased chemokine expression contribute to the pathogenesis of C. jejuni-induced enterocolitis.
Campylobacter jejuni is a leading cause of human enterocolitis, and after a 2- to 5-day incubation period, it typically causes diarrhea, cramping, abdominal pain, and fever with a duration of up to a week (14, 18). Patients with C. jejuni enterocolitis can also develop postinfectious, sometimes life-threatening, complications, including irritable bowel syndrome, Guillain-Barré syndrome, and immunoproliferative small intestinal disease (37, 41, 65). While significant advances have been made in understanding C. jejuni pathogenesis, including the complete genome sequence of type strain NCTC 11168 and more recently of strains RM1221 and 81-176 (6, 19, 20, 27, 51), the mechanism by which C. jejuni causes enterocolitis has not been fully elucidated.
C. jejuni strain 81-176 has been shown to causes enterocolitis when experimentally inoculated in human volunteers (9). This strain harbors a pVir plasmid, which is absent in strains NCTC 11168 and RM1221, encoding a type IV secretion system (TFSS) that has been shown to be involved in microbial invasion in intestinal epithelial cells (6). Using polarized T84 colonocyte monolayers, Monteville and Konkel showed that strain 81-176 preferentially invades via the basolateral surface and that invasion is facilitated by the binding of the bacteria to the cell matrix protein fibronectin (46). In addition, they showed that C. jejuni could traverse intestinal epithelia via the paracellular route (46).
Upon C. jejuni infection, increased expression of proinflammatory cytokines that are dependent on nuclear factor κB (NF-κB) and mitogen-activated protein (MAP) kinase signaling pathways has been detected in cultured epithelial cells, monocytes, and dendritic cells (24, 25, 29, 30, 33, 34, 39, 44, 57, 62). Signaling pathways activated in C. jejuni-infected cells share features with host innate immune responses mediated by members of the Toll-like receptor (TLR) and nucleotide-binding oligomerization domain (NOD) family of proteins that recognize conserved microbial components (for a review, see reference 3). The mechanism by which C. jejuni infection triggers innate immune responses in epithelial cells remains poorly understood. C. jejuni lipooligosaccharide and flagellin do not appear to be potent TLR ligands, in contrast to lipopolysaccharide and flagellin from Escherichia coli and Salmonella enterica serovar Typhimurium, respectively (5, 22, 33, 57, 62).
Unlike the robust proinflammatory responses produced in response to bacterial challenge by macrophages, intestinal epithelial cells are relatively insensitive to stimulation by microbes (for a review, see reference 1). As a result, intestinal epithelial cells are well suited to function as a physical barrier between luminal microbes and immune cell populations in the lamina propria and submucosa (17). This physical barrier is maintained by connections between adjoining epithelial cells consisting of specialized intercellular structures: tight junctions (TJ) and adherens junctions (AJ). TJ are composed of transmembrane proteins claudins, occludin, and junctional adhesion molecules (for a review, see reference 55). Connecting TJ transmembrane proteins with various regulatory molecules and the underlying actin cytoskeleton are scaffolding proteins containing PDZ domains, i.e., zonula occludens. AJ are composed of the intercellular transmembrane protein E-cadherin which, via its cytosolic domain, is linked to β-catenin. The apical actin cytoskeleton provides mechanical support to the junctional complexes and plays a regulatory role in TJ and AJ remodeling (12). Lipid rafts are submembrane domains enriched with cholesterol and sphingolipid and preferentially partitioned in the apical membrane of polarized epithelia (for a review, see reference 38). The loss of TJ barrier function has been correlated with translocation of lipid raft-associated TJ proteins, suggesting that this membrane microdomain is an integral part of TJ structure (48).
We have previously shown that C. jejuni induces severe gastritis and proximal duodenitis in immunocompromised mice exhibiting defective NF-κB expression (21). Histopathological changes in C. jejuni-infected mice are characterized by mucosal infiltration of inflammatory cells, glandular atrophy, and epithelial hyperplasia and dysplasia (21). As is often observed in enteric infection with pathogenic bacteria, host inflammatory responses induced by C. jejuni are probably due, at least in part, to a “leaky” TJ barrier allowing the influx of bacteria or microbial components into the lamina propria, activating submucosal immune cells, resulting in intestinal inflammation (17, 61). Indeed, MacCallum et al. (40) showed that C. jejuni infection leads to occludin displacement from TJ and a decrease in the transepithelial electrical resistance (TER), a measurement of TJ barrier function, in polarized intestinal Caco-2 cells. The aim of this study was to characterize the underlining TJ structural changes in model T84 colonocytes infected with C. jejuni. Polarized T84 colonocytes are widely used in studying the effects of enteropathogens on TJ functions (56) and have been used as an in vitro model to analyze the invasive properties of C. jejuni from both the apical and basolateral portions of the cell (46). Our data indicated that either apical or basolateral infection with C. jejuni in T84 cells resulted in a time-dependent loss of TER and a marked decrease in the level of hyperphosphorylated occludin. Claudin-1 was found to accumulate in lipid rafts; while the level of lipid raft-associated junctional adhesion molecule 1 (JAM-1) was decreased in infected cells. NF-κB and MAP kinases were activated shortly after C. jejuni infection, leading to increased secretion of the proinflammatory chemokine interleukin-8 (IL-8).
MATERIALS AND METHODS
Cell culture, bacterial infection, and TER measurement.
A total of 4 × 105 T84 cells (ATCC CCL-248) were seeded on collagen-coated permeable supports (6.5-mm-diameter, 0.4-μm-pore-size Transwell inserts; Corning Life Sciences, Acton, Mass.). Cells were grown in a 1:1 (volume) mixture of Dulbecco's minimal essential medium (DMEM) and F-12 medium (DMEM/F-12 medium) supplemented with 15 mM HEPES (pH 7.5), 6% fetal calf serum (Invitrogen, Carlsbad, Calif.), 100 U/ml penicillin, and 100 μg/ml streptomycin. C. jejuni strains 81-176 (9) and NCTC 11168 (51) were grown at 37°C on tryptic soy agar containing 5% defibrinated sheep red blood cells (Quad Five, Ryegate, Mont.) for 48 h in a GasPak jar filled with 10% H2, 10% CO2, and 80% N2. After 2 weeks of growth to allow development of polarized monolayers, T84 cells were incubated in serum-free and antibiotic-free DMEM/F-12 medium the day before being infected. C. jejuni was harvested from plates, resuspended in DMEM/F-12 medium, and added to either the apical or basolateral side of polarized T84 monolayers at an multiplicity of infection (MOI) of 10. Prior to infection and 24 and 48 h after infection, TER was measured using a voltohmmeter (Millicell-ERS; Millipore Corporation, Andover, Mass.).
Immunofluorescence microscopy.
After TER was measured, permeable filter supports were excised, rinsed with ice-cold phosphate-buffered saline, and immediately fixed in 4% paraformaldehyde for 10 min at room temperature. Cells were permeabilized with 0.5% Triton X-100 for 10 min at room temperature, incubated with 3% bovine serum albumin for 2 h to block nonspecific antibody binding, and then incubated overnight at 4°C with primary antibodies. Primary antibodies against occludin (Invitrogen, Carlsbad, Cal.) and zonula occludens 1 (ZO-1) (BD Transduction Laboratories, Lexington, Ky.) were used at 1:50 dilution and were detected using secondary anti-rabbit or anti-mouse antibodies conjugated to Alexa 555 or Alexa 488, respectively (Invitrogen—Molecular Probes, Inc., Eugene, Oreg.). Immunofluorescence was visualized with a laser-scanning confocal imaging system (Carl Zeiss Microimaging, Thornwood, New York).
Sucrose density gradient centrifugation.
T84 cells grown on 75-mm-diameter filter supports were lysed in MBST buffer (25 mM morpholineethanesulfonic acid [MES] [pH 6.5], 150 mM NaCl, and 1% Triton X-100 plus protease inhibitors [1 μg/ml aprotinin, 10 μg/ml leupeptin, 1 μg/ml pepstatin, and 10 μM phenylmethylsulfonyl fluoride]) at 4°C for 30 min. Cells were homogenized with a Dounce homogenizer using a tight-fitting pestle. Two milliliters of cell lysate was mixed with an equal volume of 80% sucrose in MBS buffer (25 mM MES [pH 6.5] and 150 mM NaCl) and placed in the bottom of a centrifuge tube. This was overlaid with 4 ml of MBS buffer containing first 30% sucrose and then 5% sucrose as previously described (48). Samples were centrifuged in a SW41 rotor at 275,000 × g for 20 h. One-millimeter fractions were collected from the top of the gradient (total of 12 fractions per sample). The pellet was resuspended in 1 ml of MBS buffer containing 1% sodium dodecyl sulfate (SDS), boiled for 5 min, and centrifuged at 16,000 × g for 30 min. The resulting supernatant, representing the protein extract from the pellet, was saved for subsequent analysis. Protein concentration was determined using a Dc protein assay kit (Bio-Rad Laboratories, Hercules, Calif.), and equal protein concentrations were trichloroacetic acid (final concentration of 10%) precipitated prior to being separated by SDS-polyacrylamide gel electrophoresis for Western blot analysis. Blots (polyvinylidene difluoride membrane, Immobilon-P; Millipore) were incubated with antibodies against occludin, claudin-1, JAM-1 (Invitrogen), ZO-1, E-cadherin, β-catenin, β1 integrin (BD Transduction Laboratories), or caveolin-1 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) overnight at 4°C. These were detected using species-specific secondary antibodies coupled to horseradish peroxidase (Santa Cruz Biotechnology), a chemiluminescence detection kit (Renaissance enhanced luminal reagent; Perkin-Elmer Life Science, Boston, Mass.), and Kodak BioMax MR film (Eastman Kodak, Rochester, N.Y.).
Cytokine assays.
Media from the basal aspect of polarized T84 monolayers were collected 24 h after infection and centrifuged at 16,000 × g for 5 min to remove bacteria and cell debris. The concentration of IL-8 was determined by an enzyme-linked immunosorbent assay following the manufacturer's recommendations (R & D Systems, Minneapolis, Minnesota). For a positive control, media from polarized T84 monolayers were collected 24 h after basolateral exposure to 100 ng/ml Salmonella enterica serovar Typhimurium flagellin (InvivoGen, San Diego, Calif.). Statistical significance was determined using paired Student's t test.
Electromobility shift assay.
Control and infected T84 monolayers grown on 24-mm-diameter inserts were washed three times with ice-cold phosphate-buffered saline and scraped into TNE buffer (40 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 1 mM EDTA) as described previously (35). Cells were collected by centrifugation at 2,300 × g for 30 seconds in a microcentrifuge. Cell pellets were resuspended in HKE buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, and protease inhibitors). Cell suspensions were incubated on ice for 10 min, and Nonidet P-40 was added to a final concentration of 0.5% (vol/vol) followed by an additional 2-min incubation on ice. After centrifugation at 2,300 × g for 20 seconds, supernatant (cytosol) was removed and frozen immediately on dry ice, while the pellet was resuspended in high-salt extraction buffer (20 mM HEPES [pH 7.9], 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, and protease inhibitors) and incubated on ice for 30 min. Nuclear extracts were obtained by centrifugation at 16,000 × g for 2 min and were frozen immediately on dry ice. Double-stranded oligonucleotides containing consensus sequences for NF-κB and AP-1 (Promega Corp., Madison, Wis.) were end labeled using T4 polynucleotide kinase (New England Biolabs, Inc., Ipswich, Mass.) in the presence of [γ-32P]ATP (Perkin-Elmer Life Science). End-labeled probes were purified with Quick spin G-25 columns (Roche Applied Science, Indianapolis, Ind.). The binding reaction was initiated by adding the labeled probe to nuclear extracts (15 μg) in 20 μl of binding buffer (20 mM HEPES [pH 7.5], 50 mM KCl, 2.5 mM MgCl2, 1 mM dithiothreitol, 0.2 μg/ml bovine serum albumin, and 10% glycerol) which was incubated at room temperature for 30 min. Protein-DNA complexes were separated from free probe on 5% native polyacrylamide gels, which were then dried and exposed to Kodak Biomax MR film at −80°C.
RESULTS
C. jejuni infection reduces transepithelial electrical resistance.
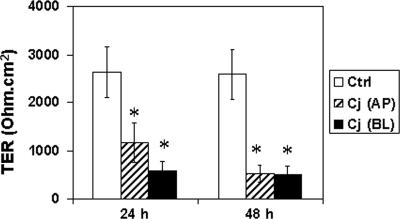
To examine the effect of C. jejuni on TJ barrier function, we measured the TER of polarized T84 monolayers after infection. Preliminary studies showed that TER decreased to 77% ± 14% and 50% ± 9% of the control value 24 h after T84 cells were infected with C. jejuni 81-176 at MOIs of 1 and 10, respectively. We also investigated whether the decrease in TER was strain specific by comparing strain 81-176 to NCTC 11168. The loss of TER in T84 cells infected with NCTC 11168 at an MOI of 10 was 88% ± 7% and 64% ± 9% (n = 6) of the control value 24 and 48 h postinfection, respectively. This effect on TER was significantly less than that caused by strain 81-176 (see below). Therefore, C. jejuni infection with strain 81-176 at an MOI of 10 was used in subsequent studies.
Monolayers infected apically with C. jejuni 81-176 at an MOI of 10 exhibited a decrease in TER by 24 h (1,179 ± 462 Ω · cm2) compared to untreated control monolayers (2,630 ± 731 Ω · cm2; Fig. 1). This significant (P < 0.05) compromise in barrier function is consistent with what has been reported for polarized Caco-2 monolayers (11, 40). Because translocation of C. jejuni to the basolateral aspect of polarized monolayers is known to occur (11, 46), we tested the effect of basolateral infection on epithelial integrity. TER decreased to 577 ± 197 Ω · cm2 by 24 h after infection, less than the TER of untreated control monolayers (P < 0.05) and less than the TER of apically infected monolayers (P < 0.05; Fig. 1). TER continued to decrease in apically infected monolayers over the next 24 h, and by 48 h after infection, there was no difference in TER between monolayers that had been infected apically or basolaterally.
FIG. 1.
Reduced transepithelial electrical resistance in T84 cells infected with C. jejuni. Confluent T84 cells grown on permeable supports were infected with C. jejuni (Cj) at an MOI of 10 from either the apical (AP) or basolateral (BL) aspect. TER (ohm · cm2) was determined at 0, 24, and 48 h postinfection. Data (means ± standard deviations [error bars]; n = 12 for each group) were analyzed by paired Student t test, and an asterisk indicates significant differences between control (Ctrl) and infected cell samples at P < 0.05. In addition, there was significant difference (P < 0.05) between apical versus basolateral infection at 24 h.
Characterization of lipid rafts isolated by sucrose density gradients.
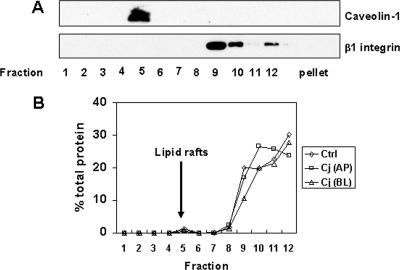
MacCallum et al. reported visual evidence for redistribution of occludin associated with a decrease in TER (40). In order to further characterize changes in TJ proteins in response to C. jejuni infection, we performed subcellular fractionation using cold detergent extraction and isopycnic sucrose gradient centrifugation. Nusrat et al. have used this method to demonstrate that TJ proteins reside in cholesterol-enriched detergent-insoluble glycolipid rafts (48). Initial isopycnic centrifugation with whole-cell lysates of uninfected T84 cells demonstrated that Triton X-100-insoluble lipid rafts appeared as a light-scattering band in fraction 5. Western blot analysis of the fractions revealed that caveolin-1 was almost exclusively localized in fraction 5 (Fig. 2A), confirming the presence of the lipid rafts in this fraction. Western blot analysis also revealed that the basolateral membrane protein β1 integrin was restricted to the high-density fractions 9 to 12 at the bottom of the gradient (Fig. 2A). C. jejuni infection did not affect the distribution of caveolin-1 or β1 integrin in the sucrose density fractions (data not shown). In addition, there was no difference in protein concentration in each fraction between infected and uninfected control monolayers (Fig. 2B).
FIG. 2.
Characterization of lipid rafts isolated by sucrose density gradient centrifugation. Lipid rafts were isolated by Triton X-100 detergent extraction and sucrose gradient centrifugation as described in Materials and Methods. Twelve 1-ml aliquots were collected from sucrose gradients, and proteins associated with the pellet were extracted with 1% SDS. (A) Equal amounts of protein from each fraction were analyzed by immunoblotting, probing with antibodies against caveolin-1 or β1 integrin, markers for lipid rafts and the basolateral membrane, respectively. Caveolin-1 was localized in fraction 5, which coincided with the light-scattering band visualized in the same fraction. The immunoblot shown is representative of the immunoblots for three experiments. (B) Protein concentration was determined by Dc protein assay and expressed as a percentage of total protein. The averages of three experiments collected from untreated control (Ctrl) cells and cells infected with C. jejuni (Cj) from either the apical (AP) or basolateral (BL) aspect are shown.
Reduction in hyperphosphorylated occludin caused by C. jejuni infection.
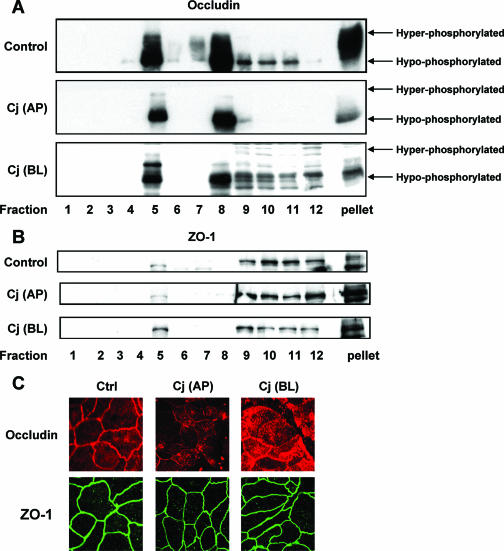
We next examined the distribution of occludin in sucrose density gradient fractions from monolayers 24 h after apical or basolateral infection with C. jejuni as well as from uninfected control monolayers. Both hyperphosphorylated and hypophosphorylated forms of occludin were found in fractions 5 and 8 and in the pellet at the bottom of the gradient (Fig. 3A). Fraction 5 and the pellet represent raft-associated and cytoskeletal occludin, respectively. The Triton X-100-soluble occludin in fraction 8 is likely to come from other subcellular compartments, including the cytosol and apical membrane. Twenty-four hours after apical infection with C. jejuni, there was almost a complete loss of hyperphosphorylated occludin from these fractions, with little change in hypophosphorylated occludin (Fig. 3A). Basolateral infection resulted in substantial but less complete loss of hyperphosphorylated occludin from these fractions (Fig. 3A). Laser-scanning confocal microscopy of uninfected control monolayers revealed a chicken wire pattern of staining for occludin in en face images consistent with its localization in TJ (Fig. 3C). Similar to what MacCallum et al. reported for apical infection of Caco-2 monolayers (40), both apical and basolateral infection of polarized T84 monolayers resulted in focal redistribution of occludin from the lateral membrane to an intracellular location (Fig. 3C).
FIG. 3.
Effects of C. jejuni on the TJ proteins occludin and ZO-1. T84 cells were infected with C. jejuni (Cj) at an MOI of 10 from either the apical (AP) or basolateral (BL) aspect for 24 h. Cell lysates were subjected to sucrose density gradient centrifugation and analyzed by immunoblotting, probing with antibodies against occludin (A) or ZO-1 (B). Occludin migrated as hyper- and hypophosphorylated forms on SDS-polyacrylamide gels. The blots shown are representative of the blots for three separate studies. (C) Control cells and T84 cells infected with C. jejuni for 24 h were fixed by paraformaldehyde immediately after infection. Cells were incubated with antibodies against occludin or ZO-1 followed by species-specific secondary antibodies coupled to Alexa 555 (red, occludin) and Alexa 488 (green, ZO-1), respectively. The micrographs shown are representative of the micrographs for three experiments.
ZO-1 is a peripheral membrane protein of tight junctions that binds to actin filaments and, as expected, was largely found in the pellet at the bottom of the gradient (Fig. 3B). A small amount of ZO-1 was associated with lipid rafts, while the balance of ZO-1 was found in Triton X-100-soluble fractions 9 to 12. There was no change in the distribution of ZO-1 in T84 cell monolayers after infection with C. jejuni compared to uninfected control monolayers, except for a slight increase in the amount of ZO-1 in rafts 24 h after basolateral infection (Fig. 3B). Laser-scanning confocal microscopy for ZO-1 confirmed this finding and revealed the same chicken wire pattern of staining in uninfected control and infected monolayers (Fig. 3C). In contrast to the total decrease in and redistribution of hyperphosphorylated occludin from TJ to cytosol, the decreased TER caused by C. jejuni infection was not associated with a change in the amount or the distribution of ZO-1 in polarized T84 monolayers.
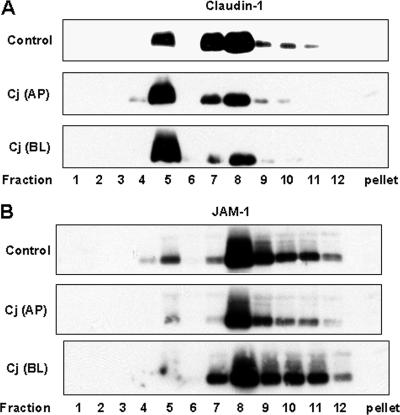
Redistribution of lipid raft-associated claudin-1 and JAM-1 caused by C. jejuni infection.
Claudin-1 and JAM-1 are transmembrane TJ proteins that, along with occludin, mediate cell adhesion and contribute to intramembrane and paracellular diffusion barriers. In uninfected control T84 monolayers, the majority of claudin-1 was found in lipid rafts and in sucrose gradient fractions 7 and 8 (Fig. 4A). The majority of JAM-1 was found in fractions 8 to 11, with a smaller amount in lipid rafts (Fig. 4B). In contrast to occludin, claudin-1 and JAM-1 were not detected in the cytoskeleton-associated pellet fraction. Twenty-four hours after apical or basolateral infection with C. jejuni, polarized T84 monolayers exhibited a concomitant increase in raft-associated claudin-1 and decrease in claudin-1 in fractions 7 and 8 (Fig. 4A). Infection also resulted in a decreased level of raft-associated JAM-1, without any obvious change in fractions 8 to 11 (Fig. 4B). In comparison, C. jejuni infection, from either the apical or basolateral aspect, did not alter the subcellular distributions of E-cadherin and β-catenin as analyzed by confocal microscopy and sucrose density gradient centrifugation (data not shown).
FIG. 4.
Equilibrium sucrose density gradient analysis of the TJ proteins claudin-1 and JAM-1. Cell lysates were prepared from control cells and T84 cells infected with C. jejuni (Cj) from either the apical (AP) or basolateral (BL) aspect for 24 h. Cell lysates were subjected to sucrose density gradient centrifugation. Equal amounts of protein from each fraction were analyzed by immunoblotting for either claudin-1 (A) or JAM-1 (B). The immunoblots presented are representative of the immunoblots for three experiments.
Induction of IL-8 in T84 cells infected with C. jejuni.
The chemokine IL-8 and other proinflammatory cytokines are believed to contribute to the development of mucosal inflammation caused by C. jejuni infection. Infection of cultured epithelial cell lines with C. jejuni has been shown to elicit IL-8 secretion (7, 24, 26, 33, 62). However, secretion of IL-8 by polarized intestinal epithelial cell monolayers in response to C. jejuni infection has not been previously reported. Therefore, we investigated the ability of apical or basolateral infection of polarized T84 monolayers with C. jejuni to activate signaling pathways leading to basolateral secretion of IL-8 24 h after infection.
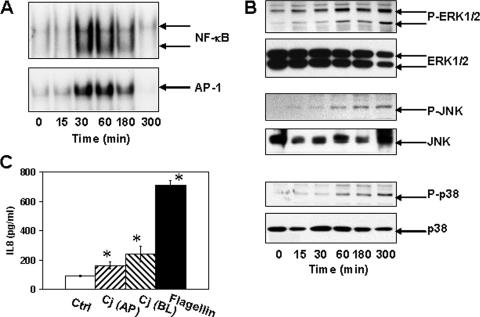
We first investigated the ability of C. jejuni infection to activate NF-κB and AP-1, two nuclear transcription factors that are important for expression of proinflammatory cytokine genes. Polarized T84 cell monolayers were infected apically with C. jejuni at an MOI of 10, and nuclear extracts were prepared 15, 30, 60, 180, or 300 min after infection. NF-κB and AP-1 DNA-binding activities were induced by 30 min after C. jejuni infection and were sustained through 180 min after infection (Fig. 5A). By 300 min after infection, both NF-κB and AP-1 activities returned to baseline. This duration of NF-κB activation is somewhat shorter than that reported for HEp-2 cells (32) but is comparable to that reported for conventional, nonpolarized T84 cells (33). We next investigated the ability of C. jejuni infection to activate MAP kinases in polarized T84 monolayers. We found that extracellular signal-regulated kinase (ERK), Jun N-terminal protein kinase (JNK), and p38 displayed time-dependent activation, as judged by phosphorylation, in infected T84 monolayers (Fig. 5B). MAP kinase activation was detected by 60 min after infection and persisted throughout the experimental period (5 h). The kinetics of ERK, JNK, and p38 activation in infected polarized T84 monolayers was slightly delayed with respect to NF-κB and AP-1 activation, but it occurred more rapidly than reported for polarized Caco-2 monolayers (39) and in a more robust and sustained manner than that reported for p38 and ERK in conventional, nonpolarized T84 monolayers, respectively (62).
FIG. 5.
Proinflammatory responses induced by C. jejuni infection. T84 cells were infected with C. jejuni at an MOI of 10 for the times indicated. (A) The binding of NF-κB and AP-1 to oligonucleotides containing respective consensus sequences was assayed by electromobility shift assay. The results shown are representative of the results for three experiments. (B) The cytosolic fraction prepared from control and C. jejuni-infected cells was analyzed for Erk1/2, JNK, and p38 phosphorylation. Immunoblots were stripped and reprobed for total Erk1/2, JNK, and p38. The immunoblots shown are representative of the immunoblots for three experiments. P-ERK1/2, phosphorylated ERK1/2. (C) T84 cells were infected with C. jejuni (Cj) at an MOI of 10 for 24 h from either the apical (AP) or basolateral aspect (BL) (n = 9). In parallel cultures, cells were exposed to 100 ng/ml of S. enterica serovar Typhimurium flagellin from the basolateral aspect for 24 h (n = 6). Media in the lower chamber were assayed for IL-8 by enzyme-linked immunosorbent assays. The mean ± standard deviation (error bar) values for IL-8 secretion are shown. An asterisk indicates significant difference (P < 0.05) between control (Ctrl) and infected cell samples. In addition, there was a significant difference (P < 0.05) in IL-8 secretion between apical and basolateral C. jejuni infection.
In addition to characterizing NF-κB, AP-1, and MAP kinase activation, we measured IL-8 secretion by polarized T84 monolayers 24 h after apical or basolateral infection with C. jejuni. IL-8 secretion was significantly greater in infected monolayers than in uninfected control monolayers, and basolateral infection induced more IL-8 secretion than infection from the apical aspect (Fig. 5C). After 24 h, neither apical nor basolateral infection with C. jejuni led to the secretion of as much IL-8 as basolateral treatment with 100 ng/ml Salmonella enterica serovar Typhimurium flagellin. These results are consistent with what has been previously reported for conventional, nonpolarized epithelial cell lines (7, 24, 26, 44, 62).
DISCUSSION
The paracellular pathway in intestinal epithelia is gated by intercellular junctions. A crucial role of these junctions is to prevent commensal and pathogenic microbes from entering the lamina propria. Despite these host defenses, pathogenic microbes have developed sophisticated strategies to traverse the epithelial barrier as a means to access an advantageous niche. This can involve the exploitation of host signaling pathways by a microbial virulence factor(s) leading to a redistribution of TJ structural proteins and compromise in barrier function. In this study, we observed a significant decrease in TER correlated with occludin redistribution and dephosphorylation in C. jejuni-infected T84 cells. The levels of lipid raft-associated claudin-1 and JAM-1 were increased and decreased, respectively; while the TJ plaque protein ZO-1 and AJ proteins E-cadherin and β-catenin were minimally affected. Our data confirm and extend previous finding in Caco-2 cells by MacCallum et al. (40) that the loss of occludin constitutes the most significant alteration in TJ transmembrane proteins.
Our findings for C. jejuni-infected T84 cells are similar to what have been reported for S. enterica serovar Typhimurium or enteropathogenic Escherichia coli (EPEC) (8, 43, 53). S. enterica serovar Typhimurium induces marked decreases in TER with concomitant selective loss of occludin, but not ZO-1, from the TJ platform (8). The increases in TJ permeability and structural alterations could be due to the contraction of the perijunctional actinomyosin ring in S. enterica serovar Typhimurium-infected cells (8, 31). EPEC-induced changes in TJ barrier function are associated with the redistribution of occludin and concomitant contraction of the perijunctional actinomyosin ring (43), which is mediated by increased myosin light-chain kinase activity that, in turn, leads to increased phosphorylation of myosin II regulatory light chain (54, 68). Our preliminary data indicated the absence of gross morphological alterations in the perijunctional actinomyosin ring in T84 cells infected with C. jejuni (data not shown). Occludin is a 60-kDa protein with multiple phosphoserine and phosphotyrosine sites. The status of occludin phosphorylation, which changes during TJ remodeling (53, 59), is mediated by protein kinase C and tyrosine kinases, while its dephosphorylation is mediated by TJ-associated protein phosphatase 2A (47, 59, 63). Perturbations in host cell signaling pathways (e.g., protein kinase C, tyrosine kinases, and/or phosphatase) most likely account for occludin redistribution and dephosphorylation in C. jejuni-infected cells.
Lipid rafts are the preferred entry sites for several invasive pathogens (Salmonella, Shigella, Listeria, and Chlamydia) (for a review, see reference 42). It is likely that C. jejuni also utilizes lipid rafts as the entry point (64). Recent data indicated that lipid rafts are required for inducing pedestal formation in host cells by attaching-effacing E. coli (52). This membrane microdomain is also an integral part of the TJ spatial organization (28, 48, 49), and loss of raft-associated JAM-1 has been reported in T84 cells exposed to gamma interferon from the basolateral aspect (13, 28, 49). In addition to increased IL-8 secretion observed in this and other studies (39, 44), elevated expression of gamma interferon-inducible protein 10, monocyte chemoattractant protein-1, growth-related oncogenes α and β, macrophage inflammatory proteins 1 and 3α have been reported in C. jejuni-infected epithelial cells (30, 33). This raises the possibility that decreases in raft-associated JAM-1 (Fig. 4) are due to an autocrine effect of increased proinflammatory cytokines in the basolateral aspect of T84 cells infected with C. jejuni. It is not immediately clear whether increased raft-associated claudin-1 observed in this study has any direct role in TJ barrier function.
Consistent with prior reports using cultured epithelial cell lines (30, 33, 39, 44, 62), we found that proinflammatory responses in C. jejuni-infected polarized T84 cells consisted of activated nuclear factors NF-κB and AP-1, increased MAP kinase phosphorylation, and elevated IL-8 secretion (Fig. 5). The host signaling pathway leading to increased proinflammatory cytokine expression induced by C. jejuni infection shares features with that of TLR activation by conserved microbial components (3). Intestinal epithelial cells, unlike cells of myeloid lineage, are relatively unresponsive to stimulation by TLR2 and TLR4 ligands, despite the presence of these receptors for microbial products (2, 15, 45). This muted response by intestinal epithelial cells is partially due to the down-regulation of TLR signaling components and/or up-regulation of TLR negative regulators (45, 50). In contrast, TLR5 is enriched in the basolateral surface of intestinal epithelial cells and is the primary microbial sensor against EPEC, enteroaggregative E. coli, and S. enterica serovar Typhimurium infection (22, 58, 66, 67). However, flagellin isolated from epsilon Proteobacteria, such as C. jejuni or Helicobacter pylori is not a potent TLR5 agonist (5, 23, 33, 62). H. pylori does activate the intracellular pattern recognition receptor NOD-1, which recognizes bacterial cell wall components delivered into gastric epithelial cells by the TFSS encoded by the H. pylori cag pathogenicity island (60).
Comparative genomic analysis reveals that C. jejuni and H. pylori encode a considerable number of orthologs, including TFSS components (19). However, C. jejuni lacks proteins orthologous to other well-characterized H. pylori virulence factors, including CagA. CagA is translocated into host cells via the cag pathogenicity island-encoded TFSS. This microbial toxin induces actin cytoskeletal rearrangement and cell scattering as well as disruption of TJ barrier by binding to ZO-1 and JAM-1 in host epithelial cells (4, 16). Recent data show that H. pylori CagA activates proinflammatory responses via a TLR-independent pathway (10). C. jejuni cytolethal distending toxin, unrelated to H. pylori CagA in amino acid sequence, induces cell cytoskeleton rearrangement and cytokine expression in nonpolarized epithelial cells (36). Therefore, the possible role of TLR-independent signaling activated by microbial virulence factors (e.g., cytolethal distending toxin) as well as the role of NOD proteins in host inflammatory responses to C. jejuni infection will need to be determined in future studies.
Prior studies indicate that C. jejuni transmigrates across intestinal epithelia via either the transcellular or paracellular pathway (46). This prompted us to compare the effects of basolateral C. jejuni infection versus the effects of apical infection on epithelial TJ function and cytokine expression. The changes in TER and IL-8 secretion 24 h postinfection were greater in cells infected from the basolateral aspect than in cells infected from the apical aspect (Fig. 1 and 5). Our data are in agreement with the prior finding (46) that C. jejuni preferentially invades polarized T84 monolayers from the basolateral surface. These data suggest that C. jejuni invasion facilitates the loss of TJ barrier function and increased cytokine expression in infected cells.
Taken together, our data lead us to propose a working model for epithelial cell-C. jejuni interactions. C. jejuni adhere and invade intestinal epithelial cells from the apical aspect. Intracellular C. jejuni then induce opening of the TJ as indicated by the significant loss of TER and occludin redistribution from the TJ platform to the cytosolic compartment. C. jejuni is then transcytosed via a “leaky” paracellular pathway to the basolateral aspect of mucosal epithelium where they continue to interact with host cells. Elevated cytokine secretion by intestinal epithelial cells, in turn, attracts neutrophils, macrophages, and lymphocytes to the site of inflammation. This “double-sided” infection accelerates and intensifies host responses leading to uncontrolled inflammation. Our observations provide new data on the pathogenesis of C. jejuni infection and will allow for more defined analysis of C. jejuni-host epithelial cell interactions.
Acknowledgments
This work was supported by NIH grant DK52413 to D.B.S.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 2 October 2006.
REFERENCES
- 1.Abreu, M. T., M. Fukata, and M. Arditi. 2005. TLR signaling in the gut in health and disease. J. Immunol. 174:4453-4460. [DOI] [PubMed] [Google Scholar]
- 2.Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609-1616. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 4.Amieva, M. R., R. Vogelmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen-Nissen, E., K. D. Smith, K. L. Strobe, S. L. Barrett, B. T. Cookson, S. M. Logan, and A. Aderem. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. USA 102:9247-9252. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacon, D. J., R. A. Alm, L. Hu, T. E. Hickey, C. P. Ewing, R. A. Batchelor, T. J. Trust, and P. Guerry. 2002. DNA sequence and mutational analyses of the pVir plasmid of Campylobacter jejuni 81-176. Infect. Immun. 70:6242-6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakhiet, M., F. S. Al-Salloom, A. Qareiballa, K. Bindayna, I. Farid, and G. A. Botta. 2004. Induction of alpha and beta chemokines by intestinal epithelial cells stimulated with Campylobacter jejuni. J. Infect. 48:236-244. [DOI] [PubMed] [Google Scholar]
- 8.Bertelsen, L. S., G. Paesold, S. L. Marcus, B. B. Finlay, L. Eckmann, and K. E. Barrett. 2004. Modulation of chloride secretory responses and barrier function of intestinal epithelial cells by the Salmonella effector protein SigD. Am. J. Physiol. Cell Physiol. 287:C939-C948. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 9.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 10.Brandt, S., T. Kwok, R. Hartig, W. Konig, and S. Backert. 2005. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 102:9300-9305. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bras, A. M., and J. M. Ketley. 1999. Transcellular translocation of Campylobacter jejuni across human polarised epithelial monolayers. FEMS Microbiol. Lett. 179:209-215. [DOI] [PubMed] [Google Scholar]
- 12.Bruewer, M., A. M. Hopkins, M. E. Hobert, A. Nusrat, and J. L. Madara. 2004. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am. J. Physiol. Cell Physiol. 287:C327-C335. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 13.Bruewer, M., A. Luegering, T. Kucharzik, C. A. Parkos, J. L. Madara, A. M. Hopkins, and A. Nusrat. 2003. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 171:6164-6172. [DOI] [PubMed] [Google Scholar]
- 14.Butzler, J. P. 2004. Campylobacter, from obscurity to celebrity. Clin. Microbiol. Infect. 10:868-876. [DOI] [PubMed] [Google Scholar]
- 15.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H. C. Reinecker, and D. K. Podolsky. 2000. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 16.Churin, Y., L. Al-Ghoul, O. Kepp, T. F. Meyer, W. Birchmeier, and M. Naumann. 2003. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell Biol. 161:249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clayburgh, D. R., L. Shen, and J. R. Turner. 2004. A porous defense: the leaky epithelial barrier in intestinal disease. Lab. Investig. 84:282-291. [DOI] [PubMed] [Google Scholar]
- 18.Crushell, E., S. Harty, F. Sharif, and B. Bourke. 2004. Enteric campylobacter: purging its secrets? Pediatr. Res. 55:3-12. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 19.Eppinger, M., C. Baar, G. Raddatz, D. H. Huson, and S. C. Schuster. 2004. Comparative analysis of four Campylobacterales. Nat. Rev. Microbiol. 2:872-885. [DOI] [PubMed] [Google Scholar]
- 20.Fouts, D. E., E. F. Mongodin, R. E. Mandrell, W. G. Miller, D. A. Rasko, J. Ravel, L. M. Brinkac, R. T. DeBoy, C. T. Parker, S. C. Daugherty, R. J. Dodson, A. S. Durkin, R. Madupu, S. A. Sullivan, J. U. Shetty, M. A. Ayodeji, A. Shvartsbeyn, M. C. Schatz, J. H. Badger, C. M. Fraser, and K. E. Nelson. 2005. Major structural differences and novel potential virulence mechanisms from the genomes of multiple campylobacter species. PLoS Biol. 3:e15. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox, J. G., A. B. Rogers, M. T. Whary, Z. Ge, N. S. Taylor, S. Xu, B. H. Horwitz, and S. E. Erdman. 2004. Gastroenteritis in NF-κB-deficient mice is produced with wild-type Campylobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72:1116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gewirtz, A. T., Y. Yu, U. S. Krishna, D. A. Israel, S. L. Lyons, and R. M. Peek, Jr. 2004. Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. J. Infect. Dis. 189:1914-1920. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 24.Hickey, T. E., S. Baqar, A. L. Bourgeois, C. P. Ewing, and P. Guerry. 1999. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect. Immun. 67:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey, T. E., G. Majam, and P. Guerry. 2005. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect. Immun. 73:5194-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hickey, T. E., A. L. McVeigh, D. A. Scott, R. E. Michielutti, A. Bixby, S. A. Carroll, A. L. Bourgeois, and P. Guerry. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 68:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofreuter, D., J. Tsai, R. O. Watson, V. Novik, B. Altman, M. Benitez, C. Clark, C. Perbost, T. Jarvie, L. Du, and J. E. Galan. 2006. Unique features of a highly pathogenic Campylobacter jejuni strain. Infect. Immun. 74:4694-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopkins, A. M., S. V. Walsh, P. Verkade, P. Boquet, and A. Nusrat. 2003. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J. Cell Sci. 116:725-742. [DOI] [PubMed] [Google Scholar]
- 29.Hu, L., M. D. Bray, M. Osorio, and D. J. Kopecko. 2006. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect. Immun. 74:2697-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu, L., and T. E. Hickey. 2005. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect. Immun. 73:4437-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jepson, M. A., H. B. Schlecht, and C. B. Collares-Buzato. 2000. Localization of dysfunctional tight junctions in Salmonella enterica serovar Typhimurium-infected epithelial layers. Infect. Immun. 68:7202-7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin, S., Y. C. Song, A. Emili, P. M. Sherman, and V. L. Chan. 2003. JlpA of Campylobacter jejuni interacts with surface-exposed heat shock protein 90alpha and triggers signalling pathways leading to the activation of NF-kappaB and p38 MAP kinase in epithelial cells. Cell. Microbiol. 5:165-174. [DOI] [PubMed] [Google Scholar]
- 33.Johanesen, P. A., and M. B. Dwinell. 2006. Flagellin-independent regulation of chemokine host defense in Campylobacter jejuni-infected intestinal epithelium. Infect. Immun. 74:3437-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones, M. A., S. Totemeyer, D. J. Maskell, C. E. Bryant, and P. A. Barrow. 2003. Induction of proinflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni. Infect. Immun. 71:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keates, A. C., S. Keates, J. H. Kwon, K. O. Arseneau, D. J. Law, L. Bai, J. L. Merchant, T. C. Wang, and C. P. Kelly. 2001. ZBP-89, Sp1, and nuclear factor-kappa B regulate epithelial neutrophil-activating peptide-78 gene expression in Caco-2 human colonic epithelial cells. J. Biol. Chem. 276:43713-43722. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 36.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354-357. [DOI] [PubMed] [Google Scholar]
- 37.Lecuit, M., E. Abachin, A. Martin, C. Poyart, P. Pochart, F. Suarez, D. Bengoufa, J. Feuillard, A. Lavergne, J. I. Gordon, P. Berche, L. Guillevin, and O. Lortholary. 2004. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N. Engl. J. Med. 350:239-248. [DOI] [PubMed] [Google Scholar]
- 38.Le Roy, C., and J. L. Wrana. 2005. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 6:112-126. [DOI] [PubMed] [Google Scholar]
- 39.MacCallum, A., G. Haddock, and P. H. Everest. 2005. Campylobacter jejuni activates mitogen-activated protein kinases in Caco-2 cell monolayers and in vitro infected primary human colonic tissue. Microbiology 151:2765-2772. [DOI] [PubMed] [Google Scholar]
- 40.MacCallum, A., S. P. Hardy, and P. H. Everest. 2005. Campylobacter jejuni inhibits the absorptive transport functions of Caco-2 cells and disrupts cellular tight junctions. Microbiology 151:2451-2458. [DOI] [PubMed] [Google Scholar]
- 41.Malinen, E., T. Rinttila, K. Kajander, J. Matto, A. Kassinen, L. Krogius, M. Saarela, R. Korpela, and A. Palva. 2005. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 100:373-382. [DOI] [PubMed] [Google Scholar]
- 42.Manes, S., G. del Real, and A. C. Martinez. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557-568. [DOI] [PubMed] [Google Scholar]
- 43.McNamara, B. P., A. Koutsouris, C. B. O'Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. 2001. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Investig. 107:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mellits, K. H., J. Mullen, M. Wand, G. Armbruster, A. Patel, P. L. Connerton, M. Skelly, and I. F. Connerton. 2002. Activation of the transcription factor NF-kappaB by Campylobacter jejuni. Microbiology 148:2753-2763. [DOI] [PubMed] [Google Scholar]
- 45.Melmed, G., L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Y. Zhou, B. Hu, M. Arditi, and M. T. Abreu. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170:1406-1415. [DOI] [PubMed] [Google Scholar]
- 46.Monteville, M. R., and M. E. Konkel. 2002. Fibronectin-facilitated invasion of T84 eukaryotic cells by Campylobacter jejuni occurs preferentially at the basolateral cell surface. Infect. Immun. 70:6665-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunbhakdi-Craig, V., T. Machleidt, E. Ogris, D. Bellotto, C. L. White III, and E. Sontag. 2002. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 158:967-978. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nusrat, A., C. A. Parkos, P. Verkade, C. S. Foley, T. W. Liang, W. Innis-Whitehouse, K. K. Eastburn, and J. L. Madara. 2000. Tight junctions are membrane microdomains. J. Cell Sci. 113:1771-1781. [DOI] [PubMed] [Google Scholar]
- 49.Nusrat, A., C. von Eichel-Streiber, J. R. Turner, P. Verkade, J. L. Madara, and C. A. Parkos. 2001. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immun. 69:1329-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otte, J. M., E. Cario, and D. K. Podolsky. 2004. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126:1054-1070. [DOI] [PubMed] [Google Scholar]
- 51.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 52.Riff, J. D., J. W. Callahan, and P. M. Sherman. 2005. Cholesterol-enriched membrane microdomains are required for inducing host cell cytoskeleton rearrangements in response to attaching-effacing Escherichia coli. Infect. Immun. 73:7113-7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakakibara, A., M. Furuse, M. Saitou, Y. Ando-Akatsuka, and S. Tsukita. 1997. Possible involvement of phosphorylation of occludin in tight junction formation. J. Cell Biol. 137:1393-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savkovic, S. D., A. Ramaswamy, A. Koutsouris, and G. Hecht. 2001. EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G890-G898. [DOI] [PubMed] [Google Scholar]
- 55.Schneeberger, E. E., and R. D. Lynch. 2004. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286:C1213-C1228. [DOI] [PubMed] [Google Scholar]
- 56.Sears, C. L. 2000. Molecular physiology and pathophysiology of tight junctions V. Assault of the tight junction by enteric pathogens. Am. J. Physiol. Gastrointest. Liver Physiol. 279:G1129-G1134. [DOI] [PubMed] [Google Scholar]
- 57.Siegesmund, A. M., M. E. Konkel, J. D. Klena, and P. F. Mixter. 2004. Campylobacter jejuni infection of differentiated THP-1 macrophages results in interleukin 1 beta release and caspase-1-independent apoptosis. Microbiology 150:561-569. [DOI] [PubMed] [Google Scholar]
- 58.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, J. A. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 105:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsukamoto, T., and S. K. Nigam. 1999. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am. J. Physiol. 276:F737-F750. [DOI] [PubMed] [Google Scholar]
- 60.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 61.Vogelmann, R., M. R. Amieva, S. Falkow, and W. J. Nelson. 2004. Breaking into the epithelial apical-junctional complex—news from pathogen hackers. Curr. Opin. Cell Biol. 16:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watson, R. O., and J. E. Galan. 2005. Signal transduction in Campylobacter jejuni-induced cytokine production. Cell. Microbiol. 7:655-665. [DOI] [PubMed] [Google Scholar]
- 63.Wong, V. 1997. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am. J. Physiol. 273:C1859-C1867. [DOI] [PubMed] [Google Scholar]
- 64.Wooldridge, K. G., P. H. Williams, and J. M. Ketley. 1996. Host signal transduction and endocytosis of Campylobacter jejuni. Microb. Pathog. 21:299-305. [DOI] [PubMed] [Google Scholar]
- 65.Yuki, N., K. Susuki, M. Koga, Y. Nishimoto, M. Odaka, K. Hirata, K. Taguchi, T. Miyatake, K. Furukawa, T. Kobata, and M. Yamada. 2004. Carbohydrate mimicry between human ganglioside GM1 and Campy-lobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc. Natl. Acad. Sci. USA 101:11404-11409. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng, H., A. Q. Carlson, Y. Guo, Y. Yu, L. S. Collier-Hyams, J. L. Madara, A. T. Gewirtz, and A. S. Neish. 2003. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 171:3668-3674. [DOI] [PubMed] [Google Scholar]
- 67.Zhou, X., J. A. Giron, A. G. Torres, J. A. Crawford, E. Negrete, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect. Immun. 71:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zolotarevsky, Y., G. Hecht, A. Koutsouris, D. E. Gonzalez, C. Quan, J. Tom, R. J. Mrsny, and J. R. Turner. 2002. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123:163-172. [DOI] [PubMed] [Google Scholar]