Abstract
The transition metal nickel plays an important role in gastric colonization and persistence of the important human pathogen Helicobacter pylori, as it is the cofactor of the abundantly produced acid resistance factor urease. Nickel uptake through the inner membrane is mediated by the NixA protein, and the expression of NixA is controlled by the NikR regulatory protein. Here we report that NikR also controls the nickel-responsive expression of the FecA3 (HP1400) and FrpB4 (HP1512) outer membrane proteins (OMPs), as well as the nickel-responsive expression of an ExbB-ExbD-TonB system, which may function in energization of outer membrane transport. Transcription and expression of the frpB4 and fecA3 genes were repressed by nickel in wild-type H. pylori 26695, but they were independent of nickel and derepressed in an isogenic nikR mutant. Both the frpB4 and fecA3 genes were transcribed from a promoter directly upstream of their start codon. Regulation by NikR was mediated via nickel-dependent binding to specific operators overlapping either the +1 or −10 sequence in the frpB4 and fecA3 promoters, respectively, and these operators contained sequences resembling the proposed H. pylori NikR recognition sequence (TATWATT-N11-AATWATA). Transcription of the HP1339-1340-1341 operon encoding the ExbB2-ExbD2-TonB2 complex was also regulated by nickel and NikR, but not by Fur and iron. In conclusion, H. pylori NikR controls nickel-responsive expression of the HP1400 (FecA3) and HP1512 (FrpB4) OMPs. We hypothesize that these two NikR-regulated OMPs may participate in the uptake of complexed nickel ions and that this process is energized by the NikR-regulated ExbB2-ExbD2-TonB2 system, another example of the specific adaptation of H. pylori to the gastric lifestyle.
The human gastric pathogen Helicobacter pylori colonizes the mucous layer overlaying the gastric epithelial cells in the human stomach. If not removed by antibiotic treatment, the infection usually remains lifelong and may progress to peptic ulcer disease or the development of adenocarcinoma of the distal stomach (22). In its niche H. pylori is exposed to hostile environmental conditions, caused by acid and changes in nutrient availability. Since about half of the world population is infected with H. pylori (22), the bacterium is clearly well adapted to the hostile conditions occurring in the gastric mucosa.
The nickel-cofactored urease and hydrogenase enzymes are major factors in gastric colonization by H. pylori (14, 26). When cytoplasmic nickel availability is insufficient, the urease and hydrogenase systems cannot be fully activated (37), leading to acid sensitivity and decreased survival and colonization of H. pylori in the gastric mucosa (14, 26). However, the bacterium also needs to prevent toxicity from high intracellular concentrations of nickel (24, 37). The intracellular concentration of nickel is therefore carefully controlled by regulation of nickel uptake and usage. In H. pylori, nickel homeostasis is controlled by the NikR (HP1338) protein, which mediates transcriptional regulation of expression of the NixA nickel uptake system and the urease operon (11, 17, 40, 46).
Nickel uptake in gram-negative bacteria is complicated by the two membrane barriers. Soluble nickel compounds can enter the periplasm via the outer membrane porins and are subsequently transported by the NixA cytoplasmic membrane protein (4, 19, 23, 45). However, it is conceivable that nickel can be complexed to eukaryotic proteins or it may be present in poorly soluble complexes, and thus, these complexes cannot reach the periplasm via the porins. Thus, the situation is similar to iron transport (2), where the soluble ferrous iron is transported by the cytoplasmic membrane FeoB transporter, but insoluble ferric iron complexes require specific, high-affinity iron uptake outer membrane transporters (2). The H. pylori genome contains six genes encoding such outer membrane proteins (OMPs) (1, 36), three annotated as orthologs of the Escherichia coli ferric citrate receptor FecA protein (47) and three annotated as orthologs of the Neisseria meningitidis FrpB protein (27). While the exact functions of the H. pylori FecA and FrpB proteins are currently unknown, analysis of their expression has shown that two out of three copies of both FecA and FrpB orthologs (HP0686, HP0807, HP0876, and HP0916/0915) display iron- and Fur-regulated expression (15, 41), as expected for iron uptake systems (2), whereas expression of the HP1400 (FecA3) and HP1512 (FrpB4) copies was iron and Fur independent (41), suggesting that these proteins may not be involved in iron transport.
Transport of complexes through the outer membrane is an energy-consuming process, and this energy is generated from the proton motive force and transduced to the OMP via the TonB-ExbB-ExbD protein complex (20, 30). H. pylori contains two genes encoding TonB orthologs, of which the tonB2 (HP1341) gene is located in an operon with genes encoding ExbB (exbB2 and HP1339) and ExbD orthologs (exbD2 and HP1340) (1, 9, 11, 36). In transcriptome studies on NikR-responsive gene regulation in H. pylori, it was reported that transcription of frpB4 and fecA3 was altered in a nikR mutant (9), but these array results were not independently confirmed and the molecular mechanism was not further investigated, and thus, it remained possible that regulation of fecA3 and frpB4 was indirect via a regulatory cascade (7, 37, 38). In the present study, it is demonstrated that expression of the FrpB4 and FecA3 proteins is repressed by nickel and that this regulation is mediated at the transcriptional level via binding of NikR to the frpB4 and fecA3 promoter regions.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
H. pylori strain 26695 (36) and its isogenic nikR (40) and fur (41) mutants were routinely cultured on Dent agar (39) at 37°C under microaerophilic conditions (10% CO2, 5% O2, and 85% N2). Broth cultures were grown in brucella broth (Difco, Sparks, MD) supplemented with 0.2% β-cyclodextrin (Fluka) (BBC) and shaken at 37°C with 40 rpm for a maximum of 24 h. NiCl2 (Sigma) was used to supplement BBC medium to achieve various nickel concentrations. Iron restriction was achieved by supplementing brucella broth with desferal (deferoxamine mesylate; Sigma) to a final concentration of 20 μM (41) before adding β-cyclodextrins. Iron-replete conditions were achieved by supplementing desferal-treated BBC with ferric chloride (Sigma) to a final concentration of 100 μM (41). E. coli strains were grown aerobically at 37°C in Luria-Bertani medium (33). When needed, growth media were supplemented with ampicillin, kanamycin, or chloramphenicol to a final concentration of 100 μg/ml, 20 μg/ml, or 20 μg/ml, respectively.
Membrane fractionation and protein analysis.
Approximately 4 × 109 H. pylori cells resuspended in 10 mM Tris-HCl, pH 8.0, were sonicated, and the supernatant was cleared from nondisrupted cells by centrifugation. The membranes present in the supernatant were subsequently pelleted in an ultracentrifuge (Beckman Optima L-080, rotor type 42.2 Ti, 155,000 × g), and subsequently the pellet was resuspended in 40 μl solubilization buffer (10 mM Tris-HCl, pH 7.5, 7 mM EDTA, 0.6% sarcosyl) (42). After a second ultracentrifugation step, the pellet containing the outer membrane fraction was resuspended in 25 μl of 10 mM Tris-HCl, pH 8.0, and was separated by sodium dodecyl sulfate (SDS)-6% polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue. The two nickel-regulated OMPs from wild-type H. pylori 26695 were subsequently cut out from the SDS- polyacrylamide gel after Coomassie blue staining and used for protein identification. The proteins were trypsin digested and analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry as described previously (41).
Purification and analysis of RNA.
Total RNA was isolated from 4 × 109 H. pylori cells using TRIzol (Gibco), according to the manufacturer's instructions. For Northern hybridization experiments, RNA was separated on 2% formaldehyde-1.5% agarose gels in 20 mM sodium phosphate buffer (pH 7), transferred to positively charged nylon membranes (Roche), and covalently bound to the membrane by cross-linking with 0.120 J/cm2 of UV light of 254-nm wavelength (39). Directly after transfer, the membranes were stained with methylene blue to confirm the integrity of the RNA samples and to confirm loading of equal amounts of RNA on the basis of the relative intensities of the 16S and 23S rRNA. The sizes of the hybridizing RNA species were calculated from comparison with a digoxigenin (DIG)-labeled marker (RNA marker I; Roche). The DIG-labeled specific RNA probes were synthesized by in vitro transcription using T7 RNA polymerase (Roche), and PCR products were amplified using the primers listed in Table 1. Detection of RNA was carried out as described previously (15, 17, 39).
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5′→3′) |
|---|---|
| frpB4-F4 | AGCCGTCTCTTAAGGGTAAC |
| frpB4-R-T7a | ctaatacgactcactatagggagaTCGCTATTGCTTGGATCTTG |
| frpB4-DFP-for | TGCTTGATTCAGCCGCTCAG |
| frpB4-DFP-revb | TGCTAGCGACAATACAAGAG |
| fecA3-F3 | GATTACCGCGCCTAAGAGTT |
| fecA3-R4-T7a | ctaatacgactcactatagggagaCTGCCTCCACCCTTGATCAC |
| fecA3-DFP-for | GCGTCAAAGAGTGTCTTGTG |
| fecA3-DFP-revb | TCCTTAGCGAACAAAGACTC |
| Hp1339-F | AGCTTTGTGGTTTGCGATTG |
| Hp1339-R-T7a | ctaatacgactcactatagggagaGTGGGAATCGCCACAGCAAG |
| Hp1340-F | AGCATCAGAAGAGGCGATGG |
| Hp1340-R-T7a | ctaatacgactcactatagggagaCTGAGCTTGCGTGGAGATGG |
| Hp1341-F | AATGCTGAGTCGGCTAAACC |
| Hp1341-R-T7a | ctaatacgactcactatagggagaGTCCGTAACGCTCCCATCAG |
Primer contains a 5′ extension with T7 promoter sequence (in lowercase letters) for the creation of an antisense RNA probe.
Primer is digoxigenin labeled.
Recombinant DNA techniques.
Restriction enzymes and DNA-modifying enzymes were used according to the manufacturer's instructions (Promega). Plasmid DNA was prepared using the Wizard system (Promega), and PCR was carried out using Taq polymerase (Promega).
Primer extension.
To map the transcriptional start site of the H. pylori frpB4 and fecA3 genes, primer extension was carried out as described previously (16). The digoxigenin-labeled primers frpB4-DFP-rev and fecA3-DFP-rev were annealed stepwise to 10 μg of total RNA from H. pylori strain 26695, and cDNA was synthesized after the addition of 5 U of avian myeloblastosis virus reverse transcriptase (Promega) and incubation for 1 h at 42°C. Nucleotide sequencing reactions were carried out with the f-mol DNA cycle sequencing system (Promega) using primer frpB4-DFP-rev on a fragment created with primers frpB4-DFP-for and frpB4-DFP-rev, as well as using primer fecA3-DFP-rev on a fragment created with primers fecA3-DFP-for and fecA3-DFP-rev (Table 1). Sequence reactions were separated on a 7% acrylamide-8 M urea sequencing gel, and then blotted onto a nylon membrane (Roche), followed by chemiluminescence DIG detection (16).
Expression and purification of H. pylori NikR.
The recombinant NikR protein was overexpressed as a fusion protein with an N-terminal Strep tag and purified using streptactin columns as described previously (17). The recombinant protein (designated Strep-NikR [17]) was over 90% pure as determined by staining with Coomassie blue following electrophoresis on SDS-12% polyacrylamide gels (8). The Strep-NikR protein preparation was stored at −80°C in small aliquots which were not refrozen after use and were used without further purification in DNase I footprinting assays.
DNase I footprinting.
Primers frpB4-DFP-for and frpB4-DFP-rev (Table 1) were used to amplify a 228-bp digoxigenin-labeled fragment of the promoter region of the frpB4 gene (PfrpB4), and primers fecA3-DFP-for and fecA3 DFP-rev (Table 1) were used to create a 351-bp digoxigenin-labeled fragment of the promoter region of the fecA3 gene (PfecA3). DNase I footprinting was performed using 721 pM and 469 pM of PfrpB4 and PfecA3, respectively. DNA fragments were incubated without or with 2.86 μM Strep-NikR protein in the presence or absence of 100 μM NiCl2 in binding buffer (10 mM HEPES [pH 7.6], 100 mM KCl, 3 mM MgCl2, and 1.5 mM CaCl2) for 30 min at 37°C. Subsequently, the DNA was digested with 0.25 U DNase I (Promega) for 1 min, and the reaction was stopped as described previously (12). Fragments were separated on a 7% acrylamide-8 M urea sequencing gel (Bio-Rad). Gels were blotted onto a positively charged nylon membrane (Roche), and then chemiluminescence DIG detection was performed (17).
RESULTS
NikR regulates transcription and expression of the fecA3 (HP1400) and frpB4 (HP1512) genes in H. pylori.
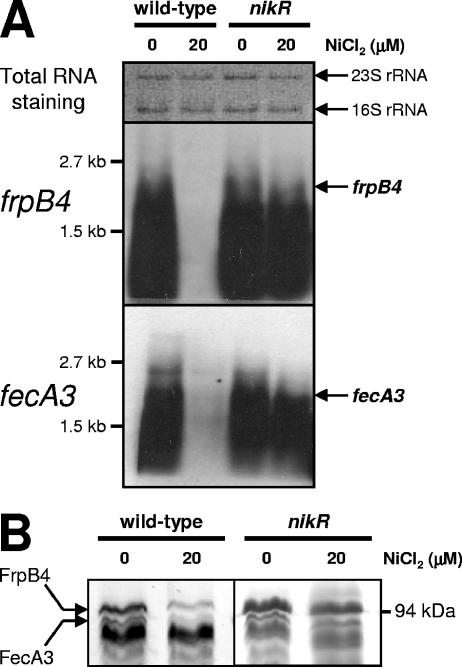
To examine the roles of NikR and nickel in the regulation of both frpB4 and fecA3, RNA from wild-type H. pylori and nikR mutant strains was isolated and hybridized to probes specific for the frpB4 and fecA3 genes (Fig. 1A). Transcription of both genes was repressed by nickel in the wild-type strain, since the transcript was not detected when the wild-type strain was grown in nickel-supplemented medium (Fig. 1A). In contrast, in the nikR mutant, transcription of both genes was constitutively high and independent of NiCl2 supplementation (Fig. 1A). The transcriptional pattern is identical to that of the NikR-regulated nixA gene (17), and this suggests that NikR may directly mediate regulation of both the fecA3 and frpB4 genes.
FIG. 1.
Transcription and expression of the H. pylori fecA3 and frpB4 genes are repressed by nickel and NikR. (A) Northern hybridization of RNA from H. pylori 26695 wild-type strain and its isogenic nikR mutant. The cells were grown in BBC medium supplemented with 0 and 20 μM NiCl2. The positions of the frpB4 and fecA3 transcripts are indicated on the right side, whereas the positions of the probes used and relevant marker sizes are shown on the left side. (B) Comparison of expression of the FrpB4 and FecA3 proteins by SDS-polyacrylamide gel electrophoresis of the outer membrane protein fraction from H. pylori 26695 wild-type and nikR mutant strains, grown in BBC medium supplemented with 0 and 20 μM NiCl2. Proteins were stained with Coomassie blue, and the FrpB4 and FecA3 proteins were identified by MALDI-TOF mass spectrometry from H. pylori strain 26695 grown without nickel supplementation.
To prove that FecA3 and FrpB4 are indeed nickel- and NikR-regulated OMPs, we isolated and compared the outer membrane fraction of the wild-type H. pylori and the nikR mutant strains. The outer membrane fraction of wild-type H. pylori 26695 contained two nickel-repressed proteins of approximately 95 kDa, and expression of these two proteins was no longer nickel repressed in the nikR mutant (Fig. 1B). The two nickel-regulated proteins of wild-type H. pylori 26695 were subsequently positively identified as FrpB4 and FecA3 by MALDI-TOF mass spectrometry (Fig. 1B).
NikR mediates repression of HP1512 (frpB4) transcription by nickel-dependent binding to the HP1512 promoter region.
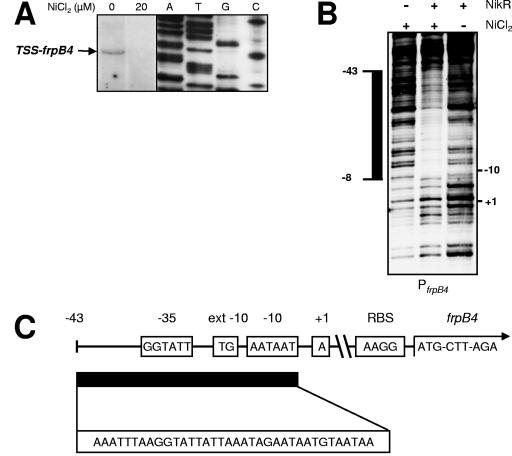
The transcription start site (TSS) of the frpB4 gene was identified with the help of primer extension (Fig. 2A). Transcription of frpB4 started at the A residue 54 bp upstream of the ATG start codon of the frpB4 gene, and the primer extension product was detected only when H. pylori 26695 was grown without nickel supplementation (Fig. 2A). The +1 position is preceded by a possible −10 promoter sequence (AATAAT) and an extended −10 region (TG at position −14) (6), whereas the −35 sequence does not resemble the E. coli consensus sequence (Fig. 2C).
FIG. 2.
NikR directly represses frpB4 transcription by nickel-dependent direct binding to a specific operator in the H. pylori frpB4 promoter region. (A) Determination of the transcriptional start site of frpB4 by primer extension, with RNA from H. pylori 26695 wild-type cells grown in medium supplemented with 0 and 20 μM NiCl2. The position of the primer extension fragment of frpB4 is marked with an arrow, and the sequence reaction products are displayed in lanes A, T, G, and C. (B) Identification of the NikR operator sequence in the frpB4 (PfrpB4) promoter by DNase footprinting in the absence (−) and presence (+) of recombinant Strep-NikR protein in the absence (−) or presence (+) of NiCl2. The protected region is indicated by a black bar on the left side of the panel. The locations of the TSS and −10 residue are also indicated. (C) Graphical representation of the frpB4 promoter region with the TSS, −10 box, extended (ext) −10 box, −35 box, ribosomal binding site, and ATG start codon of the frpB4 gene. The location of the NikR binding site is indicated by a black bar, and the sequence is shown below. −43 indicates the boundary of the NikR binding site.
Direct nickel-dependent binding of the NikR protein to the frpB4 promoter was demonstrated using DNase I footprinting (Fig. 2B). In the presence of nickel, recombinant Strep-NikR protein blocked DNase I degradation of a single 36-bp sequence (AAATTTAAGGTATTATTAAATAGAATAATGTAATAA). This sequence is located from −43 to −8 relative to the transcription start site (Fig. 2B and C) and overlaps with the putative −10 promoter region (Fig. 2B and C). The −43 to −8 region was not protected against DNase I degradation by Strep-NikR in the absence of nickel (Fig. 2B).
NikR mediates repression of HP1400 (fecA3) transcription by nickel-dependent binding to the HP1400 promoter region.
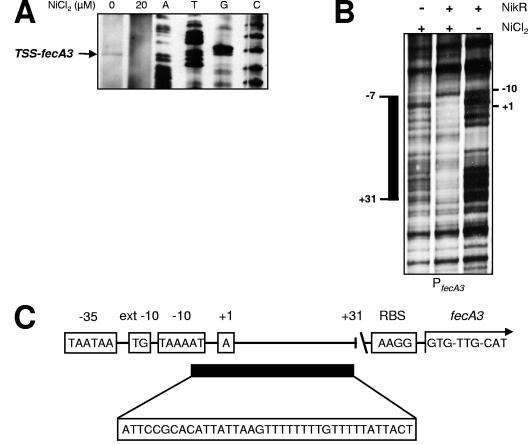
The transcription start site of the fecA3 gene was identified with the help of primer extension (Fig. 3A). Transcription of fecA3 started at the A residue 113 bp upstream of the GTG start codon of the fecA3 gene (Fig. 3A), and the primer extension product was detected only when H. pylori 26695 was grown without nickel supplementation (Fig. 3A). The +1 position is again preceded by a suitable −10 promoter region (TAAAAT [Fig. 3C]) and an extended −10 region (TG at position −14), but no discernible −35 sequence.
FIG. 3.
NikR directly represses fecA3 transcription by nickel-dependent direct binding to a specific operator in the H. pylori fecA3 promoter region. (A) The transcriptional start site of fecA3 determined by primer extension with RNA from H. pylori 26695 wild-type cells grown in medium supplemented with 0 and 20 μM NiCl2. The primer extension fragment of fecA3 is marked with an arrow, and the sequence reaction products are displayed in lanes A, T, G, and C. (B) Identification of the NikR operator sequence in the fecA3 (PfecA3) promoter by DNase footprinting in the absence (−) and presence (+) of recombinant Strep-NikR protein in the absence (−) or presence (+) of NiCl2. The protected region is indicated by a black bar on the left side of the panel, and the locations of the TSS and the −10 residue are indicated on the right side. (C) Graphical representation of the fecA3 promoter region with the TSS, −10 box, extended (ext) −10 box, and −35 box, ribosomal binding site, and GTG start codon of the fecA3 gene. The location of the NikR binding site is indicated by a black bar, and the sequence is shown below. +31 indicates the boundary of the NikR binding site.
Direct nickel-dependent binding of the NikR protein to the fecA3 promoter was demonstrated using DNase I footprinting. In the presence of nickel, Strep-NikR protein blocked DNase I degradation of a single 38-bp sequence (ATTCCGCACATTATTAAGTTTTTTTTGTTTTTATTACT) in the promoter of the fecA3 gene (Fig. 3B). This sequence is located from −7 to +31 relative to the transcription start site (Fig. 3B and C) and thus overlaps with the +1 sequence. Strep-NikR did not bind to the fecA3 promoter in the absence of nickel (Fig. 3B).
The regulation of the exbB2-exbD2-tonB2 operon is dependent on nickel and NikR, but not iron and Fur.
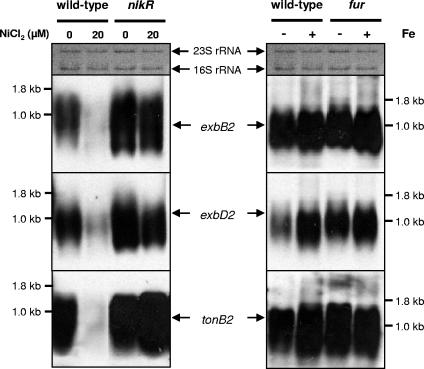
On the basis of the homology of FrpB4 and FecA3 proteins with TonB-dependent receptor proteins of other bacteria and the presence of a plug and a TonB-dependent receptor domain, as revealed by a search in the Pfam database, we predicted both FrpB4 and FecA3 proteins to be TonB-dependent transporter proteins (3). Therefore, the link between NikR and transcription of the H. pylori exbB2-exbD2-tonB2 operon (HP1339-HP1340-HP1341) was also investigated. The roles of nickel and iron on transcription of the tonB2 gene as well as those of the linked exbB2 and exbD2 genes were analyzed at the transcriptional level in H. pylori 26695 and its isogenic nikR and fur mutants. Transcription of the exbB2-exbD2-tonB2 gene cluster displayed NikR-dependent nickel-responsive repression (Fig. 4A) (9, 11), similar to the frpB4 and fecA3 genes (Fig. 1A). In contrast, all three genes were constitutively transcribed in the wild-type strain and the fur mutant, independent of iron availability (Fig. 4B).
FIG. 4.
Repression of transcription of the tonB2 operon is dependent on nickel and NikR but is not regulated by iron and Fur. (Left) Northern hybridization of RNA from H. pylori 26695 wild-type and nikR mutant cells grown in medium supplemented with 0 and 20 μM NiCl2. (Right) Northern hybridization of RNA from H. pylori 26695 wild-type and fur mutant cells grown in medium in the absence (−) or presence (+) of iron. Transferred RNA was stained by methylene blue and is included for comparison of RNA amounts. The positions of the exbB2, exbD2, and tonB2 transcripts are indicated by the black arrows between the left and right panels.
DISCUSSION
One of the adaptations of H. pylori to its gastric lifestyle has been the high-level expression of the nickel-dependent urease enzyme, which allows H. pylori to survive the acidic pH in the gastric environment, both during initial infection and chronic colonization (32). This has resulted in making scavenging and acquisition of sufficient levels of nickel a very important activity for H. pylori, and it can therefore be predicted that H. pylori has multiple mechanisms for the transport of nickel. However, uncontrolled acquisition of transition metals like nickel may lead to toxicity, as they may participate in the generation of toxic oxygen radicals or block incorporation of cofactors into enzymes (24). Therefore, acquisition, utilization, and storage of transition metals need to be carefully monitored and actively controlled, and this function is usually mediated by metal-responsive regulatory proteins. Three such proteins have been identified in H. pylori: the iron-responsive regulatory protein Fur (12, 41), the copper-responsive two-component regulatory system CrdRS (43), and the nickel-responsive regulator NikR (11, 17, 40).
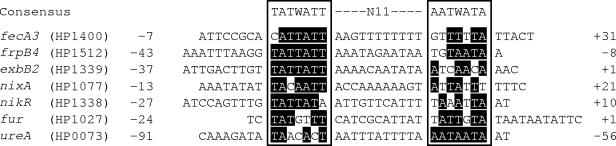
The NikR protein belongs to the ribbon-helix-helix family of transcriptional regulators, which bind to the DNA as tetramers (8, 11). It was recently demonstrated that H. pylori NikR can function both as a nickel-dependent repressor and activator of gene transcription by binding to the promoter region of its target genes (11, 17). Activation of transcription occurs when NikR binds upstream of the ureA promoter at positions −50 to −90 (11, 17), whereas repression occurs when NikR binds to the promoter region overlapping the −10 and +1 region of the nixA (17), fur, and exbB2 (11) promoters. Binding to this region is believed to prevent transcription due to competition of the regulator with RNA polymerase (8, 13). The nickel- and NikR-dependent regulation of frpB4 and fecA3 is similar to that of the nixA and exbB2-exbD2-tonB2 genes, since NikR binds at positions −43 to −8 in the promoter region of the frpB4 gene (Fig. 2) and at positions −7 to +31 in the promoter region of the fecA3 gene (Fig. 3). Therefore, in both genes either the −10 or +1 site is protected by NikR and thus blocked from binding of RNA polymerase (17). The NikR-protected operators in the frpB4 and fecA3 promoter sequences resemble the H. pylori NikR consensus sequence (TATWATT-N11-AATWATA) (Fig. 5) (11).
FIG. 5.
Alignment of the putative NikR operator sites, based on the consensus sequence proposed in reference 11. The two parts of the palindrome are boxed. The black background indicates conserved bases compared to the consensus sequence. The numbers surrounding the sequences indicate the position of the NikR binding site with respect to the +1 transcriptional start site. The W in the consensus sequence represents an A or T residue.
In the annotation of the H. pylori genome sequences, the three fecA genes are annotated as ferric iron dicitrate transporters, whereas the frpB genes are annotated as predicted iron-regulated OMPs (1, 5, 36). However, only two copies of each displayed the typical Fur-mediated iron-responsive regulatory pattern usually associated with iron acquisition systems (12, 15, 41). Since uncontrolled uptake of iron by the FrpB4 (HP1512) and FecA3 (HP1400) proteins would probably lead to iron toxicity, it could therefore be envisaged that these proteins may function in the transport of other compounds, as has been shown for TonB-dependent OMPs in other bacteria (20, 35). Indeed, in the present work it is demonstrated that both FecA3 and FrpB4 are nickel- and NikR-regulated OMPs with the help of fractionation (Fig. 1B).
The H. pylori fecA3 gene shares homology with metal-citrate uptake genes, and in Bacillus subtilis, it was demonstrated that the metal-citrate transporter CitM imports not only Ni2+-citrate but also Ni2+-isocitrate complexes (21, 44). Consistent with earlier reports (9, 11), it is also demonstrated in the present study that the exbB2, exbD2, and tonB2 genes are transcribed as an operon and that regulation of the operon is dependent on nickel and NikR (Fig. 4A), but not by Fur and iron (Fig. 4B). This complements the description of NikR binding to the exbB2 promoter (11), thus demonstrating that the exbB2-exbD2-tonB2 operon is regulated by nickel and NikR. The tonB2 operon therefore may be specific for the nickel- and NikR-dependent regulated genes. A similar system with regard to receptor specificity of multiple TonB orthologs has been described for Vibrio cholerae (25, 34). To date, the TonB complexes are known to be important for the transport of iron complexes through the outer membrane into the periplasm, which is an energy-dependent process (2, 30). As the outer membrane does not have a proton motive force, due to its permeability through pores, the proton motive force of the inner membrane is used to power many outer membrane transporters (30). It is thought that TonB is responding to the proton motive force by binding a proton and thereby changes conformation in an energized form. Energized TonB subsequently transduces the energy to the outer membrane transporter by binding to a TonB box (30).
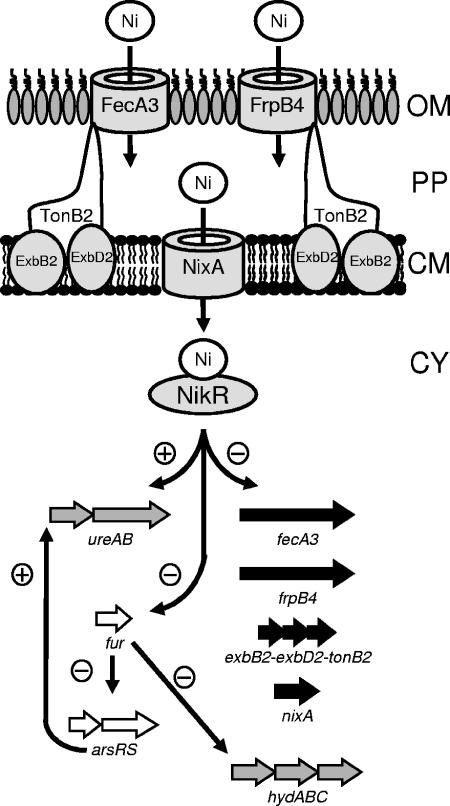
In view of the concerted regulation observed for FrpB4, FecA3, and the ExbB-ExbD-TonB system, we currently favor the model where FecA3 and FrpB4 function in nickel acquisition, as is outlined in Fig. 6. This model is hypothetical and is partially based on assumptions for which we currently lack direct experimental support, but it is consistent with the data from an independent, concurrent study of Davis and coworkers (10). They have independently identified the nickel- and NikR-responsive regulation of the frpB4 (HP1512) gene and showed that mutation of the HP1512 gene in H. pylori strain 26695 resulted in a significant decrease in urease activity and an increase in transcription of other nickel-responsive genes. Taken together, this suggests a decrease in cytoplasmic nickel availability in the HP1512 mutant compared to the wild-type strain (10). Overall, this supports our working model where FecA3 and FrpB4 are involved in transport of nickel compounds through the outer membrane (Fig. 6), but additional data are required to further establish the link between TonB-energized outer membrane transport of nickel compounds.
FIG. 6.
Graphical representation of our hypothetical model illustrating the potential links between NikR and the different NikR-regulated genes in nickel uptake and nickel utilization in H. pylori. The outer membrane (OM), cytoplasmic membrane (CM), periplasm (PP), and cytoplasm (CY) are indicated. Gray arrows represent genes involved in nickel utilization, black arrows represent genes involved in nickel transport, and white arrows represent genes involved in regulation of transcription.
The regulation of transcription of frpB1, frpB2, fecA1, and fecA2 was previously demonstrated to be dependent on iron and Fur, whereas frpB4 and fecA3 were not regulated by Fur and suggested to be constitutively transcribed (41). In a subsequent transcriptome analysis study, it was suggested that both frpB4 and fecA3 were NikR regulated (9), but these findings were not further investigated, and thus, it remained possible that the observed role of NikR was indirect, via regulation of the fur gene (7, 37, 38). In this study we have elucidated the molecular mechanism of NikR-mediated regulation of the fecA3 and frpB4 genes and have shown that NikR binds to regions overlapping either the +1 or −10 region of the respective promoters (Fig. 2 and 3). In addition, we demonstrate on the level of transcription and protein expression that NikR regulation results in nickel-responsive transcription and nickel-responsive expression (Fig. 1A and B), as was predicted but not investigated. Taken together, this not only confirms but significantly extends the previous report (9).
In conclusion, H. pylori NikR regulates transcription of the genes encoding the FecA3 and FrpB4 OMPs. So far, all genes for which NikR regulation has been confirmed at the molecular level have been demonstrated or implicated to play a role in nickel metabolism (summarized in Fig. 6). The urease system uses the majority of transported nickel, whereas the NixA protein is the major cytoplasmic membrane transporter for nickel. Furthermore, the FecA3 and FrpB4 proteins may transport complexed nickel compounds through the outer membrane, a process energized by the TonB2-ExbB2-ExbD2 complex. Finally, the nickel- and NikR-regulated fur gene (7, 11, 38) controls expression of the hydrogenase system and is involved in regulation of the HP0166-0165 regulatory system, which participates in regulation of urease and the Hpn nickel storage proteins (18, 28, 29). This allows NikR to control nickel metabolism both directly and indirectly and displays the extended repertoire of the NikR regulator compared to its E. coli counterpart, where its main function is regulation of nickel uptake in anaerobic conditions (8, 31). This adaptation may have an important function in long-term colonization by H. pylori of hostile environmental niches like the human stomach.
Acknowledgments
We thank Theo Hoogenboezem for protein identification and Harry Mobley, Gregg Davis, and Erika Flannery for helpful comments and exchange of data prior to publication.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 2 October 2006.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, et al. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 3.Bateman, A., L. Coin, R. Durbin, R. D. Finn, V. Hollich, S. Griffiths-Jones, A. Khanna, M. Marshall, S. Moxon, E. L. Sonnhammer, D. J. Studholme, C. Yeats, and S. R. Eddy. 2004. The Pfam protein families database. Nucleic Acids Res. 32:D138-D141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauerfeind, P., R. M. Garner, and L. T. Mobley. 1996. Allelic exchange mutagenesis of nixA in Helicobacter pylori results in reduced nickel transport and urease activity. Infect. Immun. 64:2877-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boneca, I. G., H. de Reuse, J. C. Epinat, M. Pupin, A. Labigne, and I. Moszer. 2003. A revised annotation and comparative analysis of Helicobacter pylori genomes. Nucleic Acids Res. 31:1704-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burr, T., J. Mitchell, A. Kolb, S. Minchin, and S. Busby. 2000. DNA sequence elements located immediately upstream of the −10 hexamer in Escherichia coli promoters: a systematic study. Nucleic Acids Res. 28:1864-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bury-Mone, S., J. M. Thiberge, M. Contreras, A. Maitournam, A. Labigne, and H. De Reuse. 2004. Responsiveness to acidity via metal ion regulators mediates virulence in the gastric pathogen Helicobacter pylori. Mol. Microbiol. 53:623-638. [DOI] [PubMed] [Google Scholar]
- 8.Chivers, P. T., and R. T. Sauer. 2000. Regulation of high-affinity nickel uptake in bacteria: Ni2+-dependent interaction of NikR with wild-type and mutant operator sites. J. Biol. Chem. 275:19735-19741. [DOI] [PubMed] [Google Scholar]
- 9.Contreras, M., J. M. Thiberge, M. A. Mandrand-Berthelot, and A. Labigne. 2003. Characterization of the roles of NikR, a nickel-responsive pleiotropic autoregulator of Helicobacter pylori. Mol. Microbiol. 49:947-963. [DOI] [PubMed] [Google Scholar]
- 10.Davis, G. S., E. L. Flannery, and H. L. T. Mobley. 2006. Helicobacter pylori HP1512 is a nickel-responsive NikR-regulated outer membrane protein. Infect. Immun. 74:6811-6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delany, I., R. Ieva, A. Soragni, M. Hilleringmann, R. Rappuoli, and V. Scarlato. 2005. In vitro analysis of protein-operator interactions of the NikR and Fur metal-responsive regulators of coregulated genes in Helicobacter pylori. J. Bacteriol. 187:7703-7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2001. The Fur repressor controls transcription of iron-activated and -repressed genes in Helicobacter pylori. Mol. Microbiol. 42:1297-1309. [DOI] [PubMed] [Google Scholar]
- 13.De Pina, K., V. Desjardin, M. A. Mandrand-Berthelot, G. Giordano, and L. F. Wu. 1999. Isolation and characterization of the nikR gene encoding a nickel-responsive regulator in Escherichia coli. J. Bacteriol. 181:670-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eaton, K. A., J. V. Gilbert, E. A. Joyce, A. E. Wanken, T. Thevenot, P. Baker, A. Plaut, and A. Wright. 2002. In vivo complementation of ureB restores the ability of Helicobacter pylori to colonize. Infect. Immun. 70:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst, F. D., S. Bereswill, B. Waidner, J. Stoof, U. Mader, J. G. Kusters, E. J. Kuipers, M. Kist, A. H. M. van Vliet, and G. Homuth. 2005. Transcriptional profiling of Helicobacter pylori Fur- and iron-regulated gene expression. Microbiology 151:533-546. [DOI] [PubMed] [Google Scholar]
- 16.Ernst, F. D., G. Homuth, J. Stoof, U. Mader, B. Waidner, E. J. Kuipers, M. Kist, J. G. Kusters, S. Bereswill, and A. H. M. van Vliet. 2005. Iron-responsive regulation of the Helicobacter pylori iron-cofactored superoxide dismutase SodB is mediated by Fur. J. Bacteriol. 187:3687-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst, F. D., E. J. Kuipers, A. Heijens, R. Sarwari, J. Stoff, C. W. Penn, J. G. Kusters, and A. H. M. van Vliet. 2005. The nickel-responsive regulator NikR controls activation and repression of gene transcription in Helicobacter pylori. Infect. Immun. 73:7252-7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsyth, M. H., P. Cao, P. P. Garcia, J. D. Hall, and T. L. Cover. 2002. Genome-wide transcriptional profiling in a histidine kinase mutant of Helicobacter pylori identifies members of a regulon. J. Bacteriol. 184:4630-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulkerson, J. F., Jr., R. M. Garner, and H. L. Mobley. 1998. Conserved residues and motifs in the NixA protein of Helicobacter pylori are critical for the high affinity transport of nickel ions. J. Biol. Chem. 273:235-241. [DOI] [PubMed] [Google Scholar]
- 20.Koebnik, R. 2005. TonB-dependent trans-envelope signalling: the exception or the rule? Trends Microbiol. 13:343-347. [DOI] [PubMed] [Google Scholar]
- 21.Krom, B. P., J. B. Warner, W. N. Konings, and J. S. Lolkema. 2000. Complementary metal ion specificity of the metal-citrate transporters CitM and CitH of Bacillus subtilis. J. Bacteriol. 182:6374-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusters, J. G., A. H. M. van Vliet, and E. J. Kuipers. 2006. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 19:449-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mobley, H. L., R. M. Garner, G. R. Chippendale, J. V. Gilbert, A. V. Kane, and A. G. Plaut. 1999. Role of Hpn and NixA of Helicobacter pylori in susceptibility and resistance to bismuth and other metal ions. Helicobacter 4:162-169. [DOI] [PubMed] [Google Scholar]
- 24.Mulrooney, S. B., and R. P. Hausinger. 2003. Nickel uptake and utilization by microorganisms. FEMS Microbiol. Rev. 27:239-261. [DOI] [PubMed] [Google Scholar]
- 25.Occhino, D. A., E. E. Wyckoff, D. P. Henderson, T. J. Wrona, and S. M. Payne. 1998. Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol. Microbiol. 29:1493-1507. [DOI] [PubMed] [Google Scholar]
- 26.Olson, J. W., and R. J. Maier. 2002. Molecular hydrogen as an energy source for Helicobacter pylori. Science 298:1788-1790. [DOI] [PubMed] [Google Scholar]
- 27.Pettersson, A., A. Maas, D. van Wassenaar, P. van der Ley, and J. Tommassen. 1995. Molecular characterization of FrpB, the 70-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect. Immun. 63:4181-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pflock, M., N. Finsterer, B. Joseph, H. Mollenkopf, T. F. Meyer, and D. Beier. 2006. Characterization of the ArsRS regulon of Helicobacter pylori, involved in acid adaptation. J. Bacteriol. 188:3449-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflock, M., S. Kennard, I. Delany, V. Scarlato, and D. Beier. 2005. Acid-induced activation of the urease promoters is mediated directly by the ArsRS two-component system of Helicobacter pylori. Infect. Immun. 73:6437-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postle, K., and R. J. Kadner. 2003. Touch and go: tying TonB to transport. Mol. Microbiol. 49:869-882. [DOI] [PubMed] [Google Scholar]
- 31.Rowe, J. L., G. L. Starnes, and P. T. Chivers. 2005. Complex transcriptional control links NikABCDE-dependent nickel transport with hydrogenase expression in Escherichia coli. J. Bacteriol. 187:6317-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachs, G., D. L. Weeks, K. Melchers, and D. R. Scott. 2003. The gastric biology of Helicobacter pylori. Annu. Rev. Physiol. 65:349-369. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Seliger, S. S., A. R. Mey, A. M. Valle, and S. M. Payne. 2001. The two TonB systems of Vibrio cholerae: redundant and specific functions. Mol. Microbiol. 39:801-812. [DOI] [PubMed] [Google Scholar]
- 35.Shultis, D. D., M. D. Purdy, C. N. Banchs, and M. C. Wiener. 2006. Outer membrane active transport: structure of the BtuB:TonB complex. Science 312:1396-1399. [DOI] [PubMed] [Google Scholar]
- 36.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 37.van Vliet, A. H. M., F. D. Ernst, and J. G. Kusters. 2004. NikR-mediated regulation of Helicobacter pylori acid adaptation. Trends Microbiol. 12:489-494. [DOI] [PubMed] [Google Scholar]
- 38.van Vliet, A. H. M., E. J. Kuipers, J. Stoof, S. W. Poppelaars, and J. G. Kusters. 2004. Acid-responsive gene induction of ammonia-producing enzymes in Helicobacter pylori is mediated via a metal-responsive repressor cascade. Infect. Immun. 72:766-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Vliet, A. H. M., E. J. Kuipers, B. Waidner, B. J. Davies, N. de Vries, C. W. Penn, C. M. J. E. Vandenbroucke-Grauls, M. Kist, S. Bereswill, and J. G. Kusters. 2001. Nickel-responsive induction of urease expression in Helicobacter pylori is mediated at the transcriptional level. Infect. Immun. 69:4891-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Vliet, A. H. M., S. W. Poppelaars, B. J. Davies, J. Stoof, S. Bereswill, M. Kist, C. W. Penn, E. J. Kuipers, and J. G. Kusters. 2002. NikR mediates nickel-responsive transcriptional induction of urease expression in Helicobacter pylori. Infect. Immun. 70:2846-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Vliet, A. H. M., J. Stoof, R. Vlasblom, S. A. Wainwright, N. J. Hughes, D. J. Kelly, S. Bereswill, J. J. Bijlsma, T. Hoogenboezem, C. M. J. E. Vandenbroucke-Grauls, M. Kist, E. J. Kuipers, and J. G. Kusters. 2002. The role of the ferric uptake regulator (Fur) in regulation of Helicobacter pylori iron uptake. Helicobacter 7:237-244. [DOI] [PubMed] [Google Scholar]
- 42.van Vliet, A. H. M., K. G. Wooldridge, and J. M. Ketley. 1998. Iron-responsive gene regulation in a Campylobacter jejuni fur mutant. J. Bacteriol. 180:5291-5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waidner, B., K. Melchers, F. N. Stahler, M. Kist, and S. Bereswill. 2005. The Helicobacter pylori CrdRS two-component regulation system (HP1364/HP1365) is required for copper-mediated induction of the copper resistance determinant CrdA. J. Bacteriol. 187:4683-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warner, J. B., and J. S. Lolkema. 2002. Growth of Bacillus subtilis on citrate and isocitrate is supported by the Mg2+-citrate transporter CitM. Microbiology 148:3405-3412. [DOI] [PubMed] [Google Scholar]
- 45.Wolfram, L., and P. Bauerfeind. 2002. Conserved low-affinity nickel-binding amino acids are essential for the function of the nickel permease NixA of Helicobacter pylori. J. Bacteriol. 184:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfram, L., E. Haas, and P. Bauerfeind. 2006. Nickel represses the synthesis of the nickel permease NixA of Helicobacter pylori. J. Bacteriol. 188:1245-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimmermann, L., K. Hantke, and V. Braun. 1984. Exogenous induction of the iron dicitrate transport system of Escherichia coli K-12. J. Bacteriol. 159:271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]