Abstract
CXCL16 is a recently discovered multifaceted chemokine that has been shown not only to recruit activated T lymphocytes but also to play a direct role in the binding and phagocytosis of bacteria by professional antigen-presenting cells. In this study, we investigated the role of CXCL16 in vivo in the regulation of the immune response using a murine model of Salmonella enterica serovar Enteritidis infection. The expression of CXCL16 was strongly upregulated in the spleens and livers of animals developing an immune response to a primary acute infection but not in the Peyer's patches. Animals developing a secondary response after reexposure to the bacteria displayed a similar pattern of expression. During the primary response, prior treatment with neutralizing antibodies to CXCL16 induced a significant increase in bacterial burden in the spleen and liver. The production of gamma interferon (IFN-γ) by the lymphocytes in the spleen was decreased by anti-CXCL16 treatment. In comparison, during the secondary response, anti-CXCL16 treatment also significantly increased bacterial burden in both the spleen and liver but had no effect on IFN-γ production. No role was found for CXCL16 in the production of antibody against SefA, a major surface antigen of S. enteritidis. Together, these results demonstrate a role for CXCL16 in the control of bacterial colonization of target organs and, more specifically, in the regulation of the cell-mediated arm of the primary response to S. enteritidis.
Salmonellae continue to be a major health problem worldwide. They comprise a group of intracellular gram-negative bacteria that are disseminated via contaminated food or water and lead to a range of diseases. Over the past decade, Salmonella enterica serovar Enteritidis has become the most frequently reported forms of Salmonella infections in Europe and North and South America (24). The use of an attenuated strain, S. enterica serovar Enteritidis 11RX (20, 23), allows the in vivo study of the immune response using a murine model of infection.
Chemokines are small chemotactic cytokines that have the ability to direct the migration of leukocytes both in normal homeostatic conditions and during inflammatory reactions and are crucial for effective recruitment and orchestration of the immune response. The majority of chemokines are functionally classified as “inflammatory/inducible” and are responsible for the control of the recruitment of effector cells to peripheral sites of infection (7). However, with over 40 ligands and 20 receptors in the chemokine gene superfamily, considerable study is required to elucidate the role of individual chemokines and receptors in the generation of immunity to a range of infectious agents, including salmonellae. Identifying the key chemokines regulating the antibacterial response to serovar Enteritidis is an important objective since it may provide avenues to enhance therapeutic strategies against a range of bacteria including Salmonella species.
A role for specific chemokines in the control of bacterial infection such as that by Salmonella subspecies has recently been uncovered primarily through in vitro studies, yet there have been few in vivo studies to confirm these in vitro findings. In a recent study using a mouse model of acute primary serovar Enteritidis infection, we demonstrated a role for CCL3 and CCL20 in the control of bacterial multiplication and in the effective development of the humoral and the cell-mediated immune responses, respectively (5). Using a model of infection of newly hatched chickens with serovar Enteritidis, Whithanage et al. observed a strong increase in CXCL8, CCL3, and CCL4 expression in some target organs, associated with proinflammatory cytokine upregulation and signs of inflammation (28, 29). In a murine knockout model, CCL2 was also found to have an important role in the control of S. enterica infection (4).
CXCL16 is a recently characterized chemokine, presenting an atypical structure and several properties that make it likely to be involved in the organization of the immune response against bacterial infection. First, CXCL16 is expressed on the cell surface as a transmembrane molecule with a chemokine domain linked to a mucin-like stalk (27). It is present at the surface of antigen-presenting cells such as macrophages and dendritic cells, where the chemokine domain plays a role in the adhesion and phagocytosis of both gram-negative and gram-positive bacteria (22). Second, the chemokine domain of CXCL16 can be shed from the surface, leading to the formation of a classic soluble chemokine gradient (9). Soluble CXCL16 is chemotactic for activated Th1-polarized lymphocytes producing gamma interferon (IFN-γ) and Tc1-polarized lymphocytes displaying a cytotoxic effector phenotype, both of which express its sole known receptor CXCR6 (11).
Because of its potential actions at the level of direct clearance of live bacteria and in the recruitment of activated subpopulations of T lymphocytes, we investigated the role of CXCL16 both in the primary immune response to serovar Enteritidis, as well as in the organization of the secondary immune response in previously immunized animals. Our results demonstrate an important role for CXCL16 in the overall control of serovar Enteritidis infection in the spleen and liver and a differential involvement in the organization of cell-mediated immunity during the primary and secondary immune responses.
MATERIALS AND METHODS
Animals.
Six- to eight-week-old female BALB/c mice were obtained from the Central Animal House at the University of Adelaide, Adelaide, South Australia. Animals were housed in conventional mouse rooms at Adelaide University where they were provided with food and water ad libitum.
Reagents.
The anti-CXCL16 antibody used in the present study was protein A purified from polyclonal antisera raised in rabbits against the chemokine domain (amino acids 1 to 88) of synthetic murine CXCL16 (kindly provided by I. Clark-Lewis, University of British Columbia, Vancouver, Canada). The S. enterica serovar Enteritidis strain 11RX was obtained from stocks within the School of Molecular and Biomedical Science at the University of Adelaide. SefA protein was purified from S. enteritidis 11RX as previously described (20).
CXCR6-expressing cell line.
Murine CXCR6 was PCR amplified from a spinal cord unligated cDNA library obtained from a C57BL/6 mouse at peak disease of experimental autoimmune encephalomyelitis. The expected 1,068-bp product generated was subsequently digested and ligated into the pEF-IRES-Puro6 vector for strong and constitutive mammalian expression (10), and its sequence was confirmed. The vector was used to generate a cell line stably expressing murine CXCR6 by electroporation of the pre-B cell line B300.19, as well as a control cell line containing the empty plasmid only. Selection of the transfectants was achieved by adding 3 μg of puromycin/ml into the culture medium 2 days after electroporation. The clones were then evaluated by reverse transcription-PCR amplification of the product and by chemotaxis assay.
Flow cytometry assay.
For detection of intracellular IFN-γ within leukocyte subpopulations, 2 × 106 cells isolated from spleen homogenates of normal rabbit immunoglobulin G (NRIgG)- or anti-CXCL16-treated animals were cultured for 72 h in presence of formalin-killed 11RX (3 × 108 CFU) or phosphate-buffered saline (PBS) control. Cells were then washed and incubated for 5 h with Golgi-Stop (BD Biosciences, Franklin Lakes, NJ) at 10 μl/ml culture and washed, and Fc receptors were blocked with murine gamma globulin (Rockland, Gilbertsville, PA) for 30 min at room temperature. Cells were then incubated with either fluorescein isothiocyanate (FITC)-conjugated monoclonal rat anti-murine CD3, DX5 (BD Biosciences), or F4/80 (Caltag Laboratories) antibodies or biotinylated monoclonal rat anti-murine Ly-6G (BD Biosciences) antibodies for 30 min at room temperature. Biotinylated antibodies were detected by incubation with FITC-conjugated streptavidin (Rockland) for 30 min at 4°C. Cells were then washed, fixed, and permeabilized in accordance with BD Cytofix/Cytoperm kit recommendations, blocked with rat gamma-globulin (Rockland) for 20 min at room temperature, and then stained with phycoerythrin (PE)-conjugated monoclonal rat anti-murine IFN-γ (BD Biosciences) for 30 min at 4°C. All cells were acquired and analyzed with FACSCanto and CellQuest (BD Biosciences).
For the quantitation of leukocyte subpopulations in target organ homogenates, single cell suspensions were prepared from the spleen, Peyer's patches, and liver; red blood cells were removed; and Fc receptors were blocked with murine gamma globulin (Rockland) for 30 min at room temperature. Cells were washed and incubated with either FITC-conjugated monoclonal rat anti-murine CD3, DX5 (BD Biosciences), or F4/80 (Caltag Laboratories) antibodies; PE-conjugated monoclonal rat anti-murine CD11c (BD Biosciences) antibodies; or biotinylated monoclonal rat anti-murine Ly-6G (BD Biosciences) antibodies for 30 min at room temperature. Biotinylated antibodies were detected by incubation with PE-conjugated streptavidin (Rockland) for 30 min at 4°C. Cells were washed, fixed with 1% paraformaldehyde, and acquired and analyzed using FACScanto and CellQuest (BD Biosciences).
Chemotaxis assay.
Single cell suspensions of 5 × 106 cells/ml were prepared in RPMI 1640 medium supplemented with 0.5% bovine serum albumin, 20 mM l-glutamine, penicillin, and gentamicin and then incubated for 30 min at 37°C with Calcein at a final concentration of 2 μM. Cells were then washed twice, resuspended at 107 cells/ml, and subjected to Transwell chemotaxis assays as previously described (13) with a range of concentrations of murine CXCL16. For inhibition experiments, anti-CXCL16 antibodies or NRIgG control antibodies were added at various concentrations to the bottom wells prior to starting the assay.
Serovar Enteritidis infection.
For primary infection experiments, serovar Enteritidis 11RX at various doses (104, 3 × 104, 105, and 3 × 105 CFU) or diluent (endotoxin-free PBS) was injected into the peritoneal cavity of BALB/c mice as previously described (1). On day 5 after infection, the spleens and livers were surgically removed, washed, and immediately homogenized. Homogenates were prepared by passing sterilely removed tissues through a cell dissociation sieve (Sigma, St. Louis, MO) using a syringe plunger. Homogenates were then centrifuged for 5 min at 3,000 rpm, and supernatants were retained and adjusted to 1 ml with sterile PBS before storage at −20°C. For each animal, four to five Peyer's patches were also collected and processed as described above. In the experiments involving anti-chemokine antibody treatment, 500 μg of rabbit polyclonal anti-mouse CXCL16 or NRIgG control was injected intraperitoneally (i.p.) 24 h prior to bacterial infection. For the study of the secondary immune response to serovar Enteritidis infection, mice were injected i.p. with 104 CFU and given 6 weeks to clear the infection. Mice were then reinfected i.p. with 107 CFU and sacrificed on day 5 after reinfection. The spleens, livers, and Peyer's patches were collected and handled as described above. In the experiments involving anti-chemokine antibody treatment, 500 μg of rabbit polyclonal anti-mouse CXCL16 or NRIgG control was injected i.p. 24 h prior to reinfection.
Viable bacterial counts.
Serial dilutions of the homogenates from the spleens, Peyer's patches, and livers were performed in Luria broth (LB) media and assayed for CFU on LB solid agar from growth at 37°C overnight.
ELISA.
Murine CXCL16, CXCL9, CXCL10, and CXCL11 enzyme-linked immunosorbent assays (ELISAs) were performed with matched-pair antibodies obtained from R&D Systems (Jomar diagnostics, Adelaide, Australia). Murine IFN-γ and interleukin-4 (IL-4) ELISAs were purchased from Pharmingen (Becton Dickinson). Murine IL-12p70 ELISA was purchased from eBioscience (Jomar Diagnostics). Streptavidin-peroxidase conjugate (Rockland) and Fast OPD substrate (Sigma) were used according to the manufacturers' recommendations. Homogenate samples were used neat (undiluted) in all ELISA. The limits of detection were 125 pg/ml for CXCL16 and CXCL11, 62.5 pg/ml for CXCL10, 39.1 pg/ml for IFN-γ, 31.2 pg/ml for IL-4, 15.6 pg/ml for CXCL9, and 7.8 pg/ml for IL-12p70. It should be noted that whereas the values presented for the spleens correspond to whole organs, the values for livers have been normalized to the quantity of tissue retrieved during microdissection. On average, this value corresponded to 178.8 ± 29 mg per liver. Detection of anti-SefA antibody in sera was performed by using a direct ELISA protocol as previously described (5). In the case of primary-infection experiments, for each isotype, the mean optical density for naive noninfected mouse serum was subtracted to each individual data obtained (1.3, 0.16, 0.37, and 0.57 for IgM, IgA, IgG1, and IgG2a, respectively [n = 6]). In the case of secondary-infection experiments, the mean optical density value for 6-week-postinfection mouse serum was subtracted to each individual data obtained (0.78, 0.1, 0.19, and 0.25 for IgM, IgA, IgG1, and IgG2a, respectively [n = 6]).
Statistical analysis.
Statistical analysis was performed by using the Student t test for all experiments, except for the bacterial burden evaluation, in which the nonparametric Mann-Whitney U test was used. The level of statistical significance attained is indicated in the figure legends.
RESULTS
CXCL16 expression is upregulated in vivo during primary and secondary S. enterica serovar Enteritidis infections.
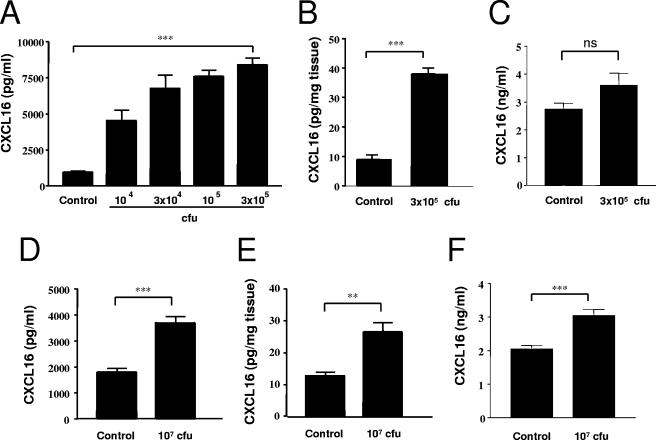
The expression of soluble CXCL16 was measured by ELISA in the spleens, livers, Peyer's patches, and sera of mice infected with serovar Enteritidis. Day 5 postinfection was selected as representative of an acute primary infection, as previously determined (5). As shown in Fig. 1A, whereas naive animals (control) displayed a low basal level of CXCL16 expression in the spleen, production was strongly and significantly increased in animals infected with serovar Enteritidis. This increase was dose dependent, with higher chemokine concentrations measured in response to higher bacterial doses. For the optimal sublethal infection dose of 3 × 105 CFU, the level of CXCL16 increased approximately ninefold over control levels. In a similar manner, the level of CXCL16 in the livers of infected animals was increased fourfold over naive controls (Fig. 1B). No significant difference was observed in the sera (Fig. 1C).
FIG. 1.
Expression of CXCL16 in vivo in response to serovar Enteritidis infection. The production of soluble CXCL16 was measured by sandwich ELISA in homogenates of the spleen (A) and liver (B) before exposure (control) or 5 days after i.p. injection of a range of CFU of serovar Enteritidis. Mice were also infected with serovar Enteritidis (104 CFU i.p.) and given 6 weeks to clear the primary infection. Mice were then sacrificed (control) or reinfected with 107 CFU i.p., and spleen (D) and liver (E) homogenates were assessed for soluble CXCL16 expression. Sera from controls and infected mice (C, primary; F, secondary) were also analyzed. The data represent the means ± the standard errors of the mean (SEM) from at least 14 mice. Significant differences from control value: **, P < 0.01; ***, P < 0.001 (Student t test). ns, not significantly different from control.
In order to study CXCL16 expression during the secondary immune response in mice previously immunized with serovar Enteritidis, the clearance of bacteria after a low dose (104 CFU i.p.) in a range of organs was monitored in a series of preliminary experiments. We found that 6 weeks postinfection only small numbers of serovar Enteritidis could occasionally be observed after plating of organ homogenates (<50 CFU for the whole spleen and <1 CFU per mg tissue for the liver, Peyer's patches, and peritoneal cavity washout [data not shown]). Therefore, this time point was selected for reinfection with a range of bacterial doses. A dose of 107 CFU was eventually selected because it was the highest nonlethal dose that induced strong reinfection (data not shown). At day 5 after reinfection, the concentration of CXCL16 in the spleen and liver was significantly increased (Fig. 1D and E, respectively) compared to immunized controls, but at reduced levels compared to those attained during primary infection (2- and 2.5-fold increases for the spleen and liver, respectively). In contrast to primary infection, concentrations of CXCL16 in the sera of animal undergoing a secondary infection were also significantly increased compared to the controls (Fig. 1F).
Of note, no change in the concentration of CXCL16 in the Peyer's patches was observed between the controls and the infected animals during either primary or secondary infection (data not shown), although as observed in a previous study bacterial colonization was also very intense in the Peyer's patches (5). Interestingly, at 6 weeks after primary infection, while the concentration of CXCL16 in the liver had returned to basal level, the concentration of CXCL16 in the spleen remained twice that measured in naive controls, possibly as a result of a very low residual bacterial presence in this organ.
CXCL9 is upregulated during serovar Enteritidis infection while levels of CXCL10 and CXCL11 are unchanged.
In parallel to the measurement of CXCL16 in target organs following primary and secondary infection with serovar Enteritidis, we also evaluated the levels of other type-1 related chemokines, namely, the ligands for CXCR3: CXCL9, CXLC10, and CXCL11. As shown in Table 1, no change in the level of CXCL10 or CXCL11 was observed in the spleen and liver at day 5 after primary exposure to serovar Enteritidis or at day 5 after the reinfection of previously immunized animals (secondary infection) compared to the appropriate controls. In contrast, the level of CXCL9 expression was significantly increased in the spleens and livers of infected animals compared to controls, during both primary and secondary infections.
TABLE 1.
Expression of CXCR3 ligands during S. enterica serovar Enteritidis infection
| Infection type and chemokine | Mean chemokine concn ± SEMa in:
|
|||
|---|---|---|---|---|
| Spleen (ng/ml)
|
Liver (pg/ml)
|
|||
| Control | Serovar Enteritidis | Control | Serovar Enteritidis | |
| Primary infection | ||||
| CXCL9 | 2.5 ± 0.2 | 9.8 ± 0.2† | 11.2 ± 0.4 | 39.6 ± 1.9† |
| CXCL10 | 0.23 ± 0.04 | 0.26 ± 0.03 | 5.1 ± 1.8 | 4.2 ± 0.8 |
| CXCL11 | 0.95 ± 0.24 | 0.79 ± 0.09 | 14.2 ± 2.7 | 13.4 ± 2.3 |
| Secondary infection | ||||
| CXCL9 | 4.2 ± 0.3 | 5.6 ± 0.4* | 14.2 ± 2.5 | 31.0 ± 3.4* |
| CXCL10 | 0.21 ± 0.01 | 0.24 ± 0.05 | 7.5 ± 1.4 | 8.1 ± 0.9 |
| CXCL11 | 0.67 ± 0.05 | 0.67 ± 0.04 | 18.9 ± 4.1 | 17.9 ± 1.7 |
Chemokine concentrations are expressed as ng/ml and pg/mg tissue at day 5 after infection for the spleen and liver, respectively (n = 14 to 16). *, P < 0.01; †, P < 0.001 (versus control).
Anti-CXCL16 antibody production and characterization.
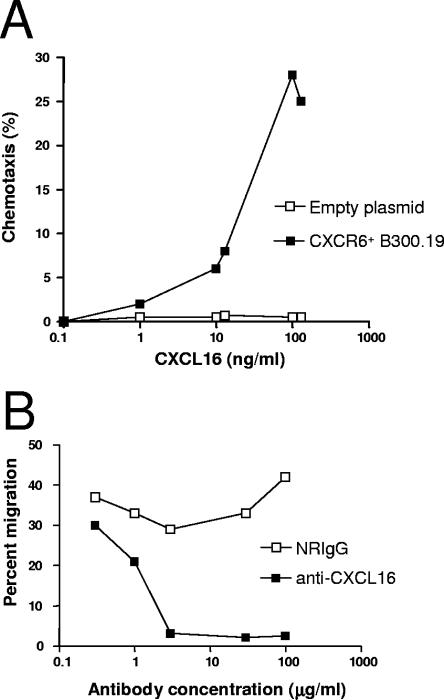
To facilitate the characterization of the neutralizing anti-CXCL16 antibody intended for use in the present study, CXCR6 (sole known receptor for CXCL16) was cloned and expressed in the B300.19 cell line. The cloned CXCR6 cDNA was verified by sequencing (see Materials and Methods; also, data not shown). The biological activity of the CXCR6-expressing cell line was tested in a chemotaxis assay. It responded strongly to soluble murine CXCL16, with the peak migration (∼30% migrating cells) occurring in response to 100 ng of mCXCL16/ml (Fig. 2A). Cells transfected with empty plasmid failed to respond to mCXCL16.
FIG. 2.
Characterization of polyclonal rabbit anti-CXCL16 antibodies. A B300.19 cell line was transfected with a plasmid encoding CXCR6 or an empty plasmid as control. These cell lines were evaluated for their ability to migrate toward soluble CXCL16. (A) A range of CXCL16 concentrations were used, and transfected CXCR6+ cells displayed a classic bell-shape chemotaxis profile with peak migration occurring at 100 ng of CXCL16/ml, while no migration was observed for the control. The ability of polyclonal rabbit anti-CXCL16 antibodies to block CXCL16-induced migration was tested in a chemotaxis assay. (B) Complete inhibition of CXCR6+ cell migration toward CXCL16 was obtained with concentrations of anti-CXCL16 of ≥30 μg/ml. These data are representative of three experiments performed with similar results.
The CXCR6-expressing cell line was used to evaluate the neutralizing ability of a rabbit anti-mCXCL16 antibodies in a chemotaxis assay. As shown in Fig. 2B, when a 50% effective chemotactic concentration of 30 ng of mCXCL16/ml was used, the anti-CXCL16 antibodies inhibited the migration of the CXCR6-expressing cells in a dose-dependent manner compared to control rabbit IgG. Concentrations of 3 μg of anti-CXCL16 antibodies/ml and above entirely neutralized mCXCL16-induced migration.
CXCL16 is involved in the control of bacterial burden.
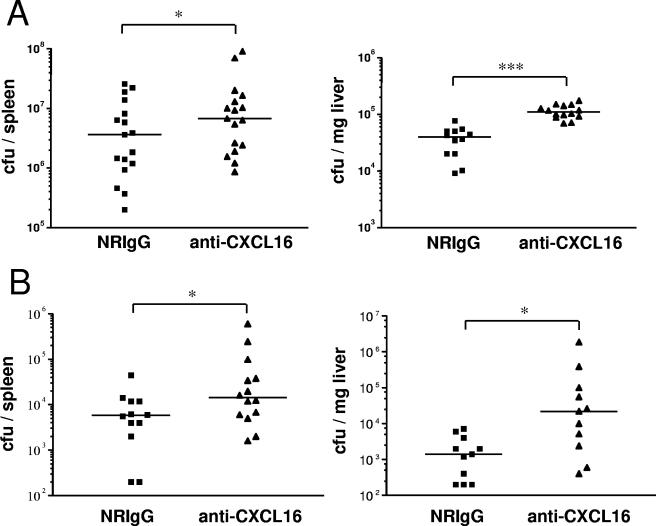
The role of CXCL16 during primary and secondary infections with serovar Enteritidis was further investigated using the neutralizing polyclonal rabbit antibody described above. In order to assess the importance of CXCL16 in the control of bacterial burden, the number of bacteria in the spleens and livers of animals undergoing a primary or secondary infection with serovar Enteritidis and treated with NRIgG or anti-CXCL16 antibody was monitored. As shown in Fig. 3A, neutralization of CXCL16 during primary infection led to a significant increase in the number of bacteria both in the spleen and in the liver compared to the NRIgG-treated control group. Similarly, in the case of secondary infection, anti-CXCL16 treatment led to a significant increase in bacterial burden in both organs compared to the control group (Fig. 3B). In contrast, no difference in bacterial load was observed in the Peyer's patches between the two treatment groups (data not shown).
FIG. 3.
CXCL16 controls the bacterial burden in the spleen and liver. Mice were pretreated with 500 μg of protein A-purified NRIgG or anti-CXCL16 the evening prior to i.p. injection of 3 × 105 CFU of serovar Enteritidis in the case of primary infection (A) and 107 CFU in the case of secondary infection (B), and the numbers of CFU recovered from the spleens and the livers were determined 5 days later. The data are plotted as values from individual mice (n = 12 to 17 and n = 11 to 14 for primary and secondary infections, respectively). The bars represent the median value. Significant differences from NRIgG value: *, P < 0.05; ***, P < 0.001 (Mann-Whitney U test).
CXCL16 is not involved in the humoral immune response to serovar Enteritidis.
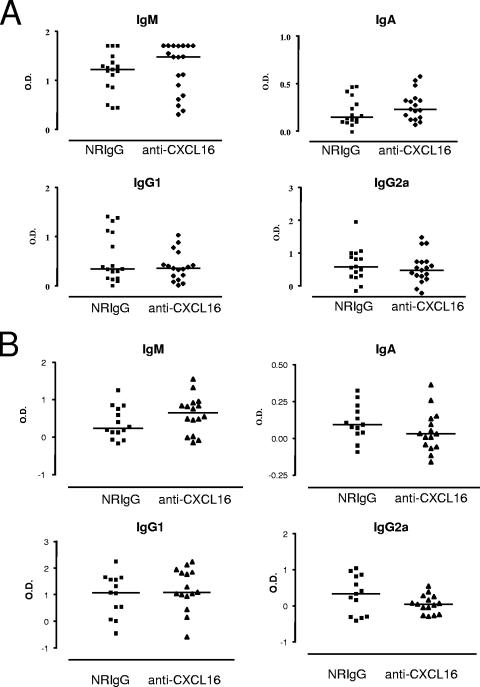
Inhibition of the control of bacterial burden could indicate a role for the CXCL16/CXCR6 system in the humoral and/or cell-mediated immune response to serovar Enteritidis, both of which have been implicated in the control of Salmonella infections (16). The humoral response involves the generation of antibodies against SefA, a major antigen of serovar Enteritidis. As previously observed (5), infected animals treated with the control NRIgG displayed a strong anti-SefA IgM response. Treatment with the anti-CXCL16 antibody prior to primary infection had no detectable effect on the level of anti-SefA IgM generated. The basal levels of anti-SefA IgA, IgG1, and IgG2a isoptypes in infected animals were considerably lower, and no difference was observed between the NRIgG- and anti-CXCL16-treated groups in the primary response (Fig. 4A). In the case of the secondary immune response, measurement of anti-SefA isotype levels in the sera of the animals at day 5 after reinfection revealed low levels of anti-SefA IgM, IgA, and IgG2a but a strong anti-SefA IgG1 response. However, in keeping with the results obtained during the study of the primary immune response, no difference with respect to antibody generation was observed between the NRIgG- and the anti-CXCL16-treated groups (Fig. 4B).
FIG. 4.
CXCL16 is not involved in the regulation of the humoral immune response to serovar Enteritidis. The level of serum antibody against the bacterial surface antigen SefA was assessed by direct ELISA on day 5 after primary (A) and secondary (B) infections. The data are plotted as values for individual mice (n = 16 to 19 for primary infection; n = 13 to 16 for secondary infection) after subtraction of the respective mean values for the control animals (naive mice or 6 weeks after the primary infection; see Materials and Methods). The bars represent the median values.
In parallel with anti-Salmonella antibody isotype measurement, we evaluated the production of IL-4, a cytokine directly involved in the promotion of the humoral immune response. For both the primary and the secondary immune responses, no change in the level of IL-4 was detected in any of the target organs at day 5 postinfection compared to naive controls or between the different antibody treatment groups (data not shown).
CXCL16 regulates the cell-mediated immune response during primary infection with serovar Enteritidis.
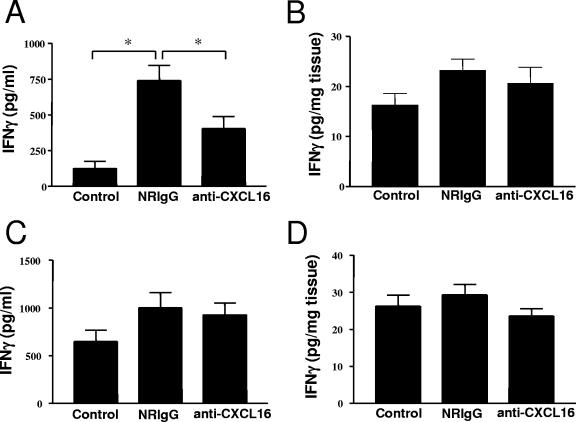
Using the neutralizing antibody treatment approach described above, the importance of CXCL16 in the cell-mediated immune response toward primary and secondary serovar Enteritidis infections was evaluated by measuring the levels of type 1 cytokines IFN-γ and IL-12p70. As previously observed in studies with this model (primary infection) (5), mice infected with serovar Enteritidis and treated with the control antibody (NRIgG) exhibited a strong IFN-γ production in the spleen but not in the liver (or Peyer's patches [data not shown]) compared to naive controls (Fig. 5A and B). In animals receiving anti-CXCL16 treatment, this increase in IFN-γ production in the spleen was significantly reduced by ca. 50% (Fig. 5A). The anti-CXCL16 treatment had no effect on the basal production of IFN-γ in the livers of infected animals (Fig. 5B). In contrast, no IL-12p70 was detected in the spleens or livers of infected mice greater than the level observed in naive mice or between the different antibody treatment groups (data not shown).
FIG. 5.
CXCL16 is involved in the regulation of the cell-mediated immune response to primary serovar Enteritidis infection. Mice were pretreated with 500 μg of protein A-purified NRIgG or anti-CXCL16 the evening prior to primary or secondary infection. The level of IFN-γ was measured in spleen (A, primary infection; C, secondary infection) and liver (B, primary infection; D, secondary infection) homogenates, respectively. The data represent the means ± the SEM (n = 10 to 13). *, Significantly different from the control or NRIgG value at P < 0.05 (Student t test).
In contrast to what we observed during primary infection, no role for CXCL16 was found in the control of cytokine production during the secondary response to Salmonella infection. No change in the level of IFN-γ production between the NRIgG- and the anti-CXCL16-treated groups was found at day 5 after reinfection in either the spleens (Fig. 5C) or the livers (Fig. 5D) of the animals. Of note, the level of IFN-γ in the spleen of immunized controls at 6 weeks after primary infection was still elevated compared to naive controls (Fig. 5A and C). No difference in IL-12p70 concentration was observed in the spleens or livers of reinfected animals compared to controls at 6 weeks after primary infection (data not shown).
CXCL16 regulates lymphocyte-derived IFN-γ production during primary serovar Enteritidis infection in the spleen.
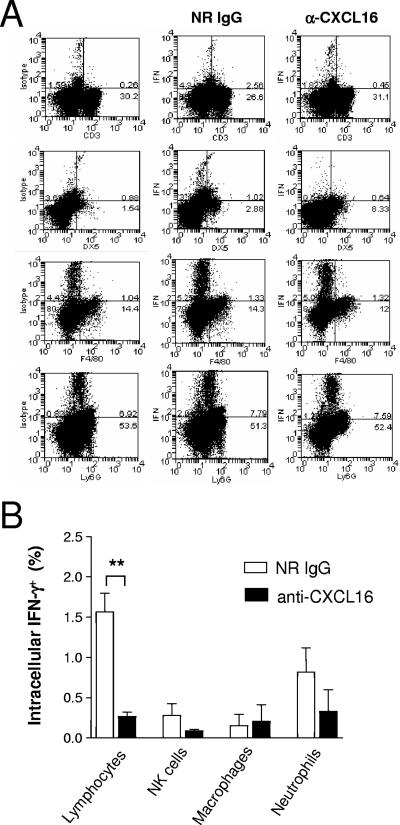
To further investigate the effect of CXCL16 on IFN-γ production during primary serovar Enteritidis infection, single cell suspensions were prepared from the spleens of mice treated with either NRIgG or anti-CXCL16 antibodies 5 days after infection with serovar Enteritidis. The percentage of cells producing IFN-γ was then measured by flow cytometry for the lymphocyte, natural killer (NK) cell, macrophage, and neutrophil subpopulations (Fig. 6A). As shown in Fig. 6B, 1.6% ± 0.2% of the lymphocytes from the spleens of mice treated with control antibodies expressed IFN-γ. In contrast, only 0.3% ± 0.05% of lymphocytes from the spleens of anti-CXCL16-treated mice expressed IFN-γ, representing a reduction of more than 80%. There was no statistical difference in the percentages of IFN-γ+ NK cells, macrophages, or neutrophils of serovar Enteritidis-infected mice treated with anti-CXCL16 compared to NRIgG-treated controls.
FIG. 6.
Anti-CXCL16 treatment inhibits IFN-γ production by spleen lymphocytes in response to primary serovar Enteritidis infection. Mice were pretreated with 500 μg of protein A-purified NRIgG or anti-CXCL16 the evening prior to primary infection with serovar Enteritidis and the level of intracellular IFN-γ was measured in the lymphocyte (CD3+), NK cell (DX5+), macrophage (F4/80+) and neutrophil (Ly6G+) populations of the spleen. (A) Representative dot plots. The upper-right quadrant shows the percentage values for double-positive cells. (B) Percentages of IFN-γ+ cells in the various splenic populations. The data represent means ± the SEM (n = 4). **, Significantly different from the NRIgG value at P < 0.01 (Student t test).
DISCUSSION
In this study, we provide the first in vivo quantitation of expression of the atypical chemokine CXCL16 during an adaptive immune response to bacteria, and we demonstrate a role for this chemokine in the generation of cell-mediated immunity against the gram-negative intracellular bacterium serovar Enteritidis via an effect on lymphocyte-derived IFN-γ production in the spleen. Our data not only provide new information on the biology of CXCL16 but, combined with our previous data (5), they also provide further insight into the role of specific chemokines in the control of Salmonella infection.
In the model used in the present study, the major target organs of serovar Enteritidis dissemination are the spleen, the liver, and the Peyer's patches. In response to primary infection by serovar Enteritidis, massive upregulation of expression of CXCL16 was observed in the spleen and liver but not in the Peyer's patches. The oral infection route is the relevant one for examining effects occurring in Peyer's patches. However, given that we find bacteria in Peyer's patches after i.p. administration, this route could influence expression of chemokines. Indeed, the expression profile is similar to that observed with respect to macrophage inflammatory protein 3α (MIP-3α)/CCL20, which was upregulated in the spleen but not in the Peyer's patches, but in contrast to MIP-1α/CCL3, which was induced in both the spleen and Peyer's patches (5). Together, these data indicate a tissue- and organ-specific regulation of the expression of these chemokines in response to i.p. injected serovar Enteritidis.
Chemokines can be classified according to a system that relates expression to function. In this context, the homeostatic/constitutive chemokines (such as SDF-1/CXCL12 or ELC/CCL19 and SLC/CCL21) are involved in the homeostatic function of the immune system, regulating lymphocyte movement in primary and secondary lymphoid organs, whereas the inflammatory/inducible chemokines (such as CXCL9 to CKCL11, CCL3, RANTES/CCL5, or MCP-1/CCL2 among many others) are involved in recruitment and/or activation of effector cells to peripheral tissues. Our data showing that expression of CXCL16 is strongly upregulated in target organs of animals infected with serovar Enteritidis classify CXCL16 as an inflammatory chemokine in the context of bacterial infection.
The results of our previous study of this model indicated that both arms of the immune response, the cell-mediated and humoral arms, are required for the control of this infection (5). In that study, neutralization of CCL3 inhibited the humoral response, whereas inhibition of CCL20 downregulated the cell-mediated response. Both treatments resulted in an increase in bacterial load, indicating the importance of CCL3 and CCL20, and both type 1 and type 2 immunity. In the present study, neutralization of CXCL16 resulted in an increase in bacterial dissemination in the spleen and the liver during primary infection. This was accompanied by a significant decrease in the level of IFN-γ produced in the spleen, indicating an important role for CXCL16 in the generation of the cell-mediated response against serovar Enteritidis. However, the treatment of mice with anti-CXCL16 had no effect on the generation of humoral immunity. These results indicate that CXCL16 and CCL20 play a similar role in the host response to serovar Enteritidis that is distinct from that of CCL3.
Our data indicating that CXCL16 regulates IFN-γ production and therefore the cell-mediated immunity during primary serovar Enteritidis infection are consistent with the results of previous studies. For instance, CXCR6, the sole known receptor for soluble CXCL16, is selectively expressed on the surface of type 1 CD4+ and CD8+ lymphocytes displaying a cytotoxic effector phenotype (11). Moreover, both soluble and membrane-bound forms of CXCL16 are strongly induced by IFN-γ, in vitro and in vivo (25, 30), and blocking CXCL16 by using a monoclonal antibody also blocks the production of IFN-γ during the course of acute and adoptive experimental autoimmune encephalomyelitis, a type 1-polarized T-cell-mediated autoimmune disease of the central nervous system (6). Together, these previous observations indicate a close relationship between the expression of CXCL16 and IFN-γ. The decrease in the level of IFN-γ production in the spleen observed in the present study could be due to effects on one or more cell types in the spleen. Indeed, primary exposure to Salmonella has been shown to trigger high levels of IFN-γ production by neutrophils and macrophages, as well as by lymphocytes and NKT cells (12). Our results show that blocking CXCL16 prior to the primary infection strongly reduced the ability of lymphocytes in the spleen to produce IFN-γ but not that of neutrophils or macrophages. Therefore, we conclude that CXCL16 has a major role in the intensity and orientation of the immune response and that it is controlling, at least in part, the production of IFN-γ by lymphocytes present in the spleen. At this stage, it is difficult to determine the precise mechanism of action of CXCL16 in this respect, although it may be acting as a costimulator of T-cell activation during antigen presentation, as has been shown for CXCL12 (17).
In the present study, we extended this model to include investigation of the role of CXCL16 in the secondary immune response to serovar Enteritidis. Although the induction of CXCL16 expression was similar to that observed in the primary response, albeit at a reduced magnitude, and the treatment of mice with neutralizing CXCL16 antibodies prior to secondary infection led to increased dissemination of bacteria in the spleen and liver, we observed a marked difference in the link between IFN-γ and CXCL16 when we compared the primary and secondary immune responses to the infection. In the spleens of mice developing an acute primary infection, the induction of IFN-γ was significantly decreased by anti-CXCL16 treatment, suggesting a strong regulatory role for CXCL16 in the cytokine balance and ultimately in establishing a type 1 microenvironment in that target organ during the primary encounter with the bacteria. In contrast, in the secondary response, the level of IFN-γ was not affected by prior treatment of the mice with anti-CXCL16 antibodies. It is established that IFN-γ is pivotal in the immune response to Salmonella infection and necessary for survival (2, 19). However, in keeping with the results of the present study, the comparison of primary and secondary immune responses to Salmonella infection by Kirby et al. demonstrated that the challenge of immune hosts resulted in considerably limited splenic IFN-γ and tumor necrosis factor alpha responses (12). The question then arises as to how anti-CXCL16 treatment is inhibiting the ability of the host to control bacterial dissemination to the spleen and the liver in the secondary response to serovar Enteritidis? The results of our previous studies indicate that humoral immunity is required for the control of this infection (5). However, anti-CXCL16 treatment had no effect on the generation of antibodies to SefA, a major surface antigen of serovar Enteritidis, so this is an unlikely explanation. Moreover, the results of experiments examining the effect of anti-CXCL16 treatment on recruitment of leukocyte subpopulations to the target organs indicate that CXCL16 does not influence the accumulation of lymphocytes, NK cells, macrophages, dendritic cells, or neutrophils in the spleen or liver (data not shown). Of potential relevance is the recent observation that, in addition to its role in the development of IFN-γ-oriented type 1 immune responses, CXCL16 plays a role in the binding and phagocytosis of bacteria (22). As a membrane-bound molecule expressed at the surface of macrophages and dendritic cells, the chemokine domain of CXCL16 specifically supports the uptake of both gram-positive and gram-negative bacteria. It is therefore possible that neutralization of CXCL16 in the secondary response is inhibiting host control of the bacteria via a direct effect on phagocytosis.
Besides CXCL16, other chemokines have been associated with type 1 IFN-γ-driven immune responses, including CXCL9, CXCL10, and CXCL11, the ligands for the CXCR3 chemokine receptor (3, 15). We therefore measured the level of CXCL9, CXCL10, and CXCL11 in the target organs during primary and secondary infections. The expression of CXCL9 was significantly upregulated during primary and secondary responses, both in the spleen and liver, whereas no change in the level of CXCL10 or CXCL11 was observed. The absence of stimulation of CXCL10 and CXCL11 production at day 5 postinfection is surprising, given their known dependence upon IFN-γ (21). Previous studies have shown that CXCL11 is expressed by macrophages in vitro after exposure to gram-negative bacteria, including S. enterica serovar Typhimurium (18), whereas CXCL10 production is increased in osteoblast cultures infected with Salmonella (8). However, the CXCR3 ligands demonstrate differential involvement in vivo in the control of the immune response to various gram-negative infections. Indeed, while CXCL10 has been shown to be comparatively more important than CXCL9 in the clearance of Klebsiella pneumoniae (31), both CXCL9 and CXCL10 levels were found to be markedly elevated in patients diagnosed with melioidosis, a severe gram-negative infection caused by the bacillus Burkholderia pseudomallei (14). Interestingly, all three CXCR3 ligands levels were increased in a murine model of intrapulmonary challenge with the gram-negative Bordetella bronchiseptica, and clearance was decreased in CXCR3−/− mice (26). These previous data therefore indicate that all three CXCR3 ligands are expressed during host responses to bacteria. However, they also indicate that the expression of these ligands is differentially regulated depending on the bacterial pathogen. In keeping with this, our results suggest that in the case of serovar Enteritidis infection, CXCL9 might be the key ligand for the recruitment and activation of CXCR3-expressing leukocytes, likely activated Th1 lymphocytes, monocytes, NK, and/or NKT cells (15). Further experiments are needed to analyze the exact role of CXCL9 and its potential synergy with the CXCL16/CXCR6 system in the control of serovar Enteritidis infection and the establishment of the cell-mediated immune response. In the same context, this model will be of interest for analyzing the possible role of proinflammatory cytokines, such as tumor necrosis factor alpha and IL-1β, in association with the modulation of CXCL16, CXCL9, and IFN-γ described here.
In summary, we demonstrate an important role for CXCL16 in the control of serovar Enteritidis infection. We show that CXCL16 is involved in the regulation of cell-mediated immunity via the production of IFN-γ during the primary response to the bacterial infection. We also show that CXCL16 is involved in the control of bacterial burden in the secondary immune response, although this is not due to a detectable effect on IFN-γ production. The difference in the mechanism of action of CXCL16 in the primary and secondary immune responses to serovar Enteritidis is likely due, at least in part, to the unique dual nature of CXCL16 as a soluble chemokine and as a membrane-bound scavenger receptor, respectively.
Editor: F. C. Fang
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Attridge, S. R., and I. Kotlarski. 1985. Local transfer of delayed-type hypersensitivity after Salmonella infection in mice. Infect. Immun. 50:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao, S., K. W. Beagley, M. P. France, J. Shen, and A. J. Husband. 2000. Interferon-gamma plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology 99:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole, K. E., C. A. Strick, T. J. Paradis, K. T. Ogborne, M. Loetscher, R. P. Gladue, W. Lin, J. G. Boyd, B. Moser, D. E. Wood, B. G. Sahagan, and K. Neote. 1998. Interferon-inducible T-cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high-affinity binding to CXCR3. J. Exp. Med. 187:2009-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Depaolo, R. W., R. Lathan, B. J. Rollins, and W. J. Karpus. 2005. The chemokine CCL2 is required for control of murine gastric Salmonella enterica infection. Infect. Immun. 73:6514-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahy, O. L., S. L. Townley, N. J. Coates, I. Clark-Lewis, and S. R. McColl. 2004. Control of Salmonella dissemination in vivo by macrophage inflammatory protein (MIP)-3α/CCL20. Lab. Investig. 84:1501-1511. [DOI] [PubMed] [Google Scholar]
- 6.Fukumoto, N., T. Shimaoka, H. Fujimura, S. Sakoda, M. Tanaka, T. Kita, and S. Yonehara. 2004. Critical roles of CXC chemokine ligand 16/scavenger receptor that binds phosphatidylserine and oxidized lipoprotein in the pathogenesis of both acute and adoptive transfer experimental autoimmune encephalomyelitis. J. Immunol. 173:1620-1627. [DOI] [PubMed] [Google Scholar]
- 7.Gale, L. M., and S. R. McColl. 1999. Chemokines: extracellular messengers for all occasions? Bioessays 21:17-28. [DOI] [PubMed] [Google Scholar]
- 8.Gasper, N. A., C. C. Petty, L. W. Schrum, I. Marriott, and K. L. Bost. 2002. Bacterium-induced CXCL10 secretion by osteoblasts can be mediated in part through Toll-like receptor 4. Infect. Immun. 70:4075-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gough, P. J., K. J. Garton, P. T. Wille, M. Rychlewski, P. J. Dempsey, and E. W. Raines. 2004. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J. Immunol. 172:3678-3685. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs, S., S. Jitrapakdee, and J. C. Wallace. 1998. Development of a bicistronic vector driven by the human polypeptide chain elongation factor 1alpha promoter for creation of stable mammalian cell lines that express very high levels of recombinant proteins. Biochem. Biophys. Res. Commun. 252:368-372. [DOI] [PubMed] [Google Scholar]
- 11.Kim, C. H., E. J. Kunkel, J. Boisvert, B. Johnston, J. J. Campbell, M. C. Genovese, H. B. Greenberg, and E. C. Butcher. 2001. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J. Clin. Investig. 107:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirby, A. C., U. Yrlid, and M. J. Wick. 2002. The innate immune response differs in primary and secondary Salmonella infection. J. Immunol. 169:4450-4459. [DOI] [PubMed] [Google Scholar]
- 13.Kohler, R. E., A. C. Caon, D. O. Willenborg, I. Clark-Lewis, and S. R. McColl. 2003. A role for macrophage inflammatory protein-3alpha/CC chemokine ligand 20 in immune priming during T cell-mediated inflammation of the central nervous system. J. Immunol. 170:6298-6306. [DOI] [PubMed] [Google Scholar]
- 14.Lauw, F. N., A. J. Simpson, J. M. Prins, S. J. van Deventer, W. Chaowagul, N. J. White, and T. van der Poll. 2000. The CXC chemokines gamma interferon (IFN-γ)-inducible protein 10 and monokine induced by IFN-γ are released during severe melioidosis. Infect. Immun. 68:3888-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loetscher, M., B. Gerber, P. Loetscher, S. A. Jones, L. Piali, I. Clark-Lewis, M. Baggiolini, and B. Moser. 1996. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T lymphocytes. J. Exp. Med. 184:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittrucker, H. W., and S. H. Kaufmann. 2000. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67:457-463. [DOI] [PubMed] [Google Scholar]
- 17.Nanki, T., and P. E. Lipsky. 2000. Cutting edge: stromal cell-derived factor-1 is a costimulator for CD4+ T-cell activation. J. Immunol. 164:5010-5014. [DOI] [PubMed] [Google Scholar]
- 18.Nau, G. J., A. Schlesinger, J. F. Richmond, and R. A. Young. 2003. Cumulative Toll-like receptor activation in human macrophages treated with whole bacteria. J. Immunol. 170:5203-5209. [DOI] [PubMed] [Google Scholar]
- 19.Nauciel, C., and F. Espinasse-Maes. 1992. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect. Immun. 60:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogunniyi, A. D., P. A. Manning, and I. Kotlarski. 1994. A Salmonella enteritidis 11RX pilin induces strong T-lymphocyte responses. Infect. Immun. 62:5376-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauty, A., M. Dziejman, R. A. Taha, A. S. Iarossi, K. Neote, E. A. Garcia-Zepeda, Q. Hamid, and A. D. Luster. 1999. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J. Immunol. 162:3549-3558. [PubMed] [Google Scholar]
- 22.Shimaoka, T., T. Nakayama, N. Kume, S. Takahashi, J. Yamaguchi, M. Minami, K. Hayashida, T. Kita, J. Ohsumi, O. Yoshie, and S. Yonehara. 2003. Cutting edge: SR-PSOX/CXC chemokine ligand 16 mediates bacterial phagocytosis by APCs through its chemokine domain. J. Immunol. 171:1647-1651. [DOI] [PubMed] [Google Scholar]
- 23.Ushiba, D., K. Saito, T. Akiyama, M. Nakano, T. Sugiyama, and S. Shirono. 1959. Studies on experimental typhoid: bacterial multiplication and host cell response after infection with Salmonella enteritidis in mice immunized with live and killed vaccines. Jpn. J. Microbiol. 3:231-242. [DOI] [PubMed] [Google Scholar]
- 24.Velge, P., A. Cloeckaert, and P. Barrow. 2005. Emergence of Salmonella epidemics: the problems related to Salmonella enterica serotype Enteritidis and multiple antibiotic resistance in other major serotypes. Vet. Res. 36:267-288. [DOI] [PubMed] [Google Scholar]
- 25.Wagsater, D., P. S. Olofsson, L. Norgren, B. Stenberg, and A. Sirsjo. 2004. The chemokine and scavenger receptor CXCL16/SR-PSOX is expressed in human vascular smooth muscle cells and is induced by interferon gamma. Biochem. Biophys. Res. Commun. 325:1187-1193. [DOI] [PubMed] [Google Scholar]
- 26.Widney, D. P., Y. Hu, A. K. Foreman-Wykert, K. C. Bui, T. T. Nguyen, B. Lu, C. Gerard, J. F. Miller, and J. B. Smith. 2005. CXCR3 and its ligands participate in the host response to Bordetella bronchiseptica infection of the mouse respiratory tract but are not required for clearance of bacteria from the lung. Infect. Immun. 73:485-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilbanks, A., S. C. Zondlo, K. Murphy, S. Mak, D. Soler, P. Langdon, D. P. Andrew, L. Wu, and M. Briskin. 2001. Expression cloning of the STRL33/BONZO/TYMSTRligand reveals elements of CC, CXC, and CX3C chemokines. J. Immunol. 166:5145-5154. [DOI] [PubMed] [Google Scholar]
- 28.Withanage, G. S., P. Kaiser, P. Wigley, C. Powers, P. Mastroeni, H. Brooks, P. Barrow, A. Smith, D. Maskell, and I. McConnell. 2004. Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar Typhimurium. Infect. Immun. 72:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Withanage, G. S., P. Wigley, P. Kaiser, P. Mastroeni, H. Brooks, C. Powers, R. Beal, P. Barrow, D. Maskell, and I. McConnell. 2005. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 73:5173-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wuttge, D. M., X. Zhou, Y. Sheikine, D. Wagsater, V. Stemme, U. Hedin, S. Stemme, G. K. Hansson, and A. Sirsjo. 2004. CXCL16/SR-PSOX is an interferon-gamma-regulated chemokine and scavenger receptor expressed in atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 24:750-755. [DOI] [PubMed] [Google Scholar]
- 31.Zeng, X., T. A. Moore, M. W. Newstead, J. C. Deng, S. L. Kunkel, A. D. Luster, and T. J. Standiford. 2005. Interferon-inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect. Immun. 73:8226-8236. [DOI] [PMC free article] [PubMed] [Google Scholar]