GUILLAIN-BARRÉ SYNDROME
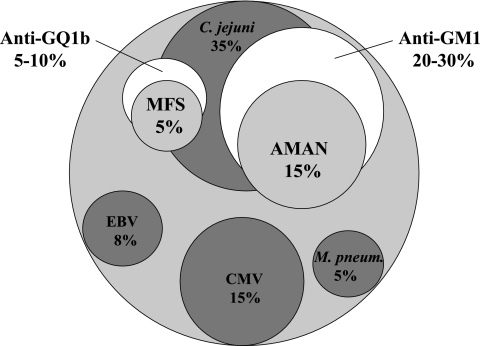
Guillain-Barré syndrome (GBS) is the most frequent cause of acute flaccid paralysis in humans, occurring with an annual incidence of 1 to 2 cases per 100,000 people. In recent years, studies have shed new light on a number of disease aspects that have enhanced the understanding of the pathogenic mechanisms of GBS. GBS is an acute inflammatory polyradiculoneuropathy that usually develops following a gastrointestinal infection. Clinical symptoms often occur 1 to 3 weeks after a bacterial or viral infection. The most commonly identified triggering agents are Campylobacter jejuni (in 13% to 39% of cases), followed by cytomegalovirus (5% to 22%), Epstein-Barr virus (1% to 13%), and Mycoplasma pneumoniae (5%), as shown in Fig. 1 (31, 70). All of these pathogens have carbohydrate sequences (antigens) in common with peripheral nerve tissue.
FIG. 1.
Preceding infections and generation of antibodies against gangliosides in GBS. The relationships among preceding infection by bacteria or viruses, antibodies against gangliosides, and GBS types (AMAN and Miller-Fisher syndrome [MFS]) are illustrated. Infections with C. jejuni, CMV, Epstein-Barr virus (EBV), and M. pneumoniae and GBS-linked antibodies against gangliosides GM1/GD1b/asialo-GM1 and GQ1b/GT1a, as well as AMAN and MFS types, are shown with the percent frequency (modified from reference 70 with permission of the publisher).
GBS is recognized as several disorders characterized by an immune-mediated attack on peripheral nerve, particularly in the myelin sheath or Schwann cells of sensory and motor nerves. These disorders are primarily classified as acute or chronic inflammatory demyelinating polyneuropathy (AIDP or CIDP), acute motor axonal neuropathy (AMAN), and acute motor and sensory axonal neuropathy (36). Although genetic predisposition has not been fully established, the AMAN type of the disease occurs more commonly in Japan and China than in North America or Europe. Pathological studies of patients with AIDP and CIDP reveal signs of primary injury to myelin in the peripheral nervous system, whereas in patients with AMAN the injury is largely confined to motor axons derived via a noninflammatory, antibody-mediated, complement-dependent mechanism.
Much of the research into GBS over the last decade has focused on the forms mediated by antiganglioside antibodies (8, 9, 88). The sera of approximately 60% of patients with GBS contain a variety of anti-glycosphingolipid (GSL) antibodies. Care, however, must be exercised in assessing the antibody data. For example, Kaida et al. (41) reported that 8 of 100 patients with GBS had no antibody reactivity, as assessed by enzyme-linked immunosorbent assay, against purified gangliosides, including GD1a and GD1b. However, after they applied a crude mixture of whole-brain gangliosides by thin-layer chromatography and overlaid the thin-layer chromatographic plate with the “antibody-negative” GBS sera, they found a strong immunoreactive band migrating between GD1a and GD1b, suggesting that the sera contained an antibody species that reacted with GD1a and GD1b in a complex form but not with either purified ganglioside alone. This result indicates that “antibody-negative” GBS sera may also react with gangliosides that are present in the form of a GD1a-GD1b ganglioside complex. Those authors observed similar results for GD1a-GM1, GM1-GT1b, and GD1b-GT1b. Thus, in sera that are antibody negative it is necessary to examine the antibody activity by appropriate ganglioside complexes and suitable methods, such as the use of liposome-incorporated GSLs. Nonetheless, measurements of these GSL antibody titers remain the most effective and reliable means for the diagnosis of GBS and in evaluating the effectiveness of treatments in clinical trials (8, 9, 87).
GANGLIOSIDE MOLECULAR MIMICRY
GBS is considered an autoimmune disease, with the immune system mistakenly attacking myelin or axons, the nerve conduits for signals to and from the brain (32). This “mistaken immune attack” may arise because the surface of C. jejuni contains polysaccharides that resemble glycoconjugates of the human nerve tissues. This resemblance has been termed “molecular mimicry,” which is defined as the dual recognition, by a single B- or T-cell receptor, of a microbe's structure and an antigen of the host, and is the mechanism by which infections trigger cross-reactive antibodies or T cells that can lead to autoimmune diseases (6).
As stated previously, GBS is recognized as several disorders characterized by immune-mediated attack on peripheral nerve. In AIDP, the myelin sheath and Schwann cells of sensory and motor nerves are targeted. AMAN and acute motor and sensory axonal neuropathy are associated with antibodies against the ganglioside component of the nerve axolemmal membrane (Fig. 2) (75, 87). Immune responses against gangliosides are suspected to originate as a result of molecular mimicry between gangliosides and lipopolysaccharides (LPSs) of C. jejuni, the most frequent infectious trigger of GBS (70). Evidence of this concept came from Moran et al. (57), who reported that the sera of rabbits immunized with ganglioside-mimicking C. jejuni lipooligosaccharides (LOSs) revealed high titers of anti-LOS antibodies that were cross-reactive with a panel of gangliosides. In addition to the above, molecular mimicry between microbial antigens and host tissues forms an attractive hypothetical mechanism for the triggering of autoimmune diseases.
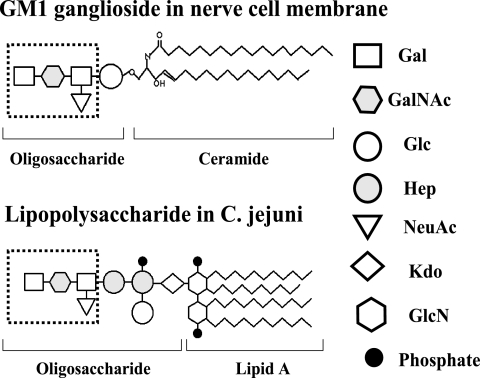
FIG. 2.
Molecular mimicry of gangliosides and C. jejuni lipopolysaccharides. Gangliosides are highly expressed in nerve cell membranes and consist of a ceramide portion and a polar head group that contains glucose (Glc), galactose (Gal), N-acetylgalactosamine (GalNAc), and N-acetylneuraminic acid (NeuAc). Lipooligosaccharide-containing ganglioside mimics are located in the outer part of the cell wall of C. jejuni. The biochemical structures of gangliosides play a crucial role in the pathogenesis of GBS.
Concerning the nature of the oligosaccharides in C. jejuni, Moran and Predergast (56) described the isolation of heat-stable (HS) antigens from C. jejuni and reported the occurrence of low-molecular-weight molecules comprised of core oligosaccharides (OS) and lipid A but devoid of O antigen chains (called LOS). One-third of C. jejuni serotypes contained high- to low-molecular-weight components that included the O antigen chain (called LPS). Thus, both LOS and LPS have been described for C. jejuni.
In contrast to the mimicry hypothesis, the appearance of naturally occurring antibodies that recognize glycans, such as the Forssman and blood group antigens, can also occur in response to intestinal or respiratory tract bacteria (76, 93). Alaniz et al. (1) presented evidence indicating a similar origin for immunoglobulin M (IgM) anti-GM1 antibodies in healthy humans. Anti-GM1 antibodies were purified from adult sera by affinity chromatography, and their ability to bind LPSs from different bacteria was tested. This highly specific preparation showed reactivity only with LPS from a strain of C. jejuni isolated from a patient with diarrhea. We conclude that normally occurring IgM antibodies are generated after birth, probably during the immune defense against specific bacterial strains.
OLIGOSACCHARIDES OF LPSs THAT MIMIC GANGLIOSIDE STRUCTURES
Increased titers of IgG anti-GM1 antibodies are associated with GBS, which in some cases is preceded by infection with C. jejuni, particularly with the Penner (PEN) 19 strain, which expresses LPS with GM1-like oligosaccharides. This raises the possibility that antiganglioside antibodies and subsequent neuropathies result from cross-reactivity to C. jejuni LPSs via molecular mimicry (48, 96, 104). Initially, Yuki et al. (96, 98) reported that the LPS of C. jejuni isolated from a patient with GBS had a GM1-like structure, suggesting that the GM1 epitope-bearing LPS may function in the production of anti-GM1 antibodies in this patient. Several serotypes of C. jejuni LPSs were reported to involve ganglioside mimicry. Anti-GM1 and anti-GD1a antibodies reacted with the LPSs of PEN 1, 4, and 19 (10, 97a). They found that the LPS fraction of C. jejuni (PEN 4) has an LPS that bears a GM1 epitope as well as an LPS that bears a GD1a epitope. However, Aspinall et al. (10) reported that the LPS of PEN 4 has a GD1a ganglioside-like structure rather than a GM1-like structure. The LPS core oligosaccharide from C. jejuni O:41 isolated from patients with GBS, now named heat-stable antigen 41 (HS:41), had GM1- and asialo-GM1-like structures (63). In addition, an LPS containing an epitope similar to GM1b was identified from C. jejuni isolated from a GBS patient with high anti-GM1b antibody titers (101). C. jejuni strains isolated from patients with Miller-Fisher syndrome had an LOS epitope mimicking GQ1b, GT1a, or GD3 (11, 67, 97). Isolates from patients with GBS more frequently had a GM1-like epitope than did isolates from patients with Miller-Fisher syndrome. GQ1b-like epitopes are present in all Miller-Fisher syndrome-associated isolates and are associated with anti-GQ1b antibody reactivity (4). More than 90% of patients with Miller-Fisher syndrome have sera with anti-GQ1b and anti-GT1a antibodies, which may also react with other disialylated gangliosides, including GD3 and GD1b. Structural studies on LPSs from neuropathy-associated C. jejuni strains have revealed GT1a-like and GD3-like core oligosaccharides (27). The LPS cores of a C. jejuni strain of serotype HS:10 isolated from a patient with Miller-Fisher syndrome have a terminal trisaccharide epitope of GD3 ganglioside (67). This trisaccharide epitope is also present in LPS cores of serotype HS:19 strains from patients with GBS (67). Goodyear et al. (27) reported that the OH4382 isolate (HS:19 serotype) and the PG836 isolate (HS:10 serotype) have an LPS that bears a GD3 ganglioside-like structure and that OH4384 has an LPS that bears a GT1a-like structure. C. jejuni, including the Miller-Fisher syndrome-associated serotypes HS:2 and HS:23, had GT1a-like and GQ1b-like oligosaccharides (59). A population of cells of C. jejuni strain 81-176 produced a mixture of LPS cores that consisted primarily of structures mimicking GM2 and GM3 gangliosides, with significantly fewer structures mimicking GD1b and GD2 (29). Anti-GQ1b IgG antibody was detected in most Miller-Fisher patients infected with C. jejuni or Haemophilus influenzae. Mass spectrometric analysis identified a C. jejuni strain (CF93-6) carrying a GT1a-like LOS that had been isolated from a patient with Miller-Fisher syndrome (44). In addition, strain OH4384 had an LPS structure that bore the GD3 or GQ1b epitope (103). The common component of GT1a and GD3 is a trisaccharide, NeuAcα2-8NeuAcα2-3Galβ1. Yuki et al. (103) reported that the GD3 trisaccharide is not essential for the development of GBS and Miller-Fisher syndrome. Ritter et al. (66) reported that C. jejuni serotypes HS:1, HS:23, and HS:36 have a GM2-like oligosaccharide structure, GalNAcβ1-4Gal(NeuAc)-Hex. C. jejuni serotype HS:19 has a GM1-like oligosaccharide in the LPS. The core oligosaccharide structures of C. jejuni LPSs from serotypes HS:1, HS:2, HS:4, HS:10, HS:19, HS:23, HS:36, and HS:41 have structures that mimic human gangliosides, including GM1, GD1a, GD2, GD3, and GM2 (57, 64). In addition, cross-reactivity of antibodies to asialo-GM2 and LOS of a C. jejuni strain from a GBS patient has been demonstrated (26). Interestingly, Watanabe et al. (86) reported that an LPS of Brucella melitensis had a GM1 ganglioside-like structure and that B. melitensis is a new etiological agent for GBS. Immunization of mice with B. melitensis induced mice to show flaccid limb weakness and ataxia-like symptoms that may be due to molecular mimicry between B. melitensis LPS containing GM1 ganglioside-like structures and peripheral nerve gangliosides. The above observations are summarized in Table 1.
TABLE 1.
Molecular mimicry between bacterial LPSs and gangliosides
| Species and Penner serotypea | Reference(s) reporting mimicry with ganglioside antigen
|
Other antigen with mimicry (reference no.) | ||||||
|---|---|---|---|---|---|---|---|---|
| GM1 | GD1a | GQ1b | GT1a | GD3 | GM2 | GD2 | ||
| C. jejuni serotypes | ||||||||
| HS:1 | 10, 57, 64 | 10, 57, 64 | 57, 64, 66 | 57, 64 | 57, 64 | |||
| HS:2 | 57, 66 | 57, 64 | 59 | 59 | 57, 64 | 57, 64 | 57, 64 | |
| HS:2 (CF93-6) | 97 | 44 | ||||||
| HS:4 | 10, 57, 64 | 10, 57, 64, 97a | 57, 64 | 57, 64 | 57, 64 | |||
| HS:10 | 57, 64 | 57, 64 | 57, 67, 64 | 57, 64 | ||||
| HS:10 (PG836) | 27 | 57, 64 | ||||||
| HS:19 | 4, 10, 48, 57, 64, 80, 96, 103 | 10, 11, 57, 64 | 57, 64, 80 | 57, 64 | ||||
| HS:19 (OH4382) | 11, 27 | |||||||
| HS:19 (OH4384) | 11 | 103 | 11, 27, 67, 103 | 102 | ||||
| HS:23 | 57, 64 | 57, 64 | 59 | 59 | 57, 64 | 57, 64, 66 | 57, 64 | |
| HS:23/HS:36 (81-176) | 29 | GM3 (29) | ||||||
| GD 1b (29) | ||||||||
| HS:36 | 57, 64 | 57, 64 | 57, 64 | 57, 64, 66 | 57, 64 | |||
| HS:41 | 57, 64, 66 | 57, 64 | 57, 64 | 57, 64 | 57, 64 | GA1 (66) | ||
| C. coli | 20 | 20 | GA1 (12) | |||||
| B. melitensis | 86 | |||||||
The serotypes of C. jejuni were determined via Penner's method with heat-stable (HS; O-antigen) serotyping.
With respect to the involvement of other Campylobacter species, Berusudsky et al. (12) isolated Campylobacter coli from a patient with severe, axonal-type GBS who had a high titer of IgG antibodies to asialo-GM1 (GA1) and GD3. They found that the LOS of C. coli induced anti-GA1 antibodies in a rat model. Thus, ganglioside cross-reactivity is not unique to C. jejuni and seems to occur in all bacterial isolates from GBS patients.
In contrast, Funakoshi et al. (20) recently isolated C. coli from two GBS patients who had anti-GM1 and anti-GD1a IgG antibodies. They found C. coli was also the causative agent for GBS but had very weak reactivities with anti-GM1 and -GD1a IgG antibodies compared to those of some GBS-related C. jejuni isolates. These two antibodies were not absorbed by the LOSs of their isolates as were those of GBS-related C. jejuni strains. These findings do not support the hypothesis of ganglioside mimicry on C. coli isolates' LOSs. In addition, a serological assay of recent C. coli infection in GBS and Miller-Fisher syndrome using the bacterial outer membrane protein as antigen revealed that 11% of GBS and 8% of Miller-Fisher patients had two or three classes of IgG, IgM, and IgA anti-C. coli antibodies. Anti-C. jejuni IgG and IgA antibody titers were significantly higher than those of anti-C. coli. This suggests that anti-C. coli antibodies may cross-react with C. jejuni proteins. Despite these uncertainties, the available data strongly favor the concept that there is an association between bacterial LPSs and nervous system gangliosides and that ganglioside mimicry may play an important role in the immunopathogenic mechanisms of GBS and related diseases.
OLIGOSACCHARIDES OF LPSs OR OTHER MOLECULES THAT MIMIC NONGANGLIOSIDE STRUCTURES
With respect to other etiological agents that may be involved in molecular mimicry in the development of GBS, Ilyas et al. (38), Terryberry et al. (78), and Yuki et al. (99) reported that the occurrence of anti-L2/HNK-1 (sulfated glucuronyl paragloboside [SGPG]) or sulfatide antibodies was more frequent in GBS patients than controls and could be important in the pathogenesis of neuropathy in some GBS patients. Human peripheral nerves contain specific glycolipid antigens, e.g., SGPG and sulfated glucuronyl lactosaminyl paragloboside, that share a common carbohydrate epitope with myelin-associated glycoproteins and other cell adhesion molecules (7, 18). These glycolipids are target antigens in demyelinating neuropathies. We first developed animal models of neuropathies by sensitizing rabbits and rats with SGPG (46, 52, 92). The sensitized animals developed clear clinical signs of neuropathies characterized by signs of delayed conduction velocity and conduction block. These models are extremely interesting and have been used extensively for studying the pathogenetic mechanisms of GBS (9).
Cytomegalovirus (CMV) infection also is a candidate for the pathogenesis of GBS (40). The infection frequently results in sensory disturbances and paresthesia of facial nerves. Visser et al. (85) noted the relationship between CMV infection and paralysis of respiratory muscle. In some patients with GBS, elevated IgM anti-CMV antibodies and high titers of IgM and IgG anti-GM2 antibodies have been reported (39). Sera from infants with symptomatic congenital CMV infection have also been shown to have anti-SGPG antibody activity, suggesting that human CMV infection may lead to the induction of anti-SGPG antibodies (60). Further studies showed that a strong correlation of anti-MAG/SGPG-positive chronic polyneuropathy exists with the presence of serum CMV DNA, suggesting that CMV infection may induce the IgM anti-MAG/SGPG antibody (100). In contrast, Sheikh et al. (71) reported that the LPS isolated from a patient with AMAN following C. jejuni infection had the L2/HNK-1 (SGPG) epitope, suggesting another type of molecular mimicry between C. jejuni LPS and the SGPG epitope. In fact, Simon-Haldi et al. (74) isolated peptide mimics of the L2/HNK-1 carbohydrate epitope. The use of recombinant DNA techniques to obtain small peptide mimics that regulate carbohydrate epitopes may offer an alternative strategy for the treatment of certain GSL antibody-mediated neurological disorders (9).
In addition, Mycoplasma pneumoniae infection may be another candidate for the pathogenesis of chronic polyneuropathy in GBS (94) in which the galactosyl β-1-3-N-acetylgalactosaminyl moiety in GA1 could be a target antigen in these patient. Serum IgG reacting with this epitope may be involved in the pathogenesis of both motor and sensory neuropathies in the patients. Galactosylceramide (GalCer) is a major GSL in nervous tissues, particularly in myelin. High titers of anti-GalCer antibodies have been found in certain patients with GBS following M. pneumoniae infection (47). At present, the carbohydrate structures in M. pneumoniae are not clearly understood, but it is possible that involvement of a GA1-like epitope or a GalCer-like epitope might be involved as a causative component in the pathogenesis of certain GBS cases.
The O-chains of a number of Helicobacter pylori strains exhibit mimicry of Lewisx and Lewisy blood group antigens. The role of this mimicry remains to be investigated but may play a role in bacterial camouflage, the induction of autoimmunity, and immune suppression in H. pylori-associated disease (55).
ANIMAL MODELS AND GANGLIOSIDE MIMICRY AFFECTING FUNCTION OF THE NEUROMUSCULAR JUNCTION
Immunization of animals with GBS-associated C. jejuni LPS can help prove whether antibodies reactive to gangliosides are induced and if the antibodies have pathogenic potential. Ang et al. (2) immunized rabbits with C. jejuni LPS from GBS-associated strains containing a GM1-like epitope. All of the rabbits produced high titers of anti-LPS antibodies that were cross-reactive with GM1, suggesting that C. jejuni strains from patients with GBS are able to induce antibodies that cross-react with GM1 and LPS. In addition, rabbits injected with LPS from one Miller-Fisher syndrome-related C. jejuni strain produced anti-GQ1b antibodies (3). All of the rabbits had elevated anti-LPS antibodies of the IgM and IgG classes, and the specificity of the cross-reactive antiganglioside response indeed corresponded with the biochemically defined mimics (5). Yuki et al. (105) reported the direct link between LPSs and the pathogenesis of GBS and demonstrated that rabbits sensitized with C. jejuni LPS developed anti-GM1 IgG antibodies and subsequent flaccid limb weakness. The rabbits also showed pathological changes in their peripheral nerves identical to changes in GBS. This observation lends strong support to the hypothesis that ganglioside mimicry is an important cause of autoimmune neuropathy. Bowes et al. (13) compared the generation of antiganglioside and anti-LPS antibodies in normal mice and GalNAc-transferase (GalNAcT) knockout mice that lack all complex gangliosides and instead express high levels of GM3 and GD3, following intraperitoneal immunization with either complex gangliosides or ganglioside-mimicking LPS. In normal mice, antibody responses to gangliosides and LPS are weak but can be enhanced by antigen format and coadministration of adjuvant to recruit T-cell help. In GalNAcT knockout mice that lack all complex gangliosides and instead express high levels of GM3 and GD3, generation of antiganglioside antibodies upon immunization with either complex gangliosides or ganglioside-mimicking LPS was greatly enhanced and exhibited class switching to T-cell-dependent IgG isotypes and immunological memory, indicating that tolerance to self-gangliosides is a major regulatory factor. These data provide strong support for the molecular mimicry hypothesis as a mechanism for inducing cross-reactive pathogenic anti-ganglioside/LPS antibodies in postinfectious neuropathies.
In contrast to the above experiments, Wirguin et al. (90) subcutaneously immunized rats, mice, and immunodeficient mice lacking in NK, CD8+, or T-cell populations with 20 μg of antigen (LPS of C. jejuni, HS:19, or GM1) emulsified in Freund's complete adjuvant and boosted on day 21 with the same dose in incomplete adjuvant. Blood samples were collected before immunization and on days 14, 28, 41, and 48 and tested by enzyme-linked immunosorbent assay for anti-keyhole limpet hemocyanin (KLH), GM1, GA1, and galactosyl ceramide antibodies. None of these animals developed significant anti-GM1 titers. Anti-GM1 antibody titers developed only after these animals were given an intraperitoneal injection of C. jejuni LPS. It should be kept in mind, however, that immunization is frequently accompanied by administration of an adjuvant such as KLH to enhance immune responses to the administered antigens. In fact, in lectin- and antibody-binding studies, Wirguin et al. (89) demonstrated that native KLH contains Gal(β1-3)GalNAc-bearing oligosaccharides and that immunization with KLH itself in Lewis rats induced the production of anti-Gal(β1-3)GalNAc antibodies. These results suggest the possibility that a glycoprotein-antigenic stimulus can induce B cells that are autoreactive to gangliosides but remain anergic (90).
The neuromuscular junction (NMJ) is rich in gangliosides, lies outside the blood-nerve barrier, and is an important site of antibody-mediated autoimmune diseases (61). In vitro electrophysiological evidence suggests muscle weakness may be affected via the action of antibodies on the NMJ (14). Antiganglioside antibodies may facilitate studies on the role of gangliosides in function by inducing specific structural and functional changes in NMJ. In pure motor chronic demyelinating polyneuropathy, the monoclonal IgM antibody binds specifically to the complex gangliosides GM2, GalNAc-GD1a, and GalNAc-GM1b, which share a common epitope of GalNAcβ1-4Gal(3-2αNeuAc)β1, and these gangliosides are localized in specific cellular components of the NMJ (68). Taguchi et al. (77) also reported that epitopes recognized with anti-GalNAc-GD1a antibodies were observed in the soma of large neurons in the anterior horn of adult spinal cord and their motor axons as well as rat ventral roots and NMJs. In addition, Yoshino et al. (95) reported GalNAc-GD1a is localized specifically in ventral spinal roots, but not in dorsal spinal roots, suggesting that GBS with anti-GalNAc-GD1a antibodies may be caused by antibody-mediated attack on motor axons. In Miller-Fisher syndrome, the serum antibody specifically binds to disialosyl epitopes on gangliosides such as GQ1b, GT1a, and GD3. Since these gangliosides are enriched in synaptic membranes, antiganglioside antibodies may target NMJ, thereby contributing to the disease symptoms (33).
To further investigate the role of structural mimicry in developing pathogenic autoantibodies, Goodyear et al. (27) immunized mice with GT1a/GD3-like C. jejuni LPS and then developed a monoclonal antibody that reacted with both the immunizing LPS and GQ1b/GT1a/GD3 gangliosides. Immunohistological studies revealed binding of the antibodies to ganglioside-rich sites, including motor nerve terminals. In ex vivo electrophysiological studies of nerve terminal functions, application of antibodies either ex vivo or in vivo via passive immunization induced massive quantal release of acetylcholine, followed by neurotransmission block. This effect was complement dependent and associated with extensive deposits of IgM and complement C3c at nerve terminals. Illa et al. (37) reported that purified anti-GM1 antibodies from patients who exhibited AMAN after immunization with a ganglioside preparation recognized epitopes at the nodes of Ranvier and at the presynaptic nerve terminals of motor end plates from human nerve biopsies. Accumulation of these antibodies at the nodes of Ranvier can cause disruption of Na+ and K+ channels and, thus, interfere with nerve conduction. Therefore, a causal link between C. jejuni infection, the presence of antiganglioside antibodies, and development of GBS is considered likely (56). Recently, Moran et al. (57) reported that rabbits immunized with ganglioside-mimicking C. jejuni LOSs presented high titers of anti-LOS antibodies in sera that were cross-reactive with a panel of gangliosides. However, non-ganglioside-mimicking C. jejuni LOSs induced a strong anti-LOS response but no antiganglioside antibodies. This result suggests that immunization with ganglioside-mimicking C. jejuni LOSs triggers the production of cross-reactive antiganglioside antibodies that recognize epitopes at the nodes of Ranvier.
Recently we reported two cases of acute or chronic demyelinating inflammatory neuropathy showing elevated titers of anti-GD3 antibodies; both types occur rarely in GBS (79). To examine the correlation between the anti-GD3 antibody titers and C. jejuni infection, we sensitized female Lewis rats with LOSs from serotype HS:19 of C. jejuni and examined changes in nerve conduction velocity and nerve conduction block (80). After 16 weeks of sensitization, animals revealed transient decreases in nerve conduction velocity and conduction blocks and high titers of anti-GD3 antibodies. These anti-GD3 antibodies also blocked the spontaneous muscle action potential of NMJs in spinal cord-muscle cell cocultures. To determine the target epitope for GD3 antibodies in causing nerve dysfunction, the LOS fraction containing the GD3 epitope was purified from the total LOSs using an anti-GD3 affinity column. Subsequently, chemical analysis of the oligosaccharide portion was performed and confirmed the presence of a GD3-like epitope with the following tetrasaccharide structure: NeuAcα2-8NeuAc2-3Galβ1-3Hep. Our data thus support the possibility of GD3 mimicry as a potential pathogenic mechanism in peripheral nerve dysfunction. These in vitro electrophysiological studies provide strong evidence that ganglioside molecular mimicry is likely responsible for muscle weaknesses, possibly via their action on the NMJ.
CAMPYLOBACTERIOSIS IS FREQUENTLY ASSOCIATED WITH GBS
An important source of bacterial infection in GBS is contaminated foodstuffs. We have reported an interesting case showing direct transmission from chicken to humans (81). Poultry are often highly colonized with C. jejuni and are a major food-borne vehicle for campylobacteriosis, which is frequently associated with GBS. In a case study, we found high titers of anti-GM1 antibodies in the serum of a laboratory worker who developed campylobacteriosis. The microbiologically confirmed strain VLA2/18 (nonserotyped) was isolated from the worker and subsequently inoculated into chickens, resulting in high titers of serum antibodies to GM1. High titers of anti-GM1 antibodies in chicken and human sera strongly inhibited spontaneous muscle action potential in an in vitro system of spinal cord and muscle cell cocultures. In addition, infection of chickens with C. jejuni strains 81116 (HS:6) and 99/419 (HS:21) or immunization with purified GM1, GM2, or GM3 resulted in elevation of serum antiganglioside antibodies with an inhibitory effect on spontaneous muscle action potentials. Immunoadsorption studies demonstrated that this inhibitory activity is due to antiganglioside antibodies. On the other hand, anti-GM1 is the only specific human serum antibody to induce an inhibitory effect on NMJs. Chicken anti-GM1 antibodies showed a strong inhibitory effect, but anti-GM2 and -GM3 had weaker inhibitory activities. Taken together, our data suggest that campylobacteriosis in chickens may provide a strong link between infection and the development of antiganglioside antibody-mediated peripheral nerve dysfunction.
GENETIC VARIABILITY OF CAMPYLOBACTER JEJUNI AND ITS RELATIONSHIP TO THE INDUCTION OF ANTIGANGLIOSIDE ANTIBODIES IN GUILLAIN-BARRÉ SYNDROME
Despite the strong association between infectious agents and inflammatory immune-mediated disorders as described in previous sections, many unanswered questions remain. First, there are millions of infections every year, and only a small number of people with an infection develop GBS. Heterogeneity in the chemical structure of the LPSs of various infectious agents may very well contribute to this low incidence. Variability in the LPS structures from different strains of infectious agents may also partially account for the development of various anticarbohydrate antibodies and ensuing clinical symptoms. To validate the molecular mimicry hypothesis, we propose that direct evidence should come from a careful study of the isolated LPS fractions of C. jejuni and other putative agents, searching for “GSL-like” epitopes. Molecular mimicry of host structures in the saccharide portion of LOSs is considered to be a virulence factor of mucosal pathogens. The pathogens then could use a mutation strategy to evade the immune response. The identification of genes involved in LOS synthesis and the study of their regulation are emerging areas of research for a better understanding of the pathogenic mechanisms used by these bacteria (23, 25). Several researchers have reported that ganglioside-like LOSs are synthesized by sialyltransferase Cst-II, β-1,4-N-acetylgalactosaminyl-transferase CgtA, and β-1,3-galactosyltransferase CgtB and that there is strong association between these genes and GBS-associated C. jejuni strains. Nachamkin (58) and Gilbert et al. (23) have applied two strategies for the cloning of four genes responsible for the biosynthesis of the GT1a ganglioside mimic in the LOS of a bacterial pathogen, C. jejuni OH4384, which has been associated with GBS. They cloned a gene encoding α-2,3-sialyltransferase, cstI, using an activity screening strategy. They also identified genes encoding CgtA, CgtB, and a bifunctional Cst-II that transfers sialic acid to O-3 of galactose and to O-8 of sialic acid that is linked α-2,3 to a galactose. Guerry et al. (28) reported the presence of three genes involved in biosynthesis of the LOS core of C. jejuni MSC57360, the type of strain of the HS:1 serotype, whose structure mimics GM2 ganglioside. Mutations of genes encoding proteins with homology to a Cst-II and a putative N-acetylmannosamine synthetase (NeuC1), part of the biosynthetic pathway of N-acetylneuraminic acid (NeuAc), produce identical phenotypes. Van Belkum et al. (82) presented the first example of a bacterial determinant associated with the pathogenesis of postinfectious acute immune-mediated neuropathy. The immune detection of a GQ1b-like epitope in the bacterial LOS moiety of GBS-related or Miller-Fisher syndrome-related strains is strongly associated with the occurrence of an anti-GQ1b antibody response in the patients. The observation of a GQ1b-like epitope in the C. jejuni LOS, the serological response in the patients, and the ubiquitous presence of the Cst-II gene indicate that the activity of the bifunctional sialic acid transferase is necessary to synthesize a GQ1b-like epitope. This eventually leads to an anti-GQ1b immune response in the host and consequent neurological symptoms. Chui et al. (17) reported the first structure of a sialyltransferase, Cst-II from C. jejuni, as a highly common food-borne pathogen. Clearly, the identification of other genes involved in LOS synthesis and the study of their regulation are of considerable interest for a better understanding of the pathogenic mechanisms used by C. jejuni (24). In this regard, Linton et al. (49) have reported that the wlaN gene product, which encodes a β-1,3-galactosyltransferase, is responsible for converting the GM2-like LOS structure to a GM1-like structure. The LPS of C. jejuni strain 81-176 expresses mainly a mixture of LPS cores containing GM2 and GM3 gangliosides (29). Genetic analyses of genes involved in the biosynthesis of the outer core of this strain revealed the presence of a homopolymeric tract of G residues within a gene encoding CgtA. Genes responsible for LOS biosynthesis appear to be essential for induction of the anti-GM1 or anti-GD1a IgG antibody and subsequent development of AMAN (105). Thus, mutants of C. jejuni in which genes involved in LOS sialylation had been knocked out had reduced reactivity with anti-GM1 sera in patients with GBS and did not induce an anti-GD1a IgG antibody response in mice. In addition, Koga et al. (45) recently examined the frequency of the C. jejuni gene cstII and the polymorphism (Asn/Thr51) that affects the expression of ganglioside-like epitopes. The strain with cstII (Asn51) regularly expressed the GQ1b epitope (83%), whereas those with cstII (Thr51) had GM1- (92%) and GD1a-like (91%) epitopes. The presence of these epitopes in bacterial LPS may very well underlie the corresponding autoantibody reactivity in patients with neuropathy.
Godschalk et al. (25) demonstrated for the first time that specific bacterial genes are crucial for induction of antiganglioside antibodies, determining the class of LOS loci (classes A to E) in a collection of 21 neuropathy-associated and 21 control C. jejuni strains isolated from patients with uncomplicated enteritis. Analyzing the expression of ganglioside-like structures in relation to the class of LOS locus, Godschalk et al. (25) found that GM1-like structures were associated with a class A locus, whereas GQ1b-like structures were predominantly expressed in strains with a class B locus. In 8 of 11 strains with a class D or E locus, ganglioside-like structures were not detected, which is in accord with the absence of genes involved in the biosynthesis or transfer of sialic acid. These results indicate that genes that are unique to the class A and B loci and genes involved in sialic acid biosynthesis or transfer may be crucial for induction of neuropathogenic cross-reactive antibodies and may be considered GBS marker genes (106) (Fig. 3).
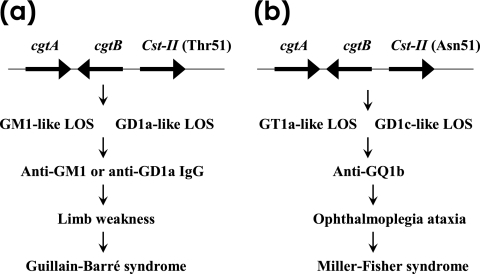
FIG. 3.
C. jejuni gene polymorphism as a determinant of clinical neuropathies after infection by the bacterium. (a) C. jejuni strains carrying cstII (Thr51) can express GM1-like or GD1a-like LOS on their cell surfaces. Infection by such C. jejuni strains can induce anti-GM1 or anti-GD1a IgG production in certain patients. Anti-GM1 or anti-GD1a IgG antibodies bind to GM1 or GD1a, respectively; these are expressed on motor nerves in the limbs and induce weakness. This binding induces the development of GBS. (b) In contrast, C. jejuni strains carrying cstII (Asn51) can express GT1a-like or GD1c-like LOS on their cell surfaces. Patients infected with C. jejuni (Asn51) more often were positive for anti-GQ1b IgG and had opthalmoplagia and ataxia (45). Anti-GQ1b IgG antibody binds to GQ1b, which is expressed on the oculomotor nerves and primary sensory neurons. This induces the development of Miller-Fisher syndrome (adopted from reference 106 with permission of the publisher).
In addition to the above, neuB1 is a gene that encodes NeuAc-synthetase and is required for the synthesis of NeuAc of C. jejuni LOS. A neuB1 mutant was constructed from a C. jejuni HS:19 wild-type strain. The mutant LOS did not bind to the cholera toxin B subunit, failed to induce anti-GM1 antibodies, and did not cause pathological changes in peripheral nerves. These data suggest that the NeuAc residue in LOS is a crucial epitope in realization of ganglioside molecular mimicry (91). Phongsisay et al. (62) recently reported that a strain of C. jejuni lacking a GM1-like epitope acquired large DNA fragments, including LPS synthesis genes, from a strain expressing GM1-like molecules and consequently transformed into potential GBS-inducible strains with a high degree of genetic and phenotypic diversity. Recently, Houliston et al. (35) identified a sialate O-acetyltransferase in the LOS biosynthesis locus of C. jejuni. Strains possessing this locus are known to produce sialylated outer core structures that mimic host gangliosides and have been implicated in triggering the onset of GBS. Fry et al. (19) reported that the C. jejuni 81116 galE gene encoding a UDP-galactose-4-epimerase, which catalyzes interconversion of UDP-galactose and UDP-glucose, is indispensable to synthesizing ganglioside-like structures of the LOS core oligosaccharide. Recently, Shu et al. (73) performed immunization experiments in guinea pig models using C. jejuni HB9313 (HS:19) and its galE mutant and showed a high titer of anti-GM1 IgG and axonal degeneration in animals sensitized with HB9313, but there was no GM1 antibody titer and no significant morphological change of the sciatic nerve of animals sensitized with the galE mutant (Table 2).
TABLE 2.
Proposed functions for GBS-related genes in the LOS biosynthesis locus of various C. jejuni strains
| Gene | Proposed function | Serotype (strain) | Class | Reference(s) |
|---|---|---|---|---|
| cstI | α-2,3-Sialylltransferase | HS:19 (OH4384) | A | 23 |
| cstII | α-2,3/α-2,8-Sialyltransferase | HS:19 (OH4384) | A | 23, 24 |
| α-2,3/α-2,8-Sialyltransferase | HS:19 (OH4384) | A | 17 | |
| α-2,3/α-2,8-Sialyltransferase | HS:19 | A | 58 | |
| cstII (Thr51) | α-2,3-Sialyltransferase | HS:19 (OH4382/OH4384) | A | 45 |
| cstII (Asn51) | α-2,3/α-2,8-Sialyltransferase | HS:19 (OH4382/OH4384) | A | 45 |
| cstIII | α-2,3-Sialyltransferase | HS:1 (MSC57360) | A | 28 |
| cgtA | β-1,4-N-Acetylgalactosaminyltransferase | HS:19 (OH4384) | A | 23 |
| β-1,4-N-Acetylgalactosaminyltransferase | HS:23/HS:36 (81176) | B | 29 | |
| β-1,4-N-Acetylgalactosaminyltransferase | HS:19 | A | 58 | |
| cgtB | β-1,3-Galactosyltransferase | HS:19 (OH4384) | A | 23 |
| β-1,3-Galactosyltransferase | HS:19 | A | 58 | |
| wlaN | β-1,3-Galactosyltransferase | NCTO 11168 | C | 49 |
| neuB1 | NeuAc synthetase | HS:19 | A | 91 |
| neuC1 | ManAc synthesis | HS:1 (MSC57360) | A | 28 |
| orf11 | O-Acetyltransferase | HS:19 | A | 35 |
| galE | UDP-glucose 4-epimerase | HS:6 | E | 19 |
GENETIC PREDISPOSITION OF GBS
Although heterogeneity in the chemical structures of the LPSs of various infectious agents contributes to the variability of bacterial virulence for GBS, the genetic susceptibility of patients to infectious agents may also contribute to differences in clinical outcomes of the patients. Studies in this area, however, are scattered and inconclusive. For example, several reports documented the involvement of host factors in certain patients that contributed to the failure to develop antiganglioside antibodies in the pathogenesis of GBS (72). Hartung and Toyka (34) reported macrophage activation in GBS in which circulating activated T lymphocytes were found, as evidenced by augmented expression of histocompatibility antigens (HLA-DR), suggesting that there is an association between GBS and HLA alleles. In HLA typing for class II alleles, Rees et al. (65) reported the association between HLA-DQB1*03 and preceding C. jejuni infection in GBS or Miller-Fisher syndrome. In AIDP patients, the DRB1*1301 allele showed a significant increase, but not in AMAN. In AMAN patients, alleles DRB1*1301-03 and DRB1*1312 are increased (54). HLA-A33, -DR15, and -DQ5 may have association with susceptibility to AIDP; on the other hand, HLA-B15 and -B35 may be associated with susceptibility to AMAN (30). Koga et al. (42) reported that HLA-B54 and -Cw1 antigens were found in GBS and Miller-Fisher patients from whom C. jejuni had been isolated more often than in the healthy controls. However, Ma et al. (50) did not find significant differences in HLA DRB1 or DQB1 alleles in Japanese cases of GBS and concluded that the roles of TCRAC, T-cell receptor beta-chain variable, or HLA class I or class II are not critical in the development of GBS. Chiba et al. (16) also reported that particular serologically defined HLA types are not preferred for the immunoresponse of anti-GQ1b IgG antibody in Miller-Fisher and GBS. A more recent study indicated that HLA-DRB1 and HLA-DQB1 alleles did not differ between GBS patients and control subjects, although the frequency of HLA-DRB1*01 was increased in patients. HLA class II genes were not found to have any association with AMAN (30) and GBS (21). However, HLA class II alleles may be a determinant in distinct subgroups of GBS (21). These reports clearly indicate the possible association of the involvement of HLA classes in the pathogenesis of GBS. They also point to an area that needs more careful studies involving more patients.
Another factor that may be involved in the immunopathogenetic mechanisms of GBS is the development of cytotoxic inflammatory cytokines. Ma et al. (51) studied genetic polymorphisms in the tumor necrosis factor (TNF) gene in Japanese patients with GBS. A significantly higher frequency of the 100-bp TNF-2α allele of the TNF-α microsatellite marker, which is associated with high TNF-α production, was found in C. jejuni-positive (Cj+) GBS patients compared to controls, suggesting the involvement of a genetic predisposition to high TNF-α secretion in the development of C. jejuni-related GBS. Schmidt-Ott (69) reported an association between 80.6% of GBS cases and Cj0017 (P39) and Cj0113 (P.18), which are encoded by C. jejuni genes. In addition, wla genes (wla cluster) that encode LOS biosynthesis (53) and the A(−670) GSNP in the promoter region of Fas and high levels of sFas (22) may be involved in the pathogenesis of GBS. Caporale et al. (15) reported the association between GBS and CD1 molecules that are major histocompatibility complex-like glycoproteins specialized in capturing and presenting a variety of glycolipids to antigen-specific T cells. CD1 genes are known to be located in human chromosome 1 (named CD1A, -B, -C, -D, and -E). Caporale et al. (15) also reported that susceptibility to GBS is associated with polymorphisms of the CD1A and CD1E genes. Oligoclonal expansion of T cells bearing particular types of T-cell receptor Vβ and Vδ genes frequently occurs in GBS and Miller-Fisher syndrome (43). Involvement of leukocyte IgG receptors in GBS has been reported. FcγRIII genotypes may represent mild disease-modifying factors in GBS (84). FcγRIIa alleles may constitute novel genetic risk markers for GBS (83). These genetic factors are summarized in Table 3.
TABLE 3.
Host risk factors for GBS and related diseases
| Host gene | Disease | Reference |
|---|---|---|
| HLA class I | ||
| A33 | AIDP | 30 |
| B15 | AMAN | 30 |
| B35 | AMAN | 30 |
| B54 | GBS/MFSa | 42 |
| Cw1 | GBS/MFS | 42 |
| HLA class II | ||
| DR | GBS | 34 |
| DR15 | AIDP | 30 |
| DRB1*1301 | AIDP | 54 |
| DRB1*1301-03 | AMAN | 54 |
| DRB1*0803 | GBS | 50 |
| DRB2*1312 | AMAN | 54 |
| DQ5 | AIDP | 30 |
| DQB1 | GBS | 50 |
| DQB1*3 | GBS/MFS | 65 |
| Cluster of differentiation | ||
| CD1A | GBS | 15 |
| CD1E | GBS | 15 |
| Apoptosis | ||
| TNF-2α | GBS (in Japan) | 51 |
| A(-670)GSNP | GBS | 22 |
| T-cell receptor | ||
| Vβ | GBS/MFS | 43 |
| Vδ | GBS/MFS | 43 |
| Fc receptor | ||
| γRIII | GBS | 84 |
| γRIII-H131 | GBS | 83 |
MFS, Miller-Fisher syndrome.
CONCLUSIONS
In conclusion, GBS and its variant, Miller-Fisher syndrome, are acute, postinfectious, autoimmune neuropathies that frequently follow C. jejuni enteritis. Increased anti-GSL antibody titers in GBS and related diseases are thought to be a result of the production of antibodies to a bacterial carbohydrate-containing surface antigen(s) that cross-reacts with the myelin sheath and the axons of nerve cells, resulting in demyelination and axonal degeneration. The pathogenesis is believed to involve molecular mimicry between epitopes on C. jejuni lipopolysaccharides and neural gangliosides, resulting in immunologic damage to the peripheral nerve. Antibody- and/or cell-mediated immune responses are believed to produce degeneration of the nerve and interruption of neurotransmission. Accumulating in vitro electrophysiological evidence suggests that ganglioside molecular mimicry may be responsible for muscle weakness, possibly via the action of antiganglioside antibodies on the neuromuscular junction. A common molecular feature in ganglioside mimicry is the presence of NeuAc in both lipopolysaccharides and gangliosides. In recent years, serological studies revealed the characterization of several C. jejuni genes involved in lipopolysaccharide biosynthesis. The presence of certain gene loci may be crucial for induction of the antiganglioside immune response that leads to GBS and related diseases. In addition, the presence of certain C. jejuni genes involved in LOS biosynthesis may be crucial for induction of the antiganglioside immune response that leads to GBS and related autoimmune neuropathies. Analysis of the expression of these genes should be helpful in designing drugs useful in treating these conditions. Understanding the gene products may also help in designing suitable vaccines for their intervention. Future studies should be directed to these important challenges.
Acknowledgments
This study was supported by NIH grant NS26994 (R.K.Y.).
We acknowledge the invaluable help provided by Rhea Markowitz in editing the manuscript.
Editor: J. B. Kaper
Footnotes
Published ahead of print on 11 September 2006.
REFERENCES
- 1.Alaniz, M. E., R. D. Lardone, S. L. Yudowski, M. I. Farace, and G. A. Nores. 2004. Normally occurring human anti-GM1 immunoglobulin M antibodies and the immune response to bacteria. Infect. Immun. 72:2148-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang, C. W., H. P. Endtz, B. C. Jacobs, J. D. Laman, M. A. de Klerk, F. G. van der Meche, and P. A. van Doorn. 2000. Campylobacter jejuni lipopolysaccharides from Guillain-Barré syndrome patients induce IgG anti-GM1 antibodies in rabbits. J. Neuroimmunol. 104:133-138. [DOI] [PubMed] [Google Scholar]
- 3.Ang, C. W., M. A. de Klerk, H. P. Endtz, B. C. Jacobs, J. D. Laman, F. G. van der Meche, and P. A. van Doorn. 2001. Guillain-Barré syndrome- and Miller Fisher syndrome-associated Campylobacter jejuni lipopolysaccharides induce anti-G M1 and anti-GQ1b antibodies in rabbits. Infect. Immun. 69:2462-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang, C. W., J. D. Laman, H. J. Willison, E. R. Wagner, H. P. Endtz, M. A. de Klerk, A. P. Tio-Gillen, N. van den Braak, B. C. Jacobs, and P. A. van Doorn. 2002. Structure of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity and clinical features of Guillain-Barré and Miller Fisher patients. Infect. Immun. 70:1202-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang, C. W., P. G. Noordzij, M. A. de Klerk, H. P. Endtz, P. A. van Doorn, and J. D. Laman. 2002. Ganglioside mimicry of Campylobacter jejuni lipopolysaccharides determines antiganglioside specificity in rabbits. Infect. Immun. 70:5081-5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang, C. W., B. C. Jacobs, and J. D. Laman. 2004. The Guillain-Barré syndrome: a true case of molecular mimicry. Trends Immunol. 25:61-66. [DOI] [PubMed] [Google Scholar]
- 7.Ariga, T., T. Kohriyama, L. Freddo, N. Latov, M. Saito, K. Kon, S. Ando, M. Suzuki, M. E. Hemling, K. E. Rinehart, S. Kusunoki, and R. K. Yu. 1987. Characterization of sulfated glucuronic acid containing glycolipids reacting with IgM M-proteins in patients with neuropathy. J. Biol. Chem. 262:848-853. [PubMed] [Google Scholar]
- 8.Ariga, T., T. Miyatake, and R. K. Yu. 2001. Recent studies on the roles of anti-glycosphingolipids in the pathogenesis of neurological diseases. J. Neurosci. Res. 65:363-370. [DOI] [PubMed] [Google Scholar]
- 9.Ariga, T., and R. K. Yu. 2005. Anti-glycolipid antibodies in Guillain-Barré syndrome and related diseases: review of clinical features and antibody specificities. J. Neurosci. Res. 80:1-17. [DOI] [PubMed] [Google Scholar]
- 10.Aspinall, G. O., A. G. McDonald, T. S. Raju, H. Pang, A. P. Moran, and J. L. Penner. 1993. Chemical structure of the core region of Campylobacter jejuni serotype O:2 lipopolysaccharides. Eur. J. Biochem. 213:1029-1037. [DOI] [PubMed] [Google Scholar]
- 11.Aspinall, G. O., A. G. McDonald, H. Pang, L. A. Kurjanczk, and J. L. Penner. 1994. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry 33:241-249. [DOI] [PubMed] [Google Scholar]
- 12.Bersudsky, M., P. Rosenberg, B. Rudensky, and I. Wirguin. 2000. Lipopolysaccharides of a Campylobacter coli isolate from a patient with Guillain-Barre syndrome display ganglioside mimicry. Neuromusc. Disorders 10:182-186. [DOI] [PubMed] [Google Scholar]
- 13.Bowes, T., E. R. Wagner, J. Boffey, D. Nicholl, L. Cochrane, M. Benboubetra, J. Conner, K. Furukawa, K. Furukawa, and H. J. Willison. 2002. Tolerance to self gangliosides in the major factor restricting the antibody response to lioppolysaccharide core oligosaccharides is Camplyobacter jejuni strains associated with Guillain-Barré syndrome. Infect. Immun. 70:5008-5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchwald, B., A. Weishaupt, K. V. Toyka, and J. Dudel. 1998. Pre- and post-synaptic blockade of neuromuscular transmission by Miller-Fisher syndrome IgG at mouse motor nerve terminals. Eur. J. Neurosci. 10:281-290. [DOI] [PubMed] [Google Scholar]
- 15.Caporale, C. M., F. Papola, M. A. Fioroni, A. Aureli, A. Giovannini, F. Notturno, D. Adorno, V. Caporale, and A. Uncini. 2006. Susceptibility to Guillain-Barré syndrome is associated to polymorphisms of GD1 genes. J. Neuroimmunol. 177:112-118. [DOI] [PubMed] [Google Scholar]
- 16.Chiba, A., S. Kusunoki, S. Kuwata, T. Juji, Y. Shibata, and I. Kanazawa. 1995. HLA and anti-GQ1b IgG antibody in Miller-Fisher syndrome and Guillain-Barré syndrome. J. Neuroimmunol. 61:85-88. [DOI] [PubMed] [Google Scholar]
- 17.Chiu, C. P., A. G. Watts, L. L. Lairson, M. Gilbert, D. Lim, W. W. Wakarchuk, S. G. Withers, and N. C. Strynadka. 2004. Structural analysis of the sialyltransferase CstII from Campylobacter jejuni in complex with a substrate analog. Nat. Struct. Mol. Biol. 11:163-170. [DOI] [PubMed] [Google Scholar]
- 18.Chou, D. K., A. A. Ilyas, J. E. Evans, C. Costello, R. H. Quarles, and F. B. Jungalwala. 1986. Structure of sulfated glucuronylglycolipids in the nervous system reacting with HNK-1 antibody and some IgM paraproteins in neuropathy. J. Biol. Chem. 261:11717-11725. [PubMed] [Google Scholar]
- 19.Fry, B. N., S. Feng, Y. Y. Chen, D. G. Newell, P. J. Coloe, and V. Korolik. 2000. The galE gene of Campylobacter jejuni is involved in lipopolysaccharide synthesis and virulence. Infect. Immun. 68:2594-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funakoshi, K., M. Koga, M. Takahashi, K. Hirata, and N. Yuki. 2006. Campylobacter coli enteritis and Guillain-Barré: no evidence of molecular mimicry and serological relationship. J. Neurol. Sci. 246:163-168. [DOI] [PubMed] [Google Scholar]
- 21.Geleijins, K., G. M. Schreuder, B. C. Jacobs, K. Sintnicolaas, R. van Koningsveld, J. Meulstee, J. D. Laman, and P. A. van Doorn. 2005. HLA class II alleles are not a general susceptibility factor in Guillain-Barré syndrome. Neurology 64:44-49. [DOI] [PubMed] [Google Scholar]
- 22.Geleijns, K., J. D. Laman, W. van Rijs, A. P. Tio-Gillen, R. O. Hintzen, P. A. van Droorn, and B. C. Jacobs. 2005. Fas polymorphisms are associated with the presence of anti-ganglioside antibodies in Guillain-Barré syndrome. J. Neuroimmunol. 161:183-189. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert, M., J.-R. Brisson, M.-F. Karwaski, J. Michniewicz, A.-M. Cunningham, Y. Wu, N. M. Young, and W. W. Wakarchuk. 2000. Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384. Identification of the glycosyltransferase genes, enzymatic synthesis of model compounds, and characterization of nanomole amounts by 600-MHz 1H and 13C NMR analysis. J. Biol. Chem. 275:3896-3906. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert, M., M.-F. Karwaski, S. Bernatchez, N. M. Young, E. Taboada, J. Michniewicz, A.-M. Cunningham, and W. W. Wakarchuk. 2002. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 277:327-337. [DOI] [PubMed] [Google Scholar]
- 25.Godschalk, P. C., A. P. Heikema, M. Gilbert, T. Komagamine, C. W. Ang, J. Glerum, D. Brochu, J. Li, N. Yuki, B. C. Jacobs, A. van Belkum, and H. P. Endtz. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in the Guillain-Barré syndrome. J. Clin. Investig. 114:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godschalk, P. C., M. Gilbert, B. C. Jacobs, T. Kramers, A. P. Tio-Gillen, C. W. Ang, N. Van den Braak, J. Li, H. A. Verbrugh, A. Van Belkum, and H. P. Endtz. 2006. Co-infection with two different Campylobacter jejuni strains in a patient with Guillain-Barre syndrome. Microbes Infect. 8:248-253. [DOI] [PubMed] [Google Scholar]
- 27.Goodyear, C. S., G. M. O'Hanlon, J. J. Plomp, E. R. Wagner, I. Morrison, J. Veitch, L. Cochrane, R. W. Bullens, P. C. Molenaar, J. Conner, and H. J. Willison. 1999. Monoclonal antibodies raised against Guillain-Barré syndrome-associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle-nerve preparations. J. Clin. Investig. 104:697-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerry, P., C. P. Ewing, T. E. Hickey, M. M. Prendergast, and A. P. Moran. 2000. Sialylation of lipooligosaccharide cores affects immunogenicity and serum resistance of Campylobacter jejuni. Infect. Immun. 68:6656-6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerry, P., C. M. Szymanski, M. M. Prendergast, T. E. Hickey, C. P. Ewing, D. L. Pattarini, and A. P. Moran. 2002. Phase variation of Campylobacter jejuni 81-176 lipopolysaccharide affects ganglioside mimicry and invasiveness in vitro. Infect. Immun. 70:787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo, L., W. Wang, C. Li, R. Liu, and G. Wang. 2002. The association between HLA typing and different subtypes of Guillain-Barré syndrome. Zhonghua Nei Ke Za Zhi. 41:381-383. [PubMed] [Google Scholar]
- 31.Hadden, R. D., H. Karch, H. P. Hartung, J. Zielasek, B. Weissbrich, J. Schubert, A. Weishaupt, D. R. Cornblath, A. V. Swan, R. A. Hughes, K. V. Toyka, et al. 2001. Preceding infections, immune factors, and outcome in Guillain-Barre syndrome. Neurology 56:758-765. [DOI] [PubMed] [Google Scholar]
- 32.Hahn, A. F. 1998. Guillain-Barré syndrome. Lancet 352:635-641. [DOI] [PubMed] [Google Scholar]
- 33.Halstead, S. K., I. Morrison, G. M. O'Hanlon, P. D. Humphreys, J. A. Goodfellow. J. J. Plomp, and H. J. Willison. 2005. Anti-disialosyl antibodies mediate selective neuronal or Schwann cell injury at mouse neuromuscular junctions. Glia 52:177-189. [DOI] [PubMed] [Google Scholar]
- 34.Hartung, H. P., and K. V. Toyka. 1990. T-cell and macrophage activation in experimental autoimmune neuritis and Guillain-Barré syndrome. Ann. Neurol. 27:S57-S63. [DOI] [PubMed] [Google Scholar]
- 35.Houliston, R. S., H. P. Endtz, N. Yuki, J. Li, H. C. Jarrell, M. Koga, A. van Belkum, M. F. Karwaski, W. W. Wakarchuk, and M. Gilbert. 2006. Identification of a sialate O-acetyltransferase from Campylobacter jejuni: demonstration of direct transfer to the C-9 position of terminal α-2,8-linked sialic acid. J. Biol. Chem. 281:11480-11486. [DOI] [PubMed] [Google Scholar]
- 36.Hughes, R. A., and C. R. Cornblath. 2005. Guillain-Barré syndrome. Lancet 366:1653-1666. [DOI] [PubMed] [Google Scholar]
- 37.Illa, I., N. Ortiz, E. Gallard, C. Juarez, J. M. Grau, and M. C. Dalakas. 1995. Acute axonal Guillain-Barré syndrome with IgG antibodies against motor axons following parenteral gangliosides. Ann. Neurol. 38:218-224. [DOI] [PubMed] [Google Scholar]
- 38.Ilyas, A. A., F. A. Mithen, M. C. Dalakas, M. Wargo, Z. W. Chen, L. Bielory, and S. D. Cook. 1991. Antibodies to sulfated glycolipids in Guillain-Barré syndrome. J. Neurol. Sci. 105:108-117. [DOI] [PubMed] [Google Scholar]
- 39.Irie, S., T. Sato, K. Nakamura, N. Kanazawa, M. Ogino, T. Nukazawa, H. Itoh, Y. Tamai, and H. Kowa. 1996. Association of anti-GM2 antibodies in Guillain-Barré syndrome with acute cytomegalovirus infection. J. Neuroimmunol. 68:19-26. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs, B. C., P. A. van Doorn, J. H. Groeneveld, A. P. Tio-Gillen, and F. G. van der Meche. 1997. Cytomegalovirus infections and anti-GM2 antibodies in Guillain-Barré syndrome. J. Neurol. Neurosurg. Psychiatry 62:641-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaida, K., D. Morita, M. Kanzaki, K. Kamakura, K. Motoyoshi, M. Hirakawa, and S. Kusunoki. 2004. Ganglioside complexes as new target antigens in Guillain-Barré syndrome. Ann. Neurol. 56:567-571. [DOI] [PubMed] [Google Scholar]
- 42.Koga, M., N. Yuki, K. Kashiwase, K. Tadokoro, T. Juji, and K. Hirata. 1998. Guillain-Barré and Fisher's syndromes subsequent to Campylobacter jejuni enteritis are associated with HLA-B54 and Cw1 independent of anti-ganglioside antibodies. J. Neuroimmunol. 88:62-66. [DOI] [PubMed] [Google Scholar]
- 43.Koga, M., N. Yuki, Y. Tsukada, K. Hirata, and Y. Matsumoto. 2003. CDR3 spectratyping analysis of the T cell receptor repertoire in Guillain-Barré and Fisher syndromes. J. Neuroimmunol. 141:112-117. [DOI] [PubMed] [Google Scholar]
- 44.Koga, M., M. Gilbert, J. Li, S. Koike, M. Takahashi, K. Furukawa, K. Hirata, and N. Yuki. 2005. Antecedent infections in Fisher syndrome; a common pathogenesis of molecular mimicry. Neurology 64:1605-1611. [DOI] [PubMed] [Google Scholar]
- 45.Koga, M., M. Takahashi, M. Matsuda, K. Hirata, and N. Yuki. 2005. Campylobacter gene polymorphism as a determinant of clinical features of Guillain-Barré syndrome. Neurology 65:1376-1381. [DOI] [PubMed] [Google Scholar]
- 46.Kohriyama, T., T. Ariga, and R. K. Yu. 1988. Preparation and characterization of antibodies against a sulfated glucuronic acid-containing glycosphingolipid. J. Neurochem. 51:869-877. [DOI] [PubMed] [Google Scholar]
- 47.Kusunoki, S., A. Chiba, S. Hitoshi, H. Takizawa, and I. Kanazawa. 1995. Anti-Gal-C antibody in autoimmune neuropathies subsequent to mycoplasma infection. Muscle Nerve 18:409-413. [DOI] [PubMed] [Google Scholar]
- 48.Lee, G., Y. Jeong, I. Wirguin, A. P. Hays, H. J. Willison, and N. Latov. 2004. Induction of human IgM and IgG anti-GM1 antibodies in transgenic mice in response to lipopolysaccharides from Campylobacter jejuni. J. Neuroimmunol. 146:63-75. [DOI] [PubMed] [Google Scholar]
- 49.Linton, D., M. Gilbert, P. G. Hitchen, A. Dell, H. R. Morris, W. W. Wakarchuk, N. A. Gregson, and B. W. Wren. 2000. Phase variation of a beta-1,3 galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni. Mol. Microbiol. 37:501-514. [DOI] [PubMed] [Google Scholar]
- 50.Ma, J. J., M. Nishimura, H. Mine, S. Kuroki, M. Nukina, M. Ohta, H. Saji, H. Obayashi, T. Saida, H. Kawakami, and T. Uchiyama. 1998. HLA and T-cell receptor gene polymorphisms in Guillain-Barré syndrome. Neurology 51:379-384. [DOI] [PubMed] [Google Scholar]
- 51.Ma, J. J., M. Nishimura, H. Mine, S. Kuroki, M. Nukina, M. Ohta, H. Saji, H. Obayashi, H. Kawakami, T. Saida, and T. Uchiyama. 1998. Genetic contribution of the tumor necrosis factor region in Guillain-Barré syndrome. Ann. Neurol. 44:815-818. [DOI] [PubMed] [Google Scholar]
- 52.Maeda, Y., C. F. Brosnan, N. Miyatani, and R. K. Yu. 1991. Preliminary studies on sensitization of Lewis rats with sulfated glucuronyl paragloboside. Brain Res. 541:254-264. [DOI] [PubMed] [Google Scholar]
- 53.Misawa, N., K. Kawashima, F. Kondo, B. M. Allos, and M. J. Blaser. 2001. DNA diversity of the wla gene cluster among serotype HS:19 and non-HS:19 Campylobacter jejuni strains. J. Endotoxin Res. 7:349-358. [PubMed] [Google Scholar]
- 54.Monos, D. S., M. Papaioakim, T. W. Ho, C. Y. Li, and G. M. McKhann. 1997. Differential distribution of HLA alleles in two forms of Guillain-Barré syndrome. J. Infect. Dis. 176(Suppl. 2):S180-S182. [DOI] [PubMed] [Google Scholar]
- 55.Moran, A. P., M. M. Predergast., and B. J. Appelmelk. 1996. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol. Med. Microbiol. 16:105-115. [DOI] [PubMed] [Google Scholar]
- 56.Moran, A. P., and M. M. Prendergast. 2001. Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: contribution of gastrointestinal infections to autoimmunity. J. Autoimmun. 16:241-256. [DOI] [PubMed] [Google Scholar]
- 57.Moran, A. P., H. Annuk, and M. M. Prendergast. 2005. Antibodies induced by ganglioside-mimicking Campylobacter jejuni lipopolysaccharides recognize epitopes at the nodes of Ranvier. J. Neuroimmunol. 165:179-185. [DOI] [PubMed] [Google Scholar]
- 58.Nachamkin, I., J. Liu, M. Li, H. Ung, A. P. Moran, M. M. Prendergast, and K. Sheikh. 2002. Campylobacter jejuni from patients with Guillain-Barré syndrome preferentially expresses a GD1a-like epitope. Infect. Immun. 70:5299-5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neisser, A., H. Bernheimer, T. Berger, A. P. Moran, and B. Schwerer. 1997. Serum antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in Miller Fisher syndrome. Infect. Immun. 65:4038-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogawa-Goto, K., K. Kubota, A. Kurotani, and T. Abe. 1994. Antibodies against sulfated glycosphingolipids of peripheral nerve myelins detected in patients with human cytomegalovirus infection. J. Neuroimmunol. 55:55-60. [DOI] [PubMed] [Google Scholar]
- 61.O'Hanlon, G. M., J. J. Plomp, M. Chakrabarti, I. Morrison, E. R. Wagner, C. S. Goodyear, X. Yin, B. D. Trapp, J. Conner, P. C. Molenaar, S. Stewart, E. G. Rowan, and H. J. Willison. 2004. Anti-GQ1b ganglioside antibodies mediate complement-dependent destruction of the motor nerve terminal. Brain 124:893-906. [DOI] [PubMed] [Google Scholar]
- 62.Phongsisay, V., V. N. Perera, and B. N. Fry. 2006. Exchange of lipooligosaccharide synthesis genes creates potential Guillain-Barré syndrome-inducible strains of Campylobacter jejuni. Infect. Immun. 74:1368-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prendergast, M. M., A. J. Lastovica, and A. P. Moran. 1998. Lipopolysaccharides from Campylobacter jejuni O:41 strains associated with Guillain-Barré syndrome exhibit mimicry of GM1 ganglioside. Infect. Immun. 66:3649-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prendergast, M. M., and A. P. Moran. 2000. Lipopolysaccharides in the development of the Guillain-Barré syndrome and Miller Fisher syndrome forms of acute inflammatory peripheral neuropathies. J. Endotoxin Res. 6:341-359. [PubMed] [Google Scholar]
- 65.Rees, J. H., R. W. Vaughan, E. Kondeatis, and R. A. Hughes. 1995. HLA-class II alleles in Guillain-Barré syndrome and Miller Fisher syndrome and their association with preceding Campylobacter jejuni infection. J. Neuroimmunol. 62:53-57. [DOI] [PubMed] [Google Scholar]
- 66.Ritter, G., S. R. Fortunato, L. Cohen, Y. Noguchi, E. M. Bernard, E. Stockert, and L. J. Old. 1996. Induction of antibodies reactive with GM2 ganglioside after immunization with lipopolysaccharides from Campylobacter jejuni. Int. J. Cancer 66:184-190. [DOI] [PubMed] [Google Scholar]
- 67.Salloway, S., L. A. Mermel, M. Seamans, G. O. Aspinall, J. E. Nam Shin, L. A. Kurjanczyk, and J. L. Penner. 1996. Miller-Fisher syndrome associated with Campylobacter jejuni bearing lipopolysaccharide molecules that mimic human ganglioside GD3. Infect. Immun. 64:2945-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santafe, M. M., M. M. Sabate, N. Garcia, N. Ortiz, M. A. Lanuza, and J. Tomas. 2005. Changes in the neuromuscular synapse induced by an antibody against gangliosides. Ann. Neurol. 57:396-407. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt-Ott, R., H. Schmidt, S. Feldmann, F., Brass, B. Krone, and U. Gross. 2006. Improved serological diagnosis stresses the major role of Campylobacter jejuni in triggering Guillain-Barré syndrome. Clin. Vaccine Immunol. 13:779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwerer, B. 2002. Antibodies against gangliosides: a link between preceding infection and immunopathogenesis of Guillain-Barré syndrome. Microbes Infect. 4:373-384. [DOI] [PubMed] [Google Scholar]
- 71.Sheikh, K. A., I. Nachamkin, T. W. Ho, C. Y. Li, G. M. McKhann, and J. W. Griffin. 1995. Penner's serotype 19 Campylobacter jejuni liposaccharide isolated from a patient with acute motor axonal neuropathy bears L2/HNK-1 and GM1 epitope. Ann. Neurol. 38(Abs.):350. [Google Scholar]
- 72.Sheikh, K. A., I. Nachamkin, T. W. Ho, H. J. Willison, J. Veitch, H. Ung, M. Nicholson, C. Y. Li, H. S. Wu, B. O. Shen, D. R. Cornblath, A. K. Asbury, G. M. McKhann, and J. W. Griffin. 1998. Campylobacter jejuni lipopolysaccharides in Guillain-Barré syndrome: molecular mimicry and host susceptibility. Neurology 51:371-378. [DOI] [PubMed] [Google Scholar]
- 73.Shu, X. M., F. C. Cai, and X. P. Zhang. 2006. Carbohydrate mimicry of Campylobacter jejuni lipooligosaccharide is critical for the induction of anti-GM1 antibody and neuropathy. Muscle Nerve 33:225-231. [DOI] [PubMed] [Google Scholar]
- 74.Simon-Haldi, M., N. Mantei, J. Franke, H. Voshol, and M. Schachner. 2002. Identification of a peptide mimic of the L2/HNK-1 carbohydrate epitope. J. Neurochem. 83:1380-1388. [DOI] [PubMed] [Google Scholar]
- 75.Solomon, T., and H. Willison. 2003. Infectious causes of acute flaccid paralysis. Curr. Opin. Infect. Dis. 16:375-381. [DOI] [PubMed] [Google Scholar]
- 76.Springer, G. F. 1971. Blood-group and Forssman antigenic determinants shared between microbes and mammalian cells. Prog. Allergy 15:9-77. [PubMed] [Google Scholar]
- 77.Taguchi, K., J. Ren, I. Utsunomiya, H. Aoyagi, N. Fujita, T. Ariga, T. Miyatake, and H. Yoshino. 2004. Neurophysiological and immunohistochemical studies on Guillain-Barré syndrome with IgG anti-GalNAc-GD1a antibodies—effects on neuromuscular transmission. J. Neurol. Sci. 225:91-98. [DOI] [PubMed] [Google Scholar]
- 78.Terryberry, J., M. Sutjita, Y. Shoenfeld, B. Gilburd, D. Tanne, M. Lorber, I. Alosachie, N. Barka, H. C. Lin, P. Youinou, et al. 1995. Myelin- and microbe-specific antibodies in Guillain-Barré syndrome. J. Clin. Lab. Anal. 9:308-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Usuki, S., J. Sanchez, T. Ariga, I. Utsunomiya, K. Taguchi, M. H. Rivner, and R. K. Yu. 2005. AIDP and CIDP having specific antibodies to the carbohydrate (-NeuAcα2-8NeuAcα2-3Galβ1-4Glc-) of gangliosides. J. Neurol. Sci. 232:37-44. [DOI] [PubMed] [Google Scholar]
- 80.Usuki, S., S. A. Thompson, M. H. Rivner, K. Taguchi, K. Shibata, T. Ariga, and R. K. Yu. 2006. Molecular mimicry: sensitization of Lewis rats with Campylobacter jejuni lipopolysaccharides induces formation of antibody toward GD3 ganglioside. J. Neurosci. Res. 83:274-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Usuki, S., K. Taguchi, S. Cawthaw, K. Shibata, T. Ariga, D. G. Newell, and R. K. Yu. 2006. Human and chicken antibodies to gangliosides following infection by Camplyobacter jejuni. Exp. Neurol. 200:50-55. [DOI] [PubMed] [Google Scholar]
- 82.Van Belkum, A., N. van den Braak, P. C. Godschalk, C. W. Ang, B. Jacobs, M. Gilbert, W. Wakarchuk, H. Verbrugh, and H. Endtz. 2001. A Campylobacter jejuni gene associated with immune-mediated neuropathy. Nat. Med. 7:752-753. [DOI] [PubMed] [Google Scholar]
- 83.Van der Pol, W. L., L. H. van den Berg, R. H. Scheepers, J. G. van der Bom, P. A. van Doorn, R. van Koningsveld, M. C. van den Broek, J. H. Wokke, and J. G. van de Winkel. 2000. IgG receptor IIa alleles determine susceptibility and severity of Guillain-Barré syndrome. Neurology 54:1661-1665. [DOI] [PubMed] [Google Scholar]
- 84.Van Sorge, N. M., W. L. van der Pol, M. D. Jansen, K. P. Geleijns, S. Kalmijn, R. A. Hughes, J. H. Rees, J. Pritchard, C. A. Vedeler, K. M. Myhr, C. Shaw, I. N. van Schaik, J. H. Wokke, P. A. van Doorn, B. C. Jacobs, J. G. van de Winkel, and L. H. van den Berg. 2005. Severity of Guillain-Barré syndrome is associated with Fc gamma receptor III polymorphisms. J. Neuroimmunol. 162:157-164. [DOI] [PubMed] [Google Scholar]
- 85.Visser, L. H., F. G. van der Meche, J. Meulstee, P. P. Rothbarth, B. C. Jacobs, P. I. Schmitz, P. A. van Doorn, et al. 1996. Cytomegalovirus infection and Guillain-Barré syndrome: the clinical, electrophysiologic, and prognostic features. Neurology 47:668-673. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe, K., S. Kim, M. Nishiguchi, H. Suzuki, and M. Watarai. 2005. Brucella melitensis infecton associated with Guillain-Barré syndrome through molecular mimicry of host structures. FEMS Immunol. Med. Microbiol. 45:121-127. [DOI] [PubMed] [Google Scholar]
- 87.Willison, H. J., and N. Yuki. 2002. Peripheral neuropathies and anti-glycolipid antibodies. Brain 125:2591-2625. [DOI] [PubMed] [Google Scholar]
- 88.Willison, H. J. 2005. The immunobiology of Guillain-Barré syndrome. J. Periph. Nerv. Syst. 10:94-112. [DOI] [PubMed] [Google Scholar]
- 89.Wirguin, I., L. Suturkova-Milosevic, C. Briani, and N. Latov. 1995. Keyhole limpet hemocyanin contains Gal(β1-3)-GalNAc determinants that are cross-reactive with the T antigen. Cancer Immunol. Immunother. 40:307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wirguin, I., C. Briani, L. Suturkova-Milosevic, T. Fisher, P. Della-Latta, P. Chalif, and N. Latov. 1997. Induction of anti-GM1 ganglioside antibodies by Campylobacter jejuni lipopolysaccharides. J. Neuroimmunol. 78:138-142. [DOI] [PubMed] [Google Scholar]
- 91.Xiang, S. L., M. Zhong, F. C. Cai, B. Deng, and X. P. Zhang. 2006. The sialic acid residue is a crucial component of C. jejuni lipopolysaccharide ganglioside mimicry in the induction Guillain-Barre syndrome. J. Neuroimmunol. 174:126-132. [DOI] [PubMed] [Google Scholar]
- 92.Yamawaki, M., A. Vasquez, A. Ben Younes, H. Yoshino, T. Kanda, T. Ariga, and R. K. Yu. 1996. Sensitization of Lewis rats with sulfoglucuronyl paragloboside: electrophysiological and immunological studies of an animal model of peripheral neuropathy. J. Neurosci. Res. 44:58-65. [DOI] [PubMed] [Google Scholar]
- 93.Yasuda, T., J. Ueno, Y. Naito, and T. Tsumita. 1982. Antiglycolipid antibodies in human sera. Adv. Exp. Med. Biol. 152:457-465. [PubMed] [Google Scholar]
- 94.Yoshino H., T. Inuzuka, and T. Miyatake. 1992. IgG antibody against GM1, GD1b and asialo-GM1 in chronic polyneuropathy following Mycoplasma pneumoniae infection. Eur. Neurol. 32:28-31. [DOI] [PubMed] [Google Scholar]
- 95.Yoshino, H., I. Utsunomiya, K. Taguchi, T. Ariga, T. Nagaoka, H. Aoyagi, A. Asano, M. Yamada, and T. Miyatake. 2005. GalNAc-GD1a is localized specifically in ventral spinal roots, but not in dorsal roots. Brain Res. 1057:177-180. [DOI] [PubMed] [Google Scholar]
- 96.Yuki, N., T. Taki, F. Inagaki, T. Kasama, M. Takahashi, K. Saito, S. Handa, and T. Miyatake. 1993. A bacterium lipopolysaccharide that elicites Guillain-Barré syndrome has a GM1 ganglioside-like structure. J. Exp. Med. 178:1771-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuki, N., T. Taki, M. Takahashi, K. Saito, H. Yoshino, T. Tai, S. Handa, and T. Miyatke. 1994. Molecular mimicry between GQ1b ganglioside and lipopolysaccharides of Campylobacter jejuni isolated from patients with Fisher's syndrome. Ann. Neurol. 36:791-793. [DOI] [PubMed] [Google Scholar]
- 97a.Yuki, N., T. Taki, M. Takahashi, K. Saito, T. Tai, T. Miyatake, and S. Handa. 1994. Penner's serotype 4 of Campylobacter jejuni has a lipopolysaccharide that bears a GM1 ganglioside epitope as well as one that bears a GD1 a epitope. Infect. Immun. 62:2101-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuki, N., Y. Ichihashi, and T. Taki. 1995. Subclass of IgG antibody to GM1 epitope-bearing lipopolysaccharide of Campylobacter jejuni in patients with Guillain-Barré syndrome. J. Neuroimmunol. 60:161-164. [DOI] [PubMed] [Google Scholar]
- 99.Yuki, N., Y. Tagawa, and S. Handa. 1996. Autoantibodies to peripheral nerve glycospingolipids SPG, SLPG and SGPG in Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy. J. Neuroimmunol. 70:1-6. [DOI] [PubMed] [Google Scholar]
- 100.Yuki, N., T. Yamamoto, and K. Hirata. 1998. Correlation between cytomegalovirus infection and IgM anti-MAG/SGPG antibody-associated neuropathy. Ann. Neurol. 44:408-410. [DOI] [PubMed] [Google Scholar]
- 101.Yuki, N., Y. Tagawa, F. Irie, Y. Hirabayashi, and S. Handa. 1997. Close association of Guillain-Barré syndrome with antibodies to minor monosialoganglioside GM1b and GM1α. J. Neuroimmunol. 74:30-34. [DOI] [PubMed] [Google Scholar]
- 102.Yuki, N., M. Koga, and K. Hirata. 2000. Is Campylobacter lipopolysaccharide bearing a GD3 epitope essential for the pathogenesis of Guillain-Barré syndrome? Acta Neurol. Scand. 102:132-134. [DOI] [PubMed] [Google Scholar]
- 103.Yuki, N. 2001. Infectious origins of, molecular mimicry in, Guillain-Barré and Fisher syndromes. Lancet Infect. Dis. 1:29-37. [DOI] [PubMed] [Google Scholar]
- 104.Yuki, N., K. Susuki, M. Koga, Y. Nishimoto, M. Odaka, K. Hirata, K. Taguchi, T. Miyatake, K. Furukawa, T. Kobata, and M. Yamada. 2004. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barré syndrome. Proc. Natl. Acad. Sci. USA 101:11404-11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuki, N., and M. Odaka. 2005. Ganglioside mimicry as a cause of Guillain-Barré syndrome. Curr. Opin. Neurol. 18:557-561. [DOI] [PubMed] [Google Scholar]
- 106.Yuki, N. 2005. Carbohydrate mimicry: a new paradigm of autoimmune diseases. Curr. Opin. Immunol. 17:577-582. [DOI] [PubMed] [Google Scholar]