Abstract
Lung surfactant protein D (SP-D) binds to Mycobacterium tuberculosis surface lipoarabinomannan and results in bacterial agglutination, reduced uptake, and inhibition of growth in human macrophages. Here we show that SP-D limits the intracellular growth of bacilli in macrophages by increasing phagosome-lysosome fusion but not by generating a respiratory burst.
Pulmonary surfactant proteins A and D (SP-A and SP-D, respectively) participate in the lung innate and adaptive immune responses against multiple pathogens through opsonization and agglutination of microorganisms, blockade of receptor-ligand interactions, and/or direct effects on host cells, particularly macrophages (5, 27). Experiments using mice with homozygous null alleles for the SP-A or SP-D genes provide direct evidence in support of these phenomena (11, 13, 17-20).
Both SP-A and SP-D have been implicated in the pathogenesis of tuberculosis. Our work has demonstrated that SP-A increases the phagocytosis of Mycobacterium tuberculosis by human macrophages by up-regulating the macrophage mannose receptor (MR) (2, 10) and reduces NADPH oxidase activity in these cells (6). In contrast, SP-D, through its carbohydrate recognition domain (CRD), binds to the terminal mannose caps of the M. tuberculosis surface lipoglycan lipoarabinomannan (ManLAM) and causes agglutination of the bacilli and inhibition of bacterial uptake by macrophages (7). However, the reduced uptake was not dependent on bacterial agglutination (8). The presence of SP-D also inhibited the intracellular growth of M. tuberculosis in macrophages (8). M. tuberculosis evades several host defense mechanisms including by inhibiting fusion of its own phagosome with lysosomes in macrophages (1) and avoiding the potential stimulation of intracellular oxidant production during phagocytosis (26).
We hypothesized that SP-D inhibits the intracellular growth of M. tuberculosis in macrophages by altering intracellular events that restrict mycobacterial growth following phagocytosis. In this study, we examined whether SP-D coating of M. tuberculosis modulates two major macrophage microbicidal mechanisms: generation of a respiratory burst and regulation of phagosome-lysosome (P-L) fusion events.
Recombinant rat SP-D (referred to as SP-D throughout the paper), produced in CHO cells and purified on a mannose-Sepharose matrix (22, 23), was used in the different assays at a concentration of 0.5 (nonagglutinating for M. tuberculosis) and/or 5.0 (agglutinating for M. tuberculosis) μg/ml. Since SP-D had been shown to inhibit the intracellular growth of M. tuberculosis in macrophages, we first determined whether SP-D had a direct microbicidal effect on M. tuberculosis by performing CFU assays following incubation of the bacilli in the absence or presence of SP-D. No significant effect of SP-D on the viability of bacteria at either 0.5 or 5.0 μg/ml was observed (n = 2; data not shown).
SP-D does not induce a respiratory burst during phagocytosis of M. tuberculosis in human macrophages.
We next determined whether phagocytosis of M. tuberculosis by human macrophages in the presence of SP-D would stimulate the production of reactive oxygen intermediates by using the 2′,7′-dichlorofluorescein (DCF) assay (6). Monocyte-derived macrophages (MDMs) were prepared from healthy human volunteers as described previously (24). MDM monolayers on tissue culture plates were preincubated with 32 mM DCF (Molecular Probes) for 30 min at 37°C followed by stimulation with phorbol myristate acetate (PMA; 1 μg/ml), opsonized zymosan (OZ) particles (multiplicity of infection, 20:1), SP-D, M. tuberculosis (multiplicity of infection, 10:1), or M. tuberculosis plus SP-D (0.5 and 5.0 μg/ml). The fluorescence emitted from the stimulated cells was monitored every 2 min for 2 h by a Fluostar32 fluorometer (BMG Lab Technologies). This assay measures production of H2O2-dependent reactive oxygen intermediates (12). PMA and OZ stimulation of MDMs resulted in progressively increased fluorescence (Fig. 1), indicating that the MDMs responded to known soluble and particulate stimuli of the oxidant burst. In contrast, neither SP-D alone, M. tuberculosis alone, nor M. tuberculosis in the presence of SP-D stimulated detectable levels of oxidants over medium-only control. These data indicate that SP-D does not stimulate a detectable respiratory burst in macrophages during phagocytosis of M. tuberculosis.
FIG. 1.
SP-D does not induce a respiratory burst in human macrophages. MDM monolayers in triplicate tissue culture wells were loaded with DCF and then stimulated with PMA, OZ, M. tuberculosis, SP-D (0.5 or 5.0 μg/ml), SP-D plus M. tuberculosis, or medium control. The fluorescence of each well was determined every 2 minutes for 120 min and expressed in relative fluorescence units. Shown are the means of triplicate determinations in one experiment representative of three.
SP-D increases the fusion of M. tuberculosis phagosomes with lysosomes in human macrophages.
Since SP-D binds to the terminal mannosyl units of ManLAM (7) and ManLAM is a bacterial determinant that inhibits M. tuberculosis phagosome maturation (9, 14), we hypothesized that binding of SP-D to the surface of M. tuberculosis would alter the surface characteristics of the bacterium and result in enhanced M. tuberculosis phagosome maturation. In order to test this hypothesis, we performed complementary transmission electron microscopy (TEM) and confocal microscopy assays to assess P-L fusion in M. tuberculosis-infected MDMs. The TEM assay relies on the labeling of lysosomes and late endosomes with horseradish peroxidase (HRP) and the subsequent visualization of these compartments in the form of a black precipitate within or separate from phagosomes following the addition of diaminobenzidine (14, 16). In the absence of HRP, there is no formation of black precipitate (data not shown). M. tuberculosis strain Erdman (ATCC 35801) was preincubated with SP-D for 1 h at 4°C before infection of the HRP-loaded MDM monolayers for 2 h at 37°C. Black precipitate within the M. tuberculosis phagosome was scored as P-L fusion. In the presence of SP-D, M. tuberculosis phagosomes demonstrated increased P-L fusion compared to the control phagosomes (Fig. 2). We found that SP-D caused relatively higher P-L fusion for phagosomes that contain single bacilli than for phagosomes containing two or more bacilli (58.2% ± 5.2% with SP-D versus 38.1% ± 2.6% without SP-D) (Fig. 2C). As the number of bacilli per phagosome increased (whether single or clumped), so did P-L fusion—an effect that was further enhanced in the presence of SP-D, except in the case of phagosomes each with three or more bacilli where the percentage of P-L fusion was very high. These data indicate that SP-D coating of M. tuberculosis has a direct effect on increasing P-L fusion of single bacilli and also may have an indirect effect by inducing agglutination of the phagocytosed bacilli to a certain extent.
FIG. 2.
SP-D increases P-L fusion of M. tuberculosis-containing phagosomes. Duplicate MDM monolayers were loaded with HRP and then incubated with M. tuberculosis in the presence or absence of SP-D. After 2 h at 37°C, the MDMs were fixed and the HRP revealed by diaminobenzidine. The MDMs were then processed for TEM. (A) TEM section showing a single bacillus contained in a phagosome that has not fused with lysosomes. (B) TEM section showing a single bacillus (arrowhead) contained within a phagosome that also contains the characteristic black HRP precipitate (arrow), indicating P-L fusion. (C) Graph showing the summary of percentages of M. tuberculosis phagosomes containing the HRP precipitate with one, two, or three or more bacilli per phagosome. The results shown are the means ± standard deviations of duplicate determinations in a representative experiment (mean ± standard deviation, n = 2, P < 0.05 for the SP-D group versus the control group for one or two bacteria).
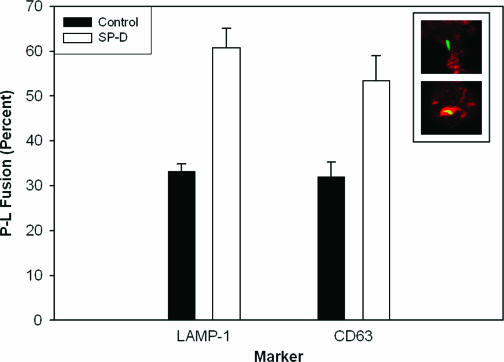
The confocal microscopy assay for P-L fusion is based on colocalization of the phagosomes with the lysosomal/late endosomal markers LAMP-1 and/or CD63 as described previously (14, 21). A green fluorescent protein-expressing M. tuberculosis H37Rv strain (M. tuberculosis-GFP; a kind gift from V. Deretic, University of New Mexico) was preincubated with SP-D before infection of the MDM monolayers as described above. The yellow appearance of individual bacilli on confocal images represents a colocalization of the bacillus (green) with the lysosomal marker (red). The association of M. tuberculosis phagosomes with LAMP-1 was significantly increased from 33.3% ± 1.5% (control) to 53.5% ± 5.5% in the presence of SP-D (Fig. 3). In separate experiments, we found that SP-D also increased the colocalization of M. tuberculosis-GFP with CD63 (Fig. 3). These data correlate well with our TEM data and together with them provide strong evidence that SP-D enhances P-L fusion of M. tuberculosis-containing phagosomes.
FIG. 3.
SP-D increases the colocalization of M. tuberculosis with lysosomal markers LAMP-1 and CD63. M. tuberculosis-GFP bacteria were incubated with or without SP-D (5.0 μg/ml) for 1 h, added to MDM monolayers for 2 h at 37°C, fixed, and then sequentially incubated with antibodies to LAMP-1 or CD63 and Texas Red anti-mouse immunoglobulin G. The MDM monolayers were then examined by confocal microscopy. The graph illustrates the percentages of single M. tuberculosis-GFP bacilli that colocalize with LAMP-1 or CD63, representing P-L fusion. The inset shows confocal micrographs of unfused (green on upper panel) and fused (yellow on lower panel) M. tuberculosis in phagosomes. Data shown are the means ± SEMs of triplicate determinations from three independent experiments (mean ± SEM, n = 3, P < 0.05, for the SP-D group versus the control group).
ManLAM beads are phagocytosed by the macrophage MR and reside in phagosomes with limited P-L fusion (14). To determine whether the effect on SP-D of increasing the P-L fusion of M. tuberculosis-containing phagosomes was related to its binding to ManLAM and disrupting the ManLAM-MR pathway, we assayed for P-L fusion of ManLAM beads or control human serum albumin (HSA) beads in the presence or absence of SP-D. Purified ManLAM from M. tuberculosis H37Rv was used to coat green fluorescent polystyrene beads (Polysciences, Inc.) as described previously (25), and the ManLAM-coated beads or HSA control beads were preincubated with SP-D before they were added to the MDM monolayers as done above with M. tuberculosis. The cells were processed (14) and analyzed by confocal microscopy. The yellow color obtained by colocalization of the green beads with the red-stained CD63 marker was scored as P-L fusion. In the absence of SP-D, P-L fusion for ManLAM bead phagosomes was 34% ± 2%, whereas in the presence of SP-D, there was a significant increase in P-L fusion (56% ± 2%, mean ± standard error of the mean [SEM]; n = 2; P < 0.05). In contrast, there was no significant difference in the level of P-L fusion with or without SP-D treatment for HSA control beads (data not shown).
To determine whether the increased P-L fusion seen with M. tuberculosis and SP-D is due primarily to bacterial agglutination which requires the collagen-like region (CLR) of SP-D (8), we performed similar experiments with M. tuberculosis using the trimeric neck CRD (NCRD) fusion protein, which lacks the CLR but contains the neck and CRD of human SP-D (4). We found that the NCRD protein also increased P-L fusion for M. tuberculosis-containing phagosomes (Fig. 4). These data indicate that the increased P-L fusion due to SP-D does not require the CLR of the protein or agglutination of the bacilli. These data, along with our previous work (7, 8), provide further evidence that SP-D influences the interaction between the macrophage and M. tuberculosis primarily via the CRD portion of the protein that binds to the surface of the bacterium via ManLAM.
FIG. 4.
NCRD increases the colocalization of M. tuberculosis-GFP with LAMP-1. M. tuberculosis-GFP bacteria were incubated with or without SP-D or NCRD (5.0 μg/ml) for 1 h, added to MDM monolayers for 2 h, fixed, and then sequentially incubated with antibodies to LAMP-1 and Texas Red anti-mouse immunoglobulin G. The MDM monolayers were then examined by confocal microscopy. The graph shows the percentages of P-L fusion of single M. tuberculosis bacilli in the absence or presence of human SP-D or its derivative NCRD. Shown are the means ± SEMs of triplicate determinations from three independent experiments (mean ± SEM, n = 3, P < 0.05, for the SP-D and NCRD groups versus the control group).
SP-D is an important member of the lung collectin family. Lung collectins are involved in the pathogenesis and host defense of many respiratory infectious diseases including tuberculosis. SP-D has been shown to reduce entry of M. tuberculosis into macrophages, agglutinate the bacteria potentially to aid their clearance from the lung, and reduce survival of the SP-D-coated bacilli that are phagocytosed by macrophages. In the current study, we provide a potential mechanism for the reduced intracellular survival of M. tuberculosis in macrophages, i.e., SP-D enhances fusion of the M. tuberculosis phagosome with lysosomes. M. tuberculosis ManLAM has been shown to be a key molecule in mediating phagosome maturation arrest (3, 9). We have previously demonstrated that the CRD of SP-D binds to the terminal mannose caps of ManLAM (7). At the same time, the macrophage MR recognizes the terminal mannose caps of ManLAM as its principal ligand in mediating phagocytosis of virulent M. tuberculosis by human macrophages (15, 24, 25). Therefore, the reduced phagocytosis of M. tuberculosis in the presence of SP-D observed in our previous study (7) is likely due to a reduction in the interaction between the ManLAM caps and the MR. Recently, we have demonstrated that the MR plays a crucial role in inhibiting maturation of phagosomes containing M. tuberculosis or ManLAM-coated beads (14). Blocking the MR activity by specific inhibitors or masking the ManLAM mannose caps by anti-ManLAM antibody redirected the particles toward other phagocytic receptors and caused higher levels of P-L fusion in macrophages (14).
Taken together, our current study clearly supports the idea that SP-D binds and masks the terminal mannose caps of ManLAM on the surface of M. tuberculosis and directs phagocytosis of the bacilli through alternative routes other than the ManLAM-MR pathway, thereby promoting phagosome maturation. Thus, our data indicate that SP-D, in contrast to SP-A, serves as an important innate host defense protein for M. tuberculosis in the lungs.
Acknowledgments
This work was supported in part by a grant from the NHLBI, NIH HL03885, and a grant from the American Lung Association of Iowa (J. S. Ferguson); NHLBI, NIH HL51990 (L. S. Schlesinger and D. R. Voelker); HL45286 and HL66235 (D. R. Voelker); NIAID, NIH 33004 and NIH 59639 (L. S. Schlesinger); and HL-44015 and HL-29594 (E. C. Crouch).
We gratefully acknowledge Jordi B. Torrelles for purifying M. tuberculosis ManLAM and coating beads; Thomas Kaufman for his technical assistance; the Central Microscopy and Imaging Facilities both at the University of Iowa and at The Ohio State University; Lee-Ann Allen, Carver College of Medicine, University of Iowa, for her assistance with confocal microscopy; and the Biosafety Level 3 Facilities at the VAMC, Iowa City, IA, and The Ohio State University.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 9 October 2006.
REFERENCES
- 1.Armstrong, J. A., and P. D. Hart. 1971. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 134:713-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beharka, A. A., C. D. Gaynor, B. K. Kang, D. R. Voelker, F. X. McCormack, and L. S. Schlesinger. 2002. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J. Immunol. 169:3565-3573. [DOI] [PubMed] [Google Scholar]
- 3.Chua, J., I. Vergne, S. Master, and V. Deretic. 2004. A tale of two lipids: Mycobacterium tuberculosis phagosome maturation arrest. Curr. Opin. Microbiol. 7:71-77. [DOI] [PubMed] [Google Scholar]
- 4.Crouch, E., Y. Tu, D. Briner, B. McDonald, K. Smith, U. Holmskov, and K. Hartshorn. 2005. Ligand specificity of human surfactant protein D: expression of a mutant trimeric collectin that shows enhanced interactions with influenza A virus. J. Biol. Chem. 280:17046-17056. [DOI] [PubMed] [Google Scholar]
- 5.Crouch, E. C. 1998. Collectins and pulmonary host defense. Am. J. Respir. Cell Mol. Biol. 19:177-201. [DOI] [PubMed] [Google Scholar]
- 6.Crowther, J. E., V. K. Kutala, P. Kuppusamy, J. S. Ferguson, A. A. Beharka, J. L. Zweier, F. X. McCormack, and L. S. Schlesinger. 2004. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J. Immunol. 172:6866-6874. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson, J. S., D. R. Voelker, F. X. McCormack, and L. S. Schlesinger. 1999. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J. Immunol. 163:312-321. [PubMed] [Google Scholar]
- 8.Ferguson, J. S., D. R. Voelker, J. A. Ufnar, and L. S. Schlesinger. 2002. Surfactant protein D inhibition of human macrophage uptake of Mycobacterium tuberculosis is independent of bacterial agglutination. J. Immunol. 168:1309-1314. [DOI] [PubMed] [Google Scholar]
- 9.Fratti, R. A., J. Chua, I. Vergne, and V. Deretic. 2003. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA 100:5437-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaynor, C. D., F. X. McCormack, D. R. Voelker, S. E. McGowan, and L. S. Schlesinger. 1995. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J. Immunol. 155:5343-5351. [PubMed] [Google Scholar]
- 11.Harrod, K. S., B. C. Trapnell, K. Otake, T. R. Korfhagen, and J. A. Whitsett. 1999. SP-A enhances viral clearance and inhibits inflammation after pulmonary adenoviral infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 277:L580-L588. [DOI] [PubMed] [Google Scholar]
- 12.Hempel, S. L., G. R. Buettner, Y. Q. O'Malley, D. A. Wessels, and D. M. Flaherty. 1999. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic. Biol. Med. 27:146-159. [DOI] [PubMed] [Google Scholar]
- 13.Hickman-Davis, J., J. Gibbs-Erwin, J. R. Lindsey, and S. Matalon. 1999. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite. Proc. Natl. Acad. Sci. USA 96:4953-4958.10220400 [Google Scholar]
- 14.Kang, B. K., A. K. Azad, J. B. Torrelles, T. M. Kaufman, A. A. Beharka, E. Tibesar, L. E. Desjardin, and L. S. Schlesinger. 2005. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 202:987-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang, B. K., and L. S. Schlesinger. 1998. Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan. Infect. Immun. 66:2769-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kielian, M. C., and Z. A. Cohn. 1980. Phagosome-lysosome fusion: characterization of intracellular membrane fusion in mouse macrophages. J. Cell Biol. 85:754-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeVine, A. M., M. D. Bruno, K. M. Huelsman, G. F. Ross, J. A. Whitsett, and T. R. Korfhagen. 1997. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J. Immunol. 158:4336-4340. [PubMed] [Google Scholar]
- 18.LeVine, A. M., J. Gwozdz, J. Stark, M. Bruno, J. Whitsett, and T. Korfhagen. 1999. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J. Clin. Investig. 103:1015-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeVine, A. M., K. E. Kurak, M. D. Bruno, J. M. Stark, J. A. Whitsett, and T. R. Korfhagen. 1998. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am. J. Respir. Cell Mol. Biol. 19:700-708. [DOI] [PubMed] [Google Scholar]
- 20.LeVine, A. M., J. A. Whitsett, J. A. Gwozdz, T. R. Richardson, J. H. Fisher, M. S. Burhans, and T. R. Korfhagen. 2000. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J. Immunol. 165:3934-3940. [DOI] [PubMed] [Google Scholar]
- 21.Malik, Z. A., G. M. Denning, and D. J. Kusner. 2000. Inhibition of Ca2+ signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages. J. Exp. Med. 191:287-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogasawara, Y., F. X. McCormack, R. J. Mason, and D. R. Voelker. 1994. Chimeras of surfactant proteins A and D identify the carbohydrate recognition domains as essential for phospholipid interaction. J. Biol. Chem. 269:29785-29792. [PubMed] [Google Scholar]
- 23.Ogasawara, Y., and D. R. Voelker. 1995. The role of the amino-terminal domain and the collagenous region in the structure and the function of rat surfactant protein D. J. Biol. Chem. 270:19052-19058. [DOI] [PubMed] [Google Scholar]
- 24.Schlesinger, L. S. 1993. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J. Immunol. 150:2920-2930. [PubMed] [Google Scholar]
- 25.Schlesinger, L. S., S. R. Hull, and T. M. Kaufman. 1994. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J. Immunol. 152:4070-4079. [PubMed] [Google Scholar]
- 26.Wayne, S., G. Denning, D. J. Kusner, T. M. Kaufman, and L. S. Schlesinger1995. Phagocytosis of virulent and attenuated strains of M. tuberculosis by human macrophages does not generate detectable superoxide anion or hydrogen peroxide. Clin. Res. 43:219A. [Google Scholar]
- 27.Wright, J. R. 1997. Immunomodulatory functions of surfactant. Physiol. Rev. 77:931-962. [DOI] [PubMed] [Google Scholar]