Abstract
Leishmania amazonensis can cause progressive disease in most inbred strains of mice. We have previously shown that L. amazonensis-infected C57BL/6 mice have profound impairments in expression of proinflammatory cytokines and chemokines and in activation of antigen-specific CD4+ T cells. These impairments are independent of interleukin-4 (IL-4) but partially due to IL-10 production. The precise mechanism of pathogenesis associated with L. amazonensis infection remains largely unresolved. Since chemokines are essential mediators of leukocyte recruitment and effector cell function, we hypothesized that these molecules are important for the initiation of early responses locally and for the eventual control of the infection. In this study, we examined the roles of CXCL10/gamma interferon-inducible protein 10 (IP-10) and CCL2/monocyte chemoattractant protein 1 (MCP-1) in the activation of the macrophage effector function in vitro and their efficacy in ameliorating infection in vivo. Bone marrow-derived macrophages of both BALB/c and C57BL/6 mice were treated with increasing concentrations of recombinant chemokines prior to infection with either stationary-phase promastigotes or tissue-derived amastigotes. We found that treatment with IP-10 or MCP-1 significantly reduced parasite burdens, in a dose-dependent manner, and triggered nitric oxide production. When susceptible C57BL/6 mice were injected locally with IP-10 following L. amazonensis infection, there was a significant delay in lesion development and a reduction in parasite burdens, accompanied by 7- and 3.5-fold increases in gamma interferon and IL-12 secretion, respectively, in restimulated lymph node cells. This study confirms that IP-10 plays a protective role in promoting the reduction of intracellular parasites and thereby opens new avenues for therapeutic control of nonhealing cutaneous leishmaniasis in the New World.
Leishmania parasites preferentially infect cells of the macrophage (Mφ) lineage and replicate within phagolysosomes, resulting in diverse clinical manifestations. The severity of disease is dependent on both the causative species of parasite and the immunological status of the host (26). Immune regulation of host responses to Leishmania has been investigated extensively in murine models of leishmaniasis. Resistance to Leishmania major in C57BL/6 (B6) or C3H mice (36) is clearly linked to a dominant Th1 response. While the susceptibility of BALB/c mice to L. major is reliant upon the early production of interleukin-4 (IL-4), which promotes lesion development during the early stages of infection, long-term persistence of L. major in resistant strains of mice appears to be mediated by IL-10 production (1).
Although the New World species L. amazonensis can cause progressive disease in a majority of inbred mouse strains, including BALB/c, B6, and C3H mice (15, 31, 39), these mice do not exhibit a classical Th2-dominant phenotype. Lesion development in L. amazonensis-infected mice is largely due to an impairment of parasite-specific Th1-cell activation (15, 31, 39). However, circulating antibodies may also contribute to L. amazonensis pathogenesis (22) via multiple mechanisms, including antibody-mediated IL-10 production (19). We and others have previously shown that RAG2−/− and major histocompatibility complex class II-negative mice are refractory to L. amazonensis infection, suggesting an essential role for CD4+ T-cell-mediated responses in the immunopathogenesis of L. amazonensis infection (39, 42).
Attempts at using proinflammatory cytokines to boost protective immunity against L. amazonensis have yielded limited success. For example, recombinant IL-12 (16) or IL-1α (46), when administered prior to L. major infection, made otherwise susceptible BALB/c mice resistant; however, exogenous IL-12 (18) or IL-1α (L. Li, R. E. Vasquez, and L. Soong, unpublished results) did not ameliorate L. amazonensis disease progression. These negative results are due in part to an IL-4-independent down-regulation of the IL-12 receptor β2 chain on CD4+ T cells, making these cells unresponsive to both intrinsic and exogenous cytokines (18). The precise mechanism leading to this IL-12 unresponsiveness remains unresolved.
Chemokines belong to a large subset of cytokines that are crucial mediators of leukocyte function, activation, and trafficking of cells involved in inflammatory responses (34). CXCL10/gamma interferon-inducible protein 10 (IP-10) binds with high affinity to CXCR3 (6), a receptor known to be expressed on several types of cells in the hematopoietic lineage, including activated and memory CD4+ and CD8+ T cells, NK cells, and some subsets of dendritic cells (27, 33, 45). Injection of IP-10 into L. major-infected BALB/c mice has been shown to induce strong NK cell recruitment and activation (28). Interestingly, L. donovani promastigotes produce a granulocytic chemotactic factor that preferentially inhibits the production of IP-10 from polymorphonuclear cells, suggesting a complex regulation of this chemokine in host-parasite interactions (44).
We have recently shown an insufficient induction of proinflammatory mediators at early stages of infection with L. amazonensis parasites and deficient priming in parasite-specific Th1 cells during the course of infection (15). We hypothesized that administration of IP-10 would skew the host's local environment towards a Th1-type immune response and therefore facilitate parasite elimination. In the present study, we show that treatment with recombinant IP-10 activated murine bone marrow-derived macrophages (BM-Mφs) to significantly reduce parasite infection in vitro and that local injection of IP-10 significantly delayed disease development in susceptible mice. This enhanced parasite killing was partially due to the production of multiple effector molecules, such as gamma interferon (IFN-γ), IL-12, and nitric oxide (NO). Collectively, the results of this study indicate that exogenous IP-10 enhances protective defense mechanisms that are critical for the control of nonhealing, New World cutaneous leishmaniasis.
MATERIALS AND METHODS
Mice.
Wild-type BALB/c, C57BL/6 (B6), C3H/HeJ (C3H), and inducible NO synthase (iNOS)-deficient (iNOS−/−) B6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). They were maintained under specific-pathogen-free conditions and used at 6 to 10 weeks of age. All protocols were approved by the Animal Care and Use Committee of the University of Texas Medical Branch (Galveston, TX).
Parasites.
L. amazonensis (MHOM/BR/77/LTB0016) parasites were maintained by regular passage through BALB/c mice. To culture parasites, 20% fetal bovine serum-supplemented (HyClone) Schneider's Drosophila medium (Invitrogen, Rockville, MD) was used at pH 7 for promastigotes and pH 5 for amastigotes. Promastigotes were cultured at 23°C. Tissue-derived amastigotes were harvested from foot tissues of infected BALB/c mice and cultured at 33°C for 48 h before in vitro infection.
Mφ culture.
BM-Mφs were generated as previously described (30). Briefly, bone marrow cells were seeded in a petri dish at 2 × 106 per 10 ml of complete Iscove's modified Eagle medium (Iscove's modified Eagle medium containing 10% fetal bovine serum, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 50 μg/ml gentamicin, and 100 U/ml penicillin) supplemented with 10% L929 culture supernatant. After 5 days, nonadherent cells were discarded, and adherent cells were maintained for an additional 4 days before being detached from the petri dish with cold phosphate-buffered saline (PBS) containing 2 mM EDTA. These cells were washed twice with warm Iscove's modified Eagle medium and were then seeded into 24-well plates at 3 × 105 cells/well.
Flow cytometric analysis of Mφ surface antigens.
The quality and quantity of BM-Mφs were assessed by fluorescence-activated cell sorting (FACS), using specific or isotype control monoclonal antibodies (MAbs) purchased from BD Biosciences (San Jose, CA) unless indicated otherwise. For blocking of nonspecific Ab binding and Fc receptors, purified anti-mouse CD16/32 (eBioscience, San Diego, CA), hamster immunoglobulin G (IgG; Pierce, Rockford, IL), and 2% rat serum (Sigma, St. Louis, MO) were used. After 30 min of being blocked on ice, cells were stained with a phycoerythrin (PE)-conjugated anti-mouse CXCR3 MAb (R&D Systems, Minneapolis, MN) and fluorescein isothiocyanate (FITC)-conjugated anti-mouse F4/80 MAb in a final volume of 200 μl for 30 min (1 μg/106 cells). To assess the general quality of BM-Mφ cultures, cells were also stained with FITC-conjugated anti-mouse CD3 and PE-conjugated anti-mouse CD8α (eBioscience). Cells were washed and analyzed on a FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ). For characterization of BM-Mφs, at least 10,000 events were collected. Data were analyzed with FlowJo software (TreeStar, San Carlos, CA). Isotype control Abs included FITC-conjugated hamster IgG1 and IgG2a and PE-conjugated rat IgG2a (eBioscience). BM-Mφ purity was determined to be ∼90%, as judged by staining with the F4/80 Mφ-specific marker. CD3+ and CD8α+ cells were undetectable via FACS analysis.
Mφ stimulation and parasite infection.
BM-Mφs were rested for at least 12 h in 24-well plates, washed once with warm medium, and then treated with recombinant IP-10, monocyte chemoattractant protein 1 (MCP-1), tumor necrosis factor alpha (TNF-α), IFN-γ (Leinco Technologies, St. Louis, MO), or lipopolysaccharide (LPS; Sigma, St. Louis, MO) at the indicated concentrations and/or in combinations for 4 h before infection with L. amazonensis promastigotes or lesion-derived amastigotes. According to the manufacturers' data sheets, endotoxin levels in these recombinants were measured to be <0.1 ng per μg. Amastigote binding to Mφs was synchronized by centrifugation of the culture plates at 100 × g for 5 min immediately after the addition of parasites. Parasite-exposed BM-Mφs were kept for 24 h at 33°C, a temperature consistent with that of Leishmania-induced cutaneous lesions, and then moved to 37°C for the rest of the observation period. Infected BM-Mφ cultures were processed to evaluate intracellular parasite burdens at 5, 24, or 48 h postinfection, as in our previous report (30). Briefly, Mφs in 24-well plates (3 wells per condition) were gently washed twice with PBS and then exposed to 0.2 ml of 0.01% sodium dodecyl sulfate (SDS) in PBS at 37°C. The process of cell lysis, which was monitored under an inverted microscope, was typically completed within 10 min. The cell suspension was immediately supplemented with 0.8 ml complete culture medium to avoid lysis of released parasites. The number of parasites per well was counted with a hemocytometer. In some cases, the numbers of intracellular parasites per cell were assessed by fluorescence microscopy, as in our previously described studies (19). Briefly, BM-Mφs were seeded into 24-well plates (3 × 105 cells/coverslip/well). Cells were left untreated or treated with either IP-10 (100 ng/ml), MCP-1 (100 ng/ml), or LPS (20 ng/ml) plus IFN-γ (20 ng/ml or 2 U/ml) for 4 h prior to infection with 2.4 × 106 stationary-phase promastigotes (at an 8:1 parasite-to-cell ratio). At 5 or 48 h, cells were fixed on a glass coverslip with methanol at 4°C for 20 min and then washed thrice with PBS. Antisera from L. amazonensis-infected BALB/c mice were diluted 1:500 in PBS and used to stain cells for 20 min at 4°C. After being washed, cells were stained with FITC-conjugated goat anti-mouse IgG (1:250) at 4°C for 20 min. Cells were counterstained with a mounting medium containing 4′,6′-2-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) and viewed under an Olympus BX51/52 fluorescence microscope (Olympus America Inc., Melville, NY). To enumerate intracellular parasites, the software-merged images from 10 random visual fields per condition were taken for counting total numbers of parasites and host cells. Data are presented as numbers of parasites per 100 Mφs and were pooled from three independent experiments (representing a total of 30 visual fields).
Mouse infection and disease evaluation.
Mice (five per group) were inoculated subcutaneously (s.c.) in the right hind foot with 2 × 105 L. amazonensis metacyclic promastigotes that were purified through negative selection with the 3A1 MAb (a generous gift from David Sacks, NIAID) as previously described (7). Lesion sizes were measured weekly with a digital caliper (Sigma, St. Louis, MO). At the indicated time points, mice were sacrificed to determine the parasite burden by a limiting dilution assay, as previously described (39). For in vivo chemokine treatment, each mouse was injected s.c. with IP-10 (100 ng in 5 μl) or PBS (5 μl) at 1, 3, and 7 days postinfection.
RNA extraction and RT-PCR analysis.
Total RNA was isolated from control or IP-10-treated BM-Mφs or whole foot tissue from sacrificed mice, using Tri reagent (Sigma). Splenocytes from naïve or L. amazonensis-infected mice were treated with LPS/IFN-γ and served as positive controls. Total RNA (100 ng) was subjected to reverse transcription-PCR (RT-PCR) analysis, with annealing temperatures of 56°C for CXCR3 and 58°C for IFN-γ and β-actin. Primer sequences (listed 5′-3′) were as follows: CXCR3 forward, GCTAGATGCCTCGGACTTTG; CXCR3 reverse, GCTGATCGTAGTTGGCTGATA (5); IFN-γ forward, CATTGAAAGCCTAGAAAGTCTG; IFN-γ reverse, CTCATGGAATGCATCCTTTTTCG (32); β-actin forward, CCAGCCTTCCTTCCTGGGTA; and β-actin reverse, CTAGAGCATTTGCGGTGCA. The expected product sizes were 557 nucleotides (nt) for CXCR3, 267 nt for IFN-γ, and 350 nt for β-actin. To determine IFN-γ expression, bands were normalized with β-actin groups, and intensities were compared to determine changes in gene expression (AlphaEase Fluor Chem 9900; Alpha Innotech, San Leandro, CA).
NO assay.
The generation of nitrite in Mφ cultures was assessed by the Griess reaction, using a nitrate/nitrite colorimetric assay kit (Caymann Chemical, Ann Arbor, MI). For each assay, Mφs (3 × 105 cells/ml) in 24-well plates were infected with 2.4 × 106 promastigotes (at an 8:1 parasite-to-cell ratio) for 48 h. Culture supernatants were incubated with the Griess reagent (1:1 [vol/vol]) for 10 min at room temperature. The absorbance was measured at 540 nm, and the nitrite concentration was determined using a standard curve of sodium nitrite and expressed as a micromolar value. In each experiment, supernatants from untreated, uninfected cells were included as negative controls.
Protein cytokine array.
In the case of in vitro assays, BM-Mφs (1 × 106 cells/well) in six-well plates were treated with 100 ng/ml of IP-10 for 4 h and then infected with 8 × 106 L. amazonensis promastigotes (8:1) for 48 h. In the case of in vivo assays, infected mice were sacrificed at 3 and 6 weeks postinfection to collect draining lymph node (LN) cells. Cells were pooled from five mice per group and cultured (5 × 106/well) in six-well plates in the presence or absence of parasite lysate (1 × 107 parasite equivalents) for 48 h. The abundance and profiles of cytokine production in supernatants were assessed by using a mouse cytokine antibody array (2.1; RayBioTech, Atlanta, GA). Briefly, membranes were incubated sequentially with blocking buffer and culture supernatants for 2 h, a cocktail of biotin-conjugated anti-cytokine Abs for 2 h, and then horseradish peroxidase-conjugated streptavidin (diluted 1:1,000) for 1 h. Detection was accomplished with the manufacturer's detection kit, and membranes were subsequently exposed to Kodak X-Omat AR films (Fisher, Philadelphia, PA). Each membrane contained positive and negative controls and allowed the simultaneous detection of more than 20 molecules (two spots per molecule). The intensity of positive controls was used to normalize membranes, and intensities of duplicate dots for each molecule were averaged for comparison (AlphaEase Fluor Chem 9900; Alpha Innotech, San Leandro, CA).
Data analysis.
To evaluate the statistical significance among different groups, a one-way analysis of variance was used with Tukey's multiple comparison test for parametric comparisons, while a Kruskal-Wallis test with Dunn's multiple comparison posttest was used for nonparametric comparisons. The tests were performed using GraphPad Prism, version 4.00, for Windows (GraphPad Software, San Diego California). Statistically significant values are referred to as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
RESULTS
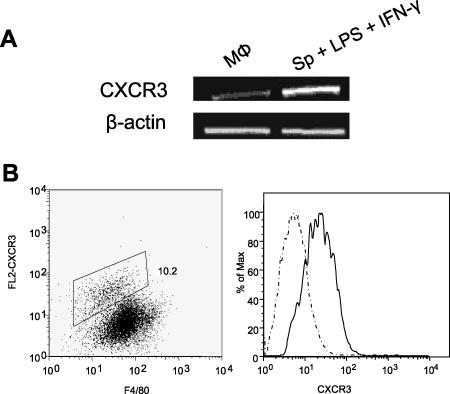
Expression of CXCR3 on murine BM-Mφs.
Although murine BM-Mφs are known to express various chemokine receptors, such as CCR2, the receptor for MCP-1, it is unclear whether these cells also express CXCR3, either constitutively or conditionally. To address this issue, we examined CXCR3 expression on BM-Mφs of BALB/c mice and used LPS/IFN-γ-treated splenocytes as positive controls for CXCR3 induction. RT-PCR analysis indicated the presence of CXCR3 mRNA in unstimulated Mφs (Fig. 1A). To confirm the surface expression of CXCR3 on BM-Mφs, we performed FACS analysis and observed a small population (∼10%) of cells that were doubly positive for both CXCR3 and the pan-Mφ marker F4/80 (Fig. 1B), suggesting the responsiveness of these cells to IP-10. However, Mφs stimulated with LPS/IFN-γ did not significantly upregulate CXCR3 expression, unlike the case in splenocytes (data not shown), which suggested that CXCR3 regulation on Mφs may differ from that observed in T cells (27).
FIG. 1.
CXCR3 is expressed on murine BM-Mφs. BM-Mφs of BALB/c mice were seeded into six-well plates (2.5 × 106 cells/well) and allowed to rest for 24 h. (A) Total RNA (100 ng) was extracted for RT-PCR analyses of CXCR3 and β-actin transcripts. Splenocytes from BALB/c mice were treated with LPS/IFN-γ and used as positive controls. (B) Mφs were collected and stained for surface expression of F4/80 and CXCR3 molecules. For flow cytometry, the single gate was set up according to the isotype control, and the number represents the percentage of cells within the gate. Staining profiles of the isotype control (dashed line) and the anti-CXCR3 group (solid line) were included in the histogram overlay representing the shift in fluorescence.
Treatment with IP-10 or MCP-1 promotes reduction of L. amazonensis promastigote and amastigote infection in vitro.
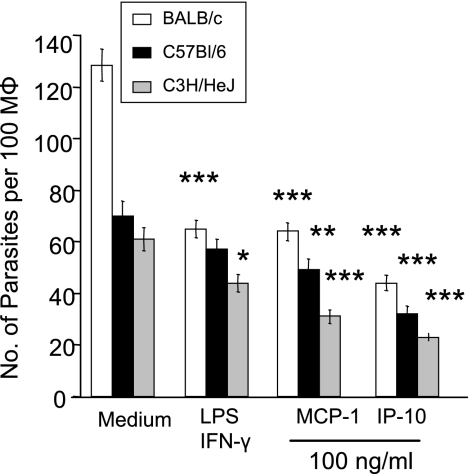
It has been reported that treatment of murine Mφs (3) or human monocytes (4) with recombinant MCP-1 or macrophage inflammatory protein 1α (MIP-1α) significantly reduces L. donovani and L. infantum parasite burdens through the induction of NO. To test whether this leishmanicidal activity can be induced in L. amazonensis infection, we evaluated the efficacy of exogenous IP-10 (1 to 200 ng/ml) in parasite killing. At 48 h postinfection, the parasite burden decreased with increasing amounts of IP-10 and MCP-1, indicating a dose-dependent effect (data not shown). Since significant reductions in the parasite burden were achieved for both tested chemokines at 100 ng/ml, this concentration was used for the subsequent studies reported herein.
As shown in Fig. 2, there was a 70% reduction in parasite loads in BALB/c Mφs (white bars) treated with IP-10 in comparison to the infection controls. Under the same conditions, cells treated with MCP-1 showed a 53% reduction in parasite load at 48 h compared to the untreated controls, which was consistent with previous reports (3, 4). From these results, IP-10 administration appeared to be more efficacious in eliminating intracellular parasites than MCP-1 treatment, while the latter was comparable to LPS/IFN-γ treatment. In addition, BM-Mφs generated from either B6 (black bars) or C3H (gray bars) mice demonstrated similar trends of parasite elimination when treated with IP-10 (Fig. 2), suggesting that this treatment is not restricted to a single mouse genetic background. Because parasite reduction was observed in C3H mice, which are known to be hyporesponsive to LPS, this rules out the possibility that endotoxin contamination acted upon BM-Mφ activation during chemokine treatment.
FIG. 2.
Pretreatment with IP-10 and MCP-1 reduces parasite burdens in Mφs. BM-Mφs of BALB/c, C57BL/6, or C3H/HeJ mice were seeded into 24-well plates (3 × 105 cells/coverslip/well). Cells were left untreated or treated with either IP-10 (100 ng/ml), MCP-1 (100 ng/ml), or LPS (20 ng/ml) plus IFN-γ (20 ng/ml) for 4 h prior to infection with 2.4 × 106 stationary-phase promastigotes (at an 8:1 parasite-to-cell ratio). All groups of cells were subsequently stained for parasites, using pooled sera from infected mice and FITC-conjugated goat anti-mouse IgG. The nuclei of the cells were stained with DAPI. Images of 10 random fields per condition were taken for counting of parasite loads. Data are presented as numbers of parasites per 100 Mφs and are shown as means ± standard deviations (SD) for three independent experiments (representing 30 fields per condition). Each group was compared to untreated controls (*, P < 0.5; **, P < 0.01; ***, P < 0.001).
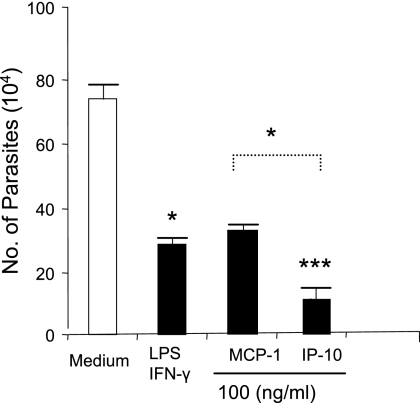
Our group and others have reported that host cells respond differentially when treated with IFN-γ and subsequently infected with either L. amazonensis promastigotes or amastigotes. Specifically, IFN-γ-treated cells carry out NO-mediated killing of promastigotes (23) but promote the growth of amastigotes (30). To determine whether IP-10 treatment is effective in eliminating amastigote infection, we assessed parasite loads at 48 h postinfection. As shown in Fig. 3, parasite loads in IP-10-treated cells (1.1 × 105 parasites/well) were not only significantly lower than those in the untreated controls (7.7 × 105 parasites/well) (P < 0.001) but also significantly lower than those observed in MCP-1-treated cells (3.0 × 105 parasites/well) (P < 0.05). Similar results were observed when Mφs of B6 mice were treated with IP-10 or MCP-1 (data not shown). Together, these results indicate the efficiency of IP-10 administration in controlling infection initiated by both L. amazonensis promastigotes and amastigotes.
FIG. 3.
Pretreatment with IP-10 and MCP-1 promotes reduction of L. amazonensis amastigotes. BM-Mφs of BALB/c mice in 24-well plates (3 × 105/well) were left untreated (white bar) or treated (black bars) with 100 ng/ml of IP-10 or MCP-1 for 4 h prior to infection with 6 × 105 tissue-derived amastigotes (at a 2:1 parasite-to-cell ratio). At 48 h postinfection, cells were lysed with 0.01% SDS, and the number of parasites per well was counted with a hemocytometer (triplicate wells per condition). Data are presented as numbers of parasites per well and shown as the means ± SD for three independent experiments (representing nine wells per condition). Each group was compared to untreated controls (*, P < 0.05; ***, P < 0.001).
Involvement of NO and Th1 cytokines in IP-10-mediated parasite reduction within Mφs.
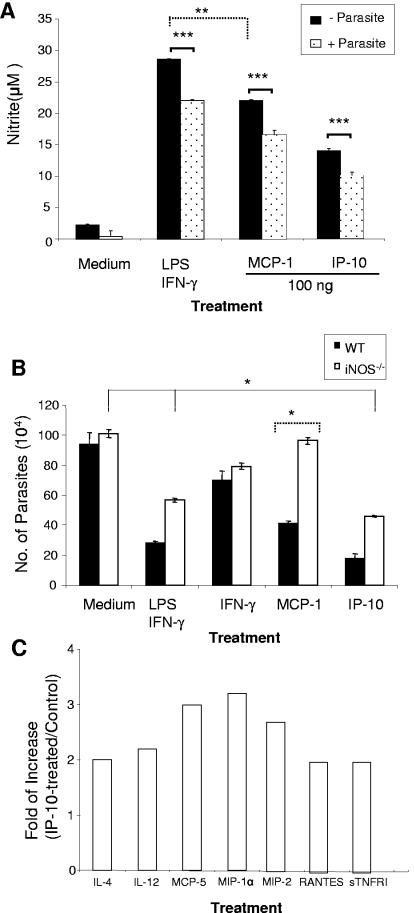
Th1 cytokine-activated Mφs are known to kill intracellular parasites through the production of toxic mediators such as NO, which is produced by iNOS, and the importance of NO in Mφ activation is well documented for animal models of Leishmania infection (3, 4). To address the role of NO in IP-10-mediated parasite reduction, we measured the amounts of nitrite in supernatants of stimulated, L. amazonensis-infected Mφs. Nitrite production in medium control cells was negligible. In the absence of parasites, treatment of cells with IP-10, MCP-1, or LPS/IFN-γ resulted in highly significant increases in NO production compared to that seen in medium control cells (P < 0.001) (Fig. 4A). Although cells treated with stimuli and then infected with parasites also presented significant amounts of NO, the levels were significantly lower in these cells than in those treated with IP-10, MCP-1, or LPS/IFN-γ in the absence of promastigotes (P < 0.001). This observation further substantiates the parasite-mediated inhibition of nitrite production seen in previous studies (24). Interestingly, IP-10 did not stimulate nitrite production in Mφs to a level comparable to those observed for the LPS/IFN-γ- and MCP-1-treated groups (P < 0.01), implying the involvement of other mechanisms in parasite killing by IP-10 treatment.
FIG. 4.
Nitric oxide and proinflammatory chemokines contribute to IP-10- and MCP-1-mediated parasite killing. (A) BM-Mφs of B6 mice (3 × 105 cells/well) in 24-well plates were left untreated or treated with the indicated stimuli, as described in the legend to Fig. 2, for 4 h prior to infection with 2.4 × 106 stationary-phase promastigotes (at an 8:1 parasite-to-cell ratio). At 48 h postinfection, supernatants from uninfected (black bars) and infected (dotted bars) groups were collected for measurement of nitrite via the Griess reagent. (B) BM-Mφs were generated from wild-type B6 mice (black bars) or iNOS−/− B6 mice (white bars) and treated with 100 ng/ml of IP-10, MCP-1, or IFN-γ for 4 h prior to infection with L. amazonensis lesion-derived amastigotes as described in the legend to Fig. 2. Cells treated with LPS (20 ng/ml) plus IFN-γ (20 ng/ml) served as positive controls. At 48 h postinfection, cells were treated with 0.01% SDS to release intracellular parasites, and the parasite number per well was counted. Data are presented as numbers of parasites per well and expressed as means ± SD for triplicate wells per condition. The iNOS−/− groups were compared with their wild-type counterparts ( , P < 0.05;
, P < 0.05; 
 , P < 0.01);
, P < 0.01); 

 , P < 0.001). The data shown are representative of three independent repeats. (C) BM-Mφs of BALB/c mice were seeded into six-well plates (1 × 106 cells/well) and infected with 8 × 106 stationary-phase promastigotes (8:1 parasite-to-cell ratio). At 48 h postinfection, cell-free supernatants were collected for the measurement of cytokine profiles via protein cytokine arrays. The intensities of protein spots for the IP-10-treated group were compared with those of the corresponding spots for the untreated controls, and data are presented as x-fold increases above the infection control levels. The data shown are the results for those molecules that displayed ≥2-fold increases over the infection control levels and are representative of three independent repeats.
, P < 0.001). The data shown are representative of three independent repeats. (C) BM-Mφs of BALB/c mice were seeded into six-well plates (1 × 106 cells/well) and infected with 8 × 106 stationary-phase promastigotes (8:1 parasite-to-cell ratio). At 48 h postinfection, cell-free supernatants were collected for the measurement of cytokine profiles via protein cytokine arrays. The intensities of protein spots for the IP-10-treated group were compared with those of the corresponding spots for the untreated controls, and data are presented as x-fold increases above the infection control levels. The data shown are the results for those molecules that displayed ≥2-fold increases over the infection control levels and are representative of three independent repeats.
To further assess the involvement of NO, we treated iNOS−/− B6 Mφs with IP-10 prior to infection. As shown in Fig. 4B, there were significant reductions of parasite loads in both wild-type and iNOS−/− Mφs treated with IP-10 when the latter cells were compared to their relevant infection controls (Fig. 4B) (P < 0.05). This is in stark contrast to the case for groups treated with MCP-1, which resulted in significant increases in parasite loads in iNOS−/− Mφs versus the findings following treatment of wild-type Mφs. This suggests that while NO is an important anti-Leishmania effector and is necessary for MCP-1-mediated reduction of the parasite burden in Mφs, it is not as crucial for IP-10 and LPS/IFN-γ treatment, despite the high NO production.
In addition to the production of reactive nitrogen species, Mφs can also deliver a wide array of antimicrobial proteins via the release of inflammatory cytokines and chemokines (8). To examine the possible contributions of these molecules, we measured the profile of cytokines and chemokines by protein cytokine arrays. In comparison to the findings for infection controls, supernatants of IP-10-treated, L. amazonensis-infected cells generated approximately threefold increases in the production of MIP-1α, CXCL2/MIP-2, and CCL12/MCP-5 as well as a twofold increase in IL-4, IL-12, CCL5/RANTES, and soluble TNF receptor 1 production (Fig. 4C). The production of other molecules was either unchanged (e.g., MCP-1) or at a level below a twofold increase (e.g., CCL20/MIP-3β). It appears that IP-10 treatment can trigger the production of a select set of inflammatory cytokines/chemokines in Mφs, suggesting that aside from NO elicitation, these molecules could potentially aid in the elimination of L. amazonensis parasites in Mφs.
Local injection of IP-10 delays lesion development in susceptible B6 mice by stimulating the production of multiple effector molecules.
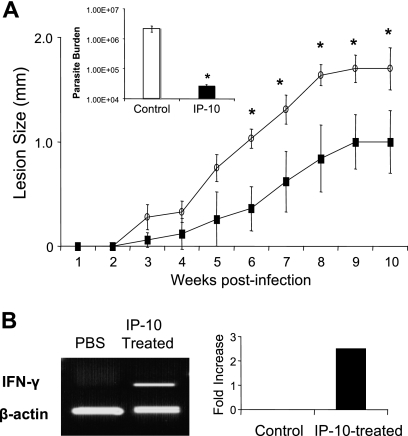
To define the in vivo function of IP-10, we infected two groups of B6 mice with 1 × 105 L. amazonensis metacyclic promastigotes. In comparison to the PBS-injected controls, IP-10-treated mice demonstrated a delay in disease onset, developed significantly smaller lesions at 6 to 10 weeks postinfection (Fig. 5A), and contained significantly lower parasite loads in foot tissues at 10 weeks postinfection (∼2 log; P < 0.05). Interestingly, we observed that IFN-γ expression at the site of infection in IP-10-treated mice was 2.5-fold higher than that in infection controls (Fig. 5B).
FIG. 5.
Local administration of IP-10 delays lesion development in susceptible B6 mice. (A) B6 mice (five per group) were infected s.c. with 2 × 105 L. amazonensis metacyclic promastigotes and treated s.c. with IP-10 (100 ng in 5 μl) or PBS on days 1, 3, and 7 of infection. Lesion size (in mm; size of the infected foot minus size of control foot for each mouse) was monitored weekly, and the data shown are the means ± SD for each group. Results are representative of three independent experiments. The parasite burden per foot was determined at 10 weeks postinfection and shown as the mean for each group.  , P < 0.05 (treated groups in comparison to the PBS controls). (B) At 10 weeks postinfection, PBS- and IP-10-treated mice were sacrificed, and total RNAs were extracted from infected foot tissues. RT-PCR analysis was performed to determine expression changes in IFN-γ. Band intensities were analyzed via spot densitometry, and expression changes were determined after normalization with β-actin.
, P < 0.05 (treated groups in comparison to the PBS controls). (B) At 10 weeks postinfection, PBS- and IP-10-treated mice were sacrificed, and total RNAs were extracted from infected foot tissues. RT-PCR analysis was performed to determine expression changes in IFN-γ. Band intensities were analyzed via spot densitometry, and expression changes were determined after normalization with β-actin.
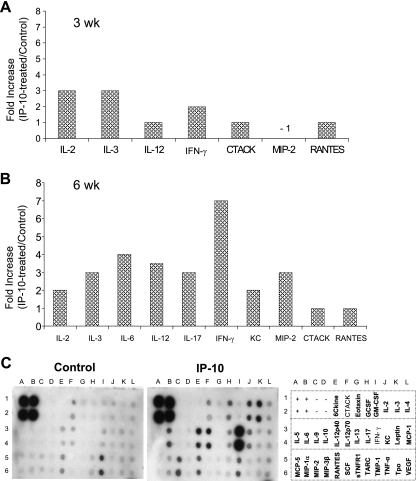
Since IP-10 treatment significantly reduced the parasite burden, we next examined the possible mechanisms underlying this protection. At 3 and 6 weeks postinfection, draining LN cells from IP-10-treated or PBS-injected L. amazonensis-infected mice were restimulated in vitro with Leishmania lysates for 48 h. Culture supernatants were analyzed for cytokine profiles using protein cytokine arrays. In comparison to that in untreated controls at 3 weeks postinfection, there was a threefold increase in the production of IL-2 and IL-3, as well as a twofold increase in IFN-γ production, in IP-10-treated mice (Fig. 6A). Except for some (onefold) increase in IL-12 and CCL5/RANTES and a decrease in CXCL2/MIP-2 in the IP-10-treated group, there were no major changes for the other tested cytokines/chemokines in the arrays. At 6 weeks, however, IP-10-treated mice displayed a unique, Th1-biased cytokine profile in comparison to that of infection controls, with a sevenfold increase in IFN-γ production, correlating with our RT-PCR finding for the lesion site (Fig. 5B), as well as three- to fourfold increases in the production of IL-3, IL-6, IL-12, IL-17, and CXCL2/MIP-2 (Fig. 6B). Evidently, IP-10 treatment triggered the up-regulation of a select set of cytokines and chemokines at 6 weeks of infection, because some molecules (e.g., IL-4, CCL3/MIP-1α, and CCL5/RANTES) showed no or marginal changes in comparison to those found in the controls (Fig. 6C). Overall, these results suggest that exogenous IP-10 stimulates effector cells to produce Th1 and other proinflammatory cytokines, which contribute to the partial control of L. amazonensis infection in vivo.
FIG. 6.
Local administration of IP-10 triggers the production of multiple Th1-favored cytokines and chemokines. B6 mice (five per group) were infected and treated as described in the legend to Fig. 5. At 3 weeks (A) and 6 weeks (B) postinfection, LN cells (5 × 106/ml/well) were collected from IP-10-treated groups or PBS controls, pooled from five mice, and stimulated with parasite antigen (1 × 107 parasite equivalents) for 48 h. Cell-free supernatants were collected for the measurement of cytokine profiles via protein cytokine arrays. The intensities of protein spots for the IP-10-treated group were compared with those of the corresponding spots for the infection controls, and data are presented as x-fold increases above the infection control levels. Data shown in panels A and B represent those molecules with ≥2-fold increases compared to the infection control levels at 3 and 6 weeks postinfection, respectively. Example membranes for infection control and IP-10 groups at 6 weeks postinfection are shown in panel C, and all tested cytokines/chemokines in the membranes are illustrated to the right. Membranes for medium controls using cells from infected mice detected no or minimal expression of cytokines and chemokines (data not shown).
DISCUSSION
The mechanism underlying nonhealing cutaneous leishmaniasis associated with L. amazonensis infection remains largely unresolved. One possibility is that L. amazonensis parasites evade the host immune response by inhibiting the early inflammatory cytokine and chemokine responses, thereby preventing the expansion of antigen-specific Th1 cells even in the absence of Th2 dominance (15). Since IP-10 is a CXC chemokine known to favor the recruitment and activation of Th1-polarized cells (35, 37, 47), we tested the prospects that exogenous IP-10 would significantly reduce parasite loads and that IP-10 injection at early stages of infection would skew immune responses and influence the outcome of L. amazonensis infection in vivo.
In the present study, we have provided evidence that exogenous IP-10 markedly enhances the responsiveness of Mφs to L. amazonensis infection. First, IP-10 treatment significantly reduces the infection prevalence and parasite loads, regardless of whether the initiation of infection occurs by promastigotes (Fig. 2) or lesion-derived amastigotes (Fig. 3). The effect of IP-10 treatment is markedly different from that of IFN-γ treatment (30), and the leishmanicidal activity of IP-10 is more potent than that of LPS/IFN-γ or MCP-1 (Fig. 2 to 4), suggesting a therapeutic potential of this chemokine in the control of Leishmania infection.
Although a wealth of information exists concerning the role of IP-10 in Th cell polarization (11, 12, 37) and the requirement for CXCR3 in the migration of activated CD4+ and CD8+ T cells to inflamed dermal sites (27), especially those induced by L. major infection (35), studies aimed at examining the role of IP-10 in L. major infection have yielded ambiguous results. For example, an early and strong induction of the IP-10 gene correlated nicely with the healing phenotype in L. major-resistant B6 mice (28, 41). A single injection of IP-10 into L. major-susceptible BALB/c mice, however, led to enhanced lesion development (45). Furthermore, it was not clear in the latter report whether heightened footpad swelling was due to increased parasite loads and/or elevated local responses. In the present study, we have shown that three injections of IP-10 at early stages of infection with L. amazonensis parasites resulted in a delayed onset of disease, reduced lesion sizes, and reduced parasite loads (Fig. 5) and that this enhanced resistance to L. amazonensis infection in IP-10-treated mice correlated with a partial reversal of immune nonresponsiveness associated with L. amazonensis infection and with the development of a Th1-biased response (Fig. 6) (15). The discrepancy between the effects of IP-10 in different models of cutaneous leishmaniasis may not be surprising, given the known differences in virulence factors of Old and New World species of Leishmania responsible for cutaneous diseases (26) as well as marked differences in the host immune responses to L. amazonensis and L. major infections (14, 15, 17, 39).
IL-4 production is often linked to the susceptibility of BALB/c mice to L. major infection; however, an IL-4-independent impairment of the host response is a hallmark of nonhealing disease caused by L. amazonensis infection (15, 17). We speculate that when given during the first few days of L. amazonensis infection, exogenous IP-10 acts on multiple cell types in the innate arm of host defense, triggering a positive loop for Th1 responses that are necessary for the control of disease progression. At the Mφ level, we have shown that IP-10 treatment stimulates the production of IL-12, CCL12/MCP-5, CCL3/MIP-1α, and CXLC2/MIP-2 (Fig. 4), which may, in turn, augment the activation of NO production and other leishmanicidal mechanisms for further reducing parasite loads. At the tissue level, exogenous IP-10 may act on multiple cell types, including Mφs, NK cells, and CD4+ and CD8+ T cells, resulting in enhanced innate immunity and parasite-specific Th1 responses in the local tissue (Fig. 5 and 6). It has been reported that s.c. injection of 1 μg of recombinant IP-10 into the footpads of BALB/c mice 2 h prior to infection with L. major resulted in markedly enhanced NK cell cytotoxic activities (28). Evidence from a visceral leishmaniasis model also supports IP-10 activation of IFN-γ-producing cells and the innate response to L. donovani (6). Studies are ongoing to further examine the effect(s) of IP-10 on dendritic cells, CD4+ and CD8+ T cells, and NK cells that may contribute to enhanced resistance in IP-10-treated mice.
Notably, we observed a threefold increase in IL-17 expression in IP-10-treated mice at 6 weeks postinfection (Fig. 6). IL-17 is the prototypical member of a new cytokine family that is involved in the proliferation, maturation, and chemotaxis of neutrophils (2, 20) and in combating intracellular microorganisms (10). IL-17 has pleiotropic activities, including the induction or amplification of the effects of TNF-α, IFN-γ, IL-1, IL-6, IL-8, IP-10, CXCL11/MIG, and MCP-1 expression and the recruitment of neutrophils (20, 40). Since IL-17 also acts on T cells as a costimulatory factor (10), more investigation is needed to determine the role of IL-17 in L. amazonensis and other Leishmania infections.
Although IP-10-treated mice mounted appreciable levels of innate responses and antigen-specific Th1 responses, these mice remained susceptible to L. amazonensis infection. Several possibilities may account for these observations. First, the half-life of recombinant IP-10 in vivo may not be sufficient to provide a long-lasting effect. The use of an IP-10/Ig fusion protein or IP-10-expressing plasmid may partially overcome this problem. In this regard, it has been reported that injection of an IP-10-encoding expression vector can redirect and promote antigen-specific, Th1-biased responses, suggesting its potential as an immunotherapy (47). Coadministration of DNAs encoding IP-10 and IL-12 has been shown to markedly enhance the antitumor efficiencies of the individual DNAs (21). Second, L. amazonensis parasites may have intrinsic, undefined mechanisms to evade cytokine- and chemokine-mediated immune responses (15). It has been suggested that L. donovani parasites secrete a chemotactic factor that preferentially inhibits IP-10 production from neutrophils, which could prevent NK cell activation (44). Finally, local injection of IP-10 may also trigger the production of multifunctional cytokines, such as IL-3, IL-6, and CXCL2/MIP-2 (Fig. 6). IL-3 and CXCL2/MIP-2 have been reported to enhance parasite loads and lesion development in mice infected with L. major parasites (9, 13, 25, 29), yet their roles in L. amazonensis infection are currently unclear. Although recombinant IL-6 has been shown to down-modulate cytokine production of human Mφs through oxygen-dependent mechanisms and to reduce host responses to Leishmania parasites (13), no significant change has been observed in L. major-infected, IL-6−/− BALB/c mice, which makes the role of IL-6 in immunity to Leishmania parasites questionable (43). Regardless of the mechanisms, it will be interesting to further examine the efficacy of the prolonged use of IP-10, alone or in combination with other cytokines, in the control of L. amazonensis infection, especially established infection.
In summary, we have shown the ability of IP-10 to activate Mφs for the production of proinflammatory mediators and the control of L. amazonensis infection in vitro. To the best of our knowledge, this is the first report examining this effect on Mφs in the context of pathogen infection. In addition to previous studies examining chemokines as potential targets for a potential anti-Leishmania therapy (38, 45), we have demonstrated that direct injection of IP-10 can overcome the deficient immune response associated with L. amazonensis infection, thereby skewing the host's cytokine profile towards a more vigorous Th1 response. While injection of recombinant IP-10 alone is insufficient to provide long-lasting protective immunity against L. amazonensis infection, this study provides important insight into mechanisms underlying the pathogenesis associated with nonhealing, New World cutaneous leishmaniasis and lays the groundwork for further study of treatment regimens.
Acknowledgments
This study was supported by NIH grant AI43003 to L.S. and by a James W. McLaughlin predoctoral fellowship to R.E.V.
We thank Li Jun Xin for his assistance with flow cytometric analysis; Jiaren Sun, John T. Sullivan, and Nanchaya Wanasen for helpful discussions and critical reviews of the manuscript; and Mardelle Susman for assisting in manuscript preparation.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettelli, E., and V. K. Kuchroo. 2005. IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J. Exp. Med. 201:169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya, S., S. Ghosh, B. Dasgupta, D. Mazumder, S. Roy, and S. Majumdar. 2002. Chemokine-induced leishmanicidal activity in murine macrophages via the generation of nitric oxide. J. Infect. Dis. 185:1704-1708. [DOI] [PubMed] [Google Scholar]
- 4.Brandonisio, O., M. A. Panaro, I. Fumarola, M. Sisto, D. Leogrande, A. Acquafredda, R. Spinelli, and V. Mitolo. 2002. Macrophage chemotactic protein-1 and macrophage inflammatory protein-1alpha induce nitric oxide release and enhance parasite killing in Leishmania infantum-infected human macrophages. Clin. Exp. Med. 2:125-129. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J., B. P. Vistica, H. Takase, D. I. Ham, R. N. Fariss, E. F. Wawrousek, C. C. Chan, J. A. DeMartino, J. M. Farber, and I. Gery. 2004. A unique pattern of up- and down-regulation of chemokine receptor CXCR3 on inflammation-inducing Th1 cells. Eur. J. Immunol. 34:2885-2894. [DOI] [PubMed] [Google Scholar]
- 6.Cotterell, S. E., C. R. Engwerda, and P. M. Kaye. 1999. Leishmania donovani infection initiates T cell-independent chemokine responses, which are subsequently amplified in a T cell-dependent manner. Eur. J. Immunol. 29:203-214. [DOI] [PubMed] [Google Scholar]
- 7.Courret, N., E. Prina, E. Mougneau, E. M. Saraiva, D. L. Sacks, N. Glaichenhaus, and J. C. Antoine. 1999. Presentation of the Leishmania antigen LACK by infected macrophages is dependent upon the virulence of the phagocytosed parasites. Eur. J. Immunol. 29:762-773. [DOI] [PubMed] [Google Scholar]
- 8.Denkers, E. Y., and B. A. Butcher. 2005. Sabotage and exploitation in macrophages parasitized by intracellular protozoans. Trends Parasitol. 21:35-41. [DOI] [PubMed] [Google Scholar]
- 9.Dumas, C., A. Muyombwe, G. Roy, C. Matte, M. Ouellette, M. Olivier, and B. Papadopoulou. 2003. Recombinant Leishmania major secreting biologically active granulocyte-macrophage colony-stimulating factor survives poorly in macrophages in vitro and delays disease development in mice. Infect. Immun. 71:6499-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaffen, S. L. 2004. Biology of recently discovered cytokines: interleukin-17—a unique inflammatory cytokine with roles in bone biology and arthritis. Arthritis Res. Ther. 6:240-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangur, V., N. P. Birmingham, and S. Thanesvorakul. 2002. Chemokines in health and disease. Vet. Immunol. Immunopathol. 86:127-136. [DOI] [PubMed] [Google Scholar]
- 12.Gu, L., S. Tseng, R. M. Horner, C. Tam, M. Loda, and B. J. Rollins. 2000. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 404:407-411. [DOI] [PubMed] [Google Scholar]
- 13.Hatzigeorgiou, D. E., S. He, J. Sobel, K. H. Grabstein, A. Hafner, and J. L. Ho. 1993. IL-6 down-modulates the cytokine-enhanced antileishmanial activity in human macrophages. J. Immunol. 151:3682-3692. [PubMed] [Google Scholar]
- 14.Ji, J., J. Masterson, J. Sun, and L. Soong. 2005. CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J. Immunol. 174:7147-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji, J., J. Sun, and L. Soong. 2003. Impaired expression of inflammatory cytokines and chemokines at early stages of infection with Leishmania amazonensis. Infect. Immun. 71:4278-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, D., M. M. Elloso, L. Showe, D. Williams, G. Trinchieri, and P. Scott. 1998. Differential regulation of the interleukin-12 receptor during the innate immune response to Leishmania major. Infect. Immun. 66:3818-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, D. E., M. R. Ackermann, U. Wille, C. A. Hunter, and P. Scott. 2002. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect. Immun. 70:2151-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, D. E., L. U. Buxbaum, and P. Scott. 2000. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J. Immunol. 165:364-372. [DOI] [PubMed] [Google Scholar]
- 19.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi, M., M. Adachi, N. Oda, F. Kokubu, and S. K. Huang. 2004. IL-17 cytokine family. J. Allergy Clin. Immunol. 114:1265-1273. [DOI] [PubMed] [Google Scholar]
- 21.Keyser, J., J. Schultz, K. Ladell, L. Elzaouk, L. Heinzerling, J. Pavlovic, and K. Moelling. 2004. IP-10-encoding plasmid DNA therapy exhibits anti-tumor and anti-metastatic efficiency. Exp. Dermatol. 13:380-390. [DOI] [PubMed] [Google Scholar]
- 22.Kima, P. E., S. L. Constant, L. Hannum, M. Colmenares, K. S. Lee, A. M. Haberman, M. J. Shlomchik, and D. McMahon-Pratt. 2000. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J. Exp. Med. 191:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemesre, J. L., D. Sereno, S. Daulouede, B. Veyret, N. Brajon, and P. Vincendeau. 1997. Leishmania spp.: nitric oxide-mediated metabolic inhibition of promastigote and axenically grown amastigote forms. Exp. Parasitol. 86:58-68. [DOI] [PubMed] [Google Scholar]
- 24.Linares, E., O. Augusto, S. C. Barao, and S. Giorgio. 2000. Leishmania amazonensis infection does not inhibit systemic nitric oxide levels elicited by lipopolysaccharide in vivo. J. Parasitol. 86:78-82. [DOI] [PubMed] [Google Scholar]
- 25.Mazingue, C., F. Cottrez-Detoeuf, J. Louis, M. Kweider, C. Auriault, and A. Capron. 1989. In vitro and in vivo effects of interleukin 2 on the protozoan parasite Leishmania. Eur. J. Immunol. 19:487-491. [DOI] [PubMed] [Google Scholar]
- 26.McMahon-Pratt, D., and J. Alexander. 2004. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 201:206-224. [DOI] [PubMed] [Google Scholar]
- 27.Mohan, K., E. Cordeiro, M. Vaci, C. McMaster, and T. B. Issekutz. 2005. CXCR3 is required for migration to dermal inflammation by normal and in vivo activated T cells: differential requirements by CD4 and CD8 memory subsets. Eur. J. Immunol. 35:1702-1711. [DOI] [PubMed] [Google Scholar]
- 28.Muller, K., G. van Zandbergen, B. Hansen, H. Laufs, N. Jahnke, W. Solbach, and T. Laskay. 2001. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med. Microbiol. Immunol. (Berlin) 190:73-76. [DOI] [PubMed] [Google Scholar]
- 29.Pompeu, M., A. L. Freitas, G. A. dosReis, and M. Barral-Netto. 1992. T-lymphocytes in experimental Leishmania amazonensis infection: comparison between immunized and naive BALB/c mice. Parasitol. Res. 78:16-22. [DOI] [PubMed] [Google Scholar]
- 30.Qi, H., J. Ji, N. Wanasen, and L. Soong. 2004. Enhanced replication of Leishmania amazonensis amastigotes in gamma interferon-stimulated murine macrophages: implications for the pathogenesis of cutaneous leishmaniasis. Infect. Immun. 72:988-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi, H., V. Popov, and L. Soong. 2001. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4(+) T cells in vivo. J. Immunol. 167:4534-4542. [DOI] [PubMed] [Google Scholar]
- 32.Reiner, S. L., S. Zheng, D. B. Corry, and R. M. Locksley. 1993. Constructing polycompetitor cDNAs for quantitative PCR. J. Immunol. Methods 165:37-46. [DOI] [PubMed] [Google Scholar]
- 33.Ritter, U., and H. Moll. 2000. Monocyte chemotactic protein-1 stimulates the killing of Leishmania major by human monocytes, acts synergistically with IFN-gamma and is antagonized by IL-4. Eur. J. Immunol. 30:3111-3120. [DOI] [PubMed] [Google Scholar]
- 34.Robertson, M. J. 2002. Role of chemokines in the biology of natural killer cells. J. Leukoc. Biol. 71:173-183. [PubMed] [Google Scholar]
- 35.Rosas, L. E., J. Barbi, B. Lu, Y. Fujiwara, C. Gerard, V. M. Sanders, and A. R. Satoskar. 2005. CXCR3-/- mice mount an efficient Th1 response but fail to control Leishmania major infection. Eur. J. Immunol. 35:515-523. [DOI] [PubMed] [Google Scholar]
- 36.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 37.Salomon, I., N. Netzer, G. Wildbaum, S. Schif-Zuck, G. Maor, and N. Karin. 2002. Targeting the function of IFN-gamma-inducible protein 10 suppresses ongoing adjuvant arthritis. J. Immunol. 169:2685-2693. [DOI] [PubMed] [Google Scholar]
- 38.Santiago, H. C., C. F. Oliveira, L. Santiago, F. O. Ferraz, D. G. de Souza, L. A. de-Freitas, L. C. Afonso, M. M. Teixeira, R. T. Gazzinelli, and L. Q. Vieira. 2004. Involvement of the chemokine RANTES (CCL5) in resistance to experimental infection with Leishmania major. Infect. Immun. 72:4918-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soong, L., C. H. Chang, J. Sun, B. J. Longley, Jr., N. H. Ruddle, R. A. Flavell, and D. McMahon-Pratt. 1997. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J. Immunol. 158:5374-5383. [PubMed] [Google Scholar]
- 40.Stamp, L. K., M. J. James, and L. G. Cleland. 2004. Interleukin-17: the missing link between T-cell accumulation and effector cell actions in rheumatoid arthritis? Immunol. Cell Biol. 82:1-9. [DOI] [PubMed] [Google Scholar]
- 41.Steigerwald, M., and H. Moll. 2005. Leishmania major modulates chemokine and chemokine receptor expression by dendritic cells and affects their migratory capacity. Infect. Immun. 73:2564-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terabe, M., T. Kuramochi, T. Hatabu, M. Ito, Y. Ueyama, K. Katakura, S. Kawazu, T. Onodera, and Y. Matsumoto. 1999. Non-ulcerative cutaneous lesion in immunodeficient mice with Leishmania amazonensis infection. Parasitol. Int. 48:47-53. [DOI] [PubMed] [Google Scholar]
- 43.Titus, R. G., G. K. DeKrey, R. V. Morris, and M. B. Soares. 2001. Interleukin-6 deficiency influences cytokine expression in susceptible BALB mice infected with Leishmania major but does not alter the outcome of disease. Infect. Immun. 69:5189-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Zandbergen, G., N. Hermann, H. Laufs, W. Solbach, and T. Laskay. 2002. Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infect. Immun. 70:4177-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vester, B., K. Muller, W. Solbach, and T. Laskay. 1999. Early gene expression of NK cell-activating chemokines in mice resistant to Leishmania major. Infect. Immun. 67:3155-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Von Stebut, E., J. M. Ehrchen, Y. Belkaid, S. L. Kostka, K. Molle, J. Knop, C. Sunderkotter, and M. C. Udey. 2003. Interleukin 1α promotes Th1 differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J. Exp. Med. 198:191-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wildbaum, G., N. Netzer, and N. Karin. 2002. Plasmid DNA encoding IFN-gamma-inducible protein 10 redirects antigen-specific T cell polarization and suppresses experimental autoimmune encephalomyelitis. J. Immunol. 168:5885-5892. [DOI] [PubMed] [Google Scholar]