Abstract
Since secondary Streptococcus pneumoniae infections greatly increase the mortality of influenza infections, we determined the relative roles of neutrophil-dependent and -independent mechanisms in increased susceptibility to S. pneumoniae during influenza infection. Mice infected with influenza for 6 days, but not 3 days, showed a significant increase in susceptibility to S. pneumoniae infection compared to mice not infected with influenza. There was significant neutrophil accumulation in the lungs of S. pneumoniae-infected mice regardless of whether or not they were infected with influenza for 3 or 6 days. Depletion of neutrophils in these mice resulted in increased susceptibility to S. pneumoniae in both the non-influenza-infected mice and mice infected with influenza for 3 days but not in the mice infected with influenza for 6 days, indicating that a prior influenza infection of 6 days may compromise neutrophil function, resulting in increased susceptibility to a S. pneumoniae infection. Neutrophils from the lungs of mice infected with influenza for 3 or 6 days exhibited functional impairment in the form of decreased phagocytosis and intracellular reactive oxygen species generation in response to S. pneumoniae. In addition, neutrophil-depleted mice infected with influenza for 6 days were more susceptible to S. pneumoniae than neutrophil-depleted mice not infected with influenza, indicating that neutrophil-independent mechanisms also contribute to influenza-induced increased susceptibility to S. pneumoniae. Pulmonary interleukin-10 levels were increased in coinfected mice infected with influenza for 6 days but not 3 days. Thus, an influenza infection of 6 days increases susceptibility to S. pneumoniae by both suppression of neutrophil function and by neutrophil-independent mechanisms such as enhanced cytokine production.
A prior influenza infection increases susceptibility to a secondary bacterial pneumonia infection, which has been associated with an increase in morbidity and mortality during influenza epidemics and pandemics (24, 25, 34, 35, 37, 40-43, 45-47, 50, 52-57). Bacteria commonly causing pneumonia in the most severely ill influenza patients are Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae. Together, influenza and secondary bacterial pneumonia were the most common cause of infectious death in the United States in 2002 (10). With the ever-increasing bacterial antibiotic resistance and the possibility of another influenza pandemic in the near future, understanding and controlling these infections is crucial.
Evidence from research, clinical studies, and epidemiological studies has shown a positive correlation between the increase in morbidity and mortality during influenza epidemics and pandemics and the associated increase in secondary S. pneumoniae infections (24, 25, 34, 35, 37, 40-43, 45-47, 50, 52-57). There are currently two hypotheses to explain the increase in susceptibility to secondary S. pneumoniae infections after an influenza infection. One hypothesis is that influenza infection alters neutrophil function, thereby reducing the effectiveness of phagocyte-mediated killing of the bacteria. The other hypothesis is that the tissue damage caused by influenza virus alters the epithelial surface of the respiratory tract, thereby exposing different surface receptors to which the S. pneumoniae adhere, and/or increasing the affinity of the S. pneumoniae for its receptors, which may result in increased growth and decreased neutrophil killing of S. pneumoniae in the respiratory tract.
The hypothesis that influenza-induced neutrophil dysfunction is the primary means of increased susceptibility to S. pneumoniae infection after influenza infection has been supported by several groups using both in vitro and in vivo models of influenza infection (1-9, 13, 39, 66). Previous studies by our laboratory have shown that neutrophils are important in resistance to S. pneumoniae infection independent of an influenza infection (23). The three major properties of the neutrophil which are crucial for bacterial clearance (chemotactic response, phagocytosis, and intracellular killing) have been shown to be altered by influenza virus, thereby potentially increasing susceptibility to S. pneumoniae infection after influenza infection due to decreased phagocytosis and killing of the bacteria by neutrophils (5, 7, 15, 39). While there have been extensive in vitro studies to support this hypothesis, the only studies examining the effects of an in vivo influenza infection on neutrophil function used blood neutrophils from an influenza-infected mouse (1, 2) or whole lungs from mice infected with both influenza and S. pneumoniae in vivo (37, 51). The effects of an in vivo influenza infection on lung neutrophil function independent of an S. pneumoniae infection have not yet been determined. Therefore, the extent to which compromised neutrophil function contributes to influenza-induced increased susceptibility to S. pneumoniae is not known.
Influenza-induced tissue damage may also play a role in increasing susceptibility to S. pneumoniae infection after influenza infection. During the initial stage of influenza infection in which viral replication occurs, the virus may alter the surface receptors expressed on the epithelial cells, such as the platelet-activating factor receptor, to which the S. pneumoniae can adhere (17-19). In addition, expression of viral glycoproteins, such as neuraminidase and hemagglutinin, on influenza-infected cells can be induced by the virus and may increase S. pneumoniae adherence (27). The influenza virus can also expose novel S. pneumoniae receptors by cleaving sialic acid on glycoproteins of host respiratory cells with its neuraminidase protein, resulting in increased pneumococcal adherence (38, 41, 46, 47, 60, 61). After replication, the virus lyses the infected cells, denuding the epithelium and exposing the basement membrane and proteins from the extracellular matrix, such as fibronectin, to which the S. pneumoniae can potentially adhere (27, 48). In addition to altering the cell surface receptors, influenza virus also causes the exposure of different cell types as the epithelium repairs itself (48). Initially, basal cells migrate to cover the surface of the exposed basement membrane, where they then differentiate into ciliated epithelial cells (33). During the entire influenza infection period, different cell types and receptors are exposed on the respiratory epithelium. The S. pneumoniae may adhere more tightly to these receptors and/or cell types, making it more difficult for neutrophils to phagocytose and kill the bacteria, thereby increasing the severity of the infection as the S. pneumoniae continues to replicate without the consequence of neutrophil-mediated killing. In addition, influenza-induced tissue damage leading to denudation of the tracheal respiratory epithelium has been shown to affect mucociliary clearance, which may allow S. pneumoniae to adhere more readily to the trachea and eventually infect the lungs, resulting in pneumonia (44, 48). Recently, it has been shown that interleukin 10 (IL-10) is increased in lungs of mice infected with influenza 14 days prior to a S. pneumoniae infection, and this increased IL-10 expression increases susceptibility to a secondary S. pneumoniae infection (63). However, the initial onset of IL-10 production after an influenza infection and the effects of the initial IL-10 expression on susceptibility to a S. pneumoniae infection have not yet been determined.
In this study, we examined the contributions of influenza-induced changes in neutrophil-dependent and -independent mechanisms in increased susceptibility to a secondary S. pneumoniae infection.
MATERIALS AND METHODS
Infectious agents.
Influenza virus A/PR/8/34 (PR8) was cultured and stored in allantoic chicken egg fluid at −80°C. Streptococcus pneumoniae type 4 (ATCC 6304) was cultured in Todd-Hewitt broth supplemented with 0.5% yeast extract at 37°C and 5% CO2. Stock cultures in logarithmic growth were frozen in 10% glycerol and stored at −80°C. CFU were determined by plating on 5% sheep blood agar plates and optical density readings.
Mice.
Female C57BL/6 mice, 6 to 12 weeks of age, from NCI-Frederick and Charles River Laboratories were used for all experiments. Animals were housed at the Animal Resource Center at Montana State University. All procedures were performed following Institutional Animal Care and Use Committee-approved protocols.
Infection model.
Mice were anesthetized with isoflurane and infected intranasally (i.n.) with 600 PFU PR8 influenza virus in 100 μl of sterile Hanks balanced salt solution (HBSS, 50 μl/nare). At day 3 or 6 after influenza infection, mice were challenged i.n. with 107 CFU of S. pneumoniae type 4 in 100 μl of sterile DPBS (Dulbecco's phosphate-buffered saline) (50 μl/nare) for 12 or 24 h. S. pneumoniae-only-infected mice were mock influenza infected i.n. with 100 μl HBSS. Influenza-only-infected mice were mock S. pneumoniae infected i.n. with 100 μl DPBS. Noninfected control mice were mock influenza infected i.n. with HBSS and mock S. pneumoniae infected i.n. with DPBS. For neutrophil depletion studies, half of the mice in each treatment group were depleted of neutrophils by intraperitoneal injection of 500 μg RB6-8C5 monoclonal antibody 16 h prior to S. pneumoniae infection (23, 30, 58). The other half of the mice in each treatment group were mock neutrophil depleted with 500 μg isotype-matched rat immunoglobulin G intraperitoneally.
Tissue preparation.
At the specified time points, mice were euthanized by deep pentobarbital anesthesia followed by exsanguination. Bronchial alveolar lavage fluids (BALF) were collected for differential cell counts and to confirm neutrophil depletions as previously described (23, 58). Lungs were collected, placed in the BALF, snap-frozen in liquid nitrogen, and stored at −80°C. To enumerate S. pneumoniae CFU, lungs were homogenized, serially diluted in 10-fold dilutions, and plated on sheep blood agar plates with neomycin using the drop plate method (29). Plates were incubated for 24 h at 37°C, 5% CO2 before CFU were counted. PFU were enumerated using the plaque assay method with Madin-Darby canine kidney cells (67).
Neutrophil ROS generation and S. pneumoniae association/phagocytosis detection.
Mice were infected with PR8 influenza virus as described above for 3 or 6 days. Control mice (mock influenza infected) received 100 μl HBSS i.n. 6 days before they were sacrificed. Lipopolysaccharide (LPS)-stimulated mice were placed in an aerosolization chamber, and LPS (10 mg/ml in 5 ml HBSS) was nebulized for 15 min. In a separate experiment, mice were infected similarly, except influenza-infected mice were also LPS stimulated as described above to control for any effects LPS may have on neutrophil function. All mice were then sacrificed 8 h after the LPS challenge. Reactive oxygen species (ROS) generation by neutrophils and S. pneumoniae association with neutrophils were then measured using two previously described methods adapted for this study as described below (16, 64).
S. pneumoniae was washed twice in DPBS, resuspended to 1 × 109 CFU/ml in 5 ml DPBS, and labeled with DiI (V22889; Molecular Probes). Unlabeled S. pneumoniae was also prepared. For opsonization, bacteria were washed twice in DPBS and resuspended at 1 × 109 CFU/ml in RPMI-H (RPMI with 10 mM HEPES) containing 25% fresh mouse serum, prepared as described below. Bacteria were then incubated at 37°C for 30 min with mild agitation. S. pneumoniae was washed twice in DPBS and resuspended at 108 CFU/ml in RPMI-H for bone marrow cells or 107 CFU/ml in RPMI-H for BALF cells. Serial dilutions were plated to confirm actual CFU, and the efficiency of labeling was checked using flow cytometry and microscopy with unlabeled bacteria and DiI-labeled bacteria.
Fresh mouse serum was collected, and it was used for S. pneumoniae opsonization and to coat sterile 96-well flat-bottom plates. Plates for the phagocytosis and ROS generation assays were coated with 20% normal mouse serum diluted in DPBS for 1 h at room temperature and washed twice with DPBS before cells were added.
Bone marrow cells were flushed from the femurs and tibias with 5 ml of HBSS with 3 mM EDTA, and BALF cells were collected by lavaging the lungs with 5 ml of HBSS with 3 mM EDTA. All samples were kept at room temperature until noted otherwise to prevent neutrophil activation by temperature changes. Cell counts and differential cell counts were done. Lungs were removed after lavaging, placed in 5 ml HBSS, snap-frozen in liquid nitrogen, and stored at −80°C for influenza plaque assays.
Red blood cells were removed from bone marrow and BALF cells by hypotonic lysis, washed, and resuspended to 107 cells/ml (bone marrow) or 106 cells/ml (BALF) in RPMI-H. To obtain sufficient cell numbers, samples were combined in sets of 2 at this point with 3 sets of 2 per treatment for a final n of 3 per group. An aliquot of each sample was removed and stained with fluorescein isothiocyanate-labeled RB6-8C5 antibody to identify neutrophils. The remainder of bone marrow samples not used in these assays were snap-frozen and stored at −80°C for plaque assays.
To detect ROS, the ROS indicator 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (C-2938; Molecular Probes) was added to the samples to a final concentration of 0.2 μM from a 10 mM stock made up in dimethyl sulfoxide, as previously described (64). For negative ROS controls, no ROS indicator was added. Samples were incubated in the dark at room temperature for 20 min before being transferred to ice.
To measure S. pneumoniae association/phagocytosis, cells were added (106 cells/100 μl for bone marrow and 105 cells/100 μl for BALF) to the wells of sterile 96-well plates previously coated with serum and kept on ice, as previously described (64). DiI-labeled S. pneumoniae was added to the wells (107 CFU/100 μl for bone marrow and 106 CFU/100 μl for BALF). For positive ROS generation controls, phorbol myristate acetate (PMA) was added to the wells (100 ng/ml). The plates were centrifuged at 400 × g for 7 min at 4°C. Samples that represented the start of the assay (time = 0 min) were placed on ice in the dark. The remaining samples were incubated at 37°C with 5% CO2 for 60 min. At the end of the designated time, samples were transferred to flow cytometry tubes and kept on ice. ROS generation and S. pneumoniae association were then measured using flow cytometry. To quench extracellular S. pneumoniae and measure phagocytosis, 100 μl trypan blue was added and the sample was reanalyzed. Neutrophils were specifically analyzed by gating for RB6-8C5 positively stained cells using the aliquots of cells described above, which did not have ROS indicator or S. pneumoniae added.
Lung cytokine levels.
Homogenized lungs were centrifuged at 300 × g for 15 min, and the supernatants were removed and analyzed using the BD Cytokine Bead Array mouse inflammation kit by following the manufacturer's instructions. Samples were analyzed by flow cytometry on a BD FACScan, and data were analyzed using the BD Cytokine Bead Array software.
Statistical analysis.
Data were analyzed using GraphPad Prism 4 software. S. pneumoniae CFU, influenza PFU, and neutrophil depletion cell count data were analyzed using the Mann-Whitney test. BALF cytokine levels and percentage of phagocytic neutrophil data were analyzed using one-way analysis of variance followed by the Bonferroni post test. All other data were analyzed using an unpaired, two-tailed t test. The minimal value of significance was set at a P value of <0.05.
RESULTS
Peak susceptibility to secondary S. pneumoniae infection occurs at 6 days after influenza infection.
Previous studies have found that susceptibility to S. pneumoniae infection is greatest at 6 to 7 days after influenza infection (14, 24, 42, 59). To validate our coinfection model, S. pneumoniae was inoculated into noninfected mice or mice infected with influenza for 3 or 6 days, and S. pneumoniae growth over 12 and 24 h was measured to determine when susceptibility to S. pneumoniae infection was greatest. We found that susceptibility to secondary S. pneumoniae infection was greatest at 6 days after influenza infection. Six-day-coinfected mice had 485.5- and 149.4-fold higher lung S. pneumoniae CFU counts at 12 and 24 h, respectively, after S. pneumoniae infection than mice infected only with S. pneumoniae (P < 0.0001) (Fig. 1A and data not shown). At 3 days after influenza infection, when influenza viral replication is at its peak, there was no significant difference in susceptibility to S. pneumoniae infection between coinfected mice and S. pneumoniae-only-infected mice at either 12 or 24 h after S. pneumoniae infection (P = 0.3109 and 0.1832, respectively) (Fig. 1A and data not shown).
FIG. 1.
S. pneumoniae CFU (A) and influenza PFU (B) in lungs after 24 h of S. pneumoniae infection for mice infected with S. pneumoniae only or infected with S. pneumoniae after 3 or 6 days of influenza infection. Data were pooled from three independent experiments with 4 to 5 mice per group per experiment. Significant differences between mice infected with S. pneumoniae only and mice coinfected with influenza and S. pneumoniae are indicated as follows: ***, P < 0.001; NS, P > 0.05. Horizontal lines represent median values for each infection. Data were analyzed using Mann-Whitney U test.
We enumerated influenza PFU in the lungs of both non-influenza-infected mice and mice infected with influenza for either 3 or 6 days to measure the growth of infectious virus particles. In the lungs of mice infected with influenza for 3 days, there were 2.3-fold more PFU than in the lungs of mice infected with influenza for 6 days (P = 0.029) (Fig. 1B). In non-influenza-infected mice, no influenza PFU were found (data not shown). Together, these data indicate that susceptibility to S. pneumoniae infection is greatest at 6 days after influenza infection. At 3 days after influenza infection, when viral replication was at its peak, susceptibility to S. pneumoniae infection was not increased compared to mice infected with S. pneumoniae only.
Effects of RB6-8C5 depletion on blood and BALF neutrophil numbers.
To determine the roles of neutrophil-dependent and -independent mechanisms of increased susceptibility to S. pneumoniae during an influenza infection, we depleted noninfected and influenza-infected mice of neutrophils by injecting the RB6-8C5 (RB6) antibody intraperitoneally 16 h before S. pneumoniae infection. To validate our neutrophil depletion method, we quantitated the number of neutrophils present in the blood and BALF of depleted and nondepleted mice. Control mice which were RB6 depleted (noninfected and influenza-only infected) had significant reductions in blood neutrophils, indicating that our depletion model was adequate for our studies. Noninfected depleted mice had a 94.4% reduction in blood neutrophils compared to nondepleted mice (P = 0.0079; data not shown), while depleted mice infected with influenza for 3 or 6 days had reductions of 72.7% and 91.4% in blood neutrophils, respectively, compared to nondepleted mice (P = 0.0159 and P = 0.0079, respectively; data not shown). In the BALF of noninfected depleted mice, no reductions were seen in neutrophil numbers, since few to no neutrophils were present in the lungs of noninfected mice whether or not they were depleted of neutrophils (data not shown). Depleted mice infected with influenza for 3 days had an 85.0% reduction in BALF neutrophils (P = 0.0159; data not shown), while those infected with influenza for 6 days had a 25.9% reduction in BALF neutrophils (P = 0.8413; data not shown) compared to nondepleted mice with similar infections.
Neutrophil depletion resulted in a significant decrease in the number of blood neutrophils compared to nondepleted mice for all groups after 12 h of S. pneumoniae infection, with reductions of 89.3% for S. pneumoniae-only-infected, 81.4% for mice coinfected for 3 days, and 61.9% for mice coinfected for 6 days (P = 0.0159, P = 0.0079, and P = 0.0159, respectively; data not shown). At 24 h after S. pneumoniae infection, mice infected only with S. pneumoniae had an 88.2% reduction and mice coinfected for 3 days had a 58.5% reduction in blood neutrophils (P = 0.0079 and P = 0.0159, respectively) (Fig. 2A). However, in mice infected with influenza for 6 days followed by S. pneumoniae for 24 h, there was not a significant difference in blood neutrophils between depleted and nondepleted mice, although there was a 31.3% reduction (P = 0.2857) (Fig. 2A). The overall reduction in blood neutrophils at 24 h after S. pneumoniae infection could be due to the overwhelming infections in the lungs recruiting all available neutrophils from the blood, resulting in temporary neutropenia.
FIG. 2.
Percentage of neutrophils in blood of total blood leukocytes (A), total number of neutrophils in BALF (B), and lung S. pneumoniae CFU (C) of nondepleted and RB6-depleted mice at 24 h after S. pneumoniae infection. Mice were infected with S. pneumoniae for 24 h after 3 or 6 days of influenza infection or were only infected with S. pneumoniae for 24 h. Data represent results from two independent experiments with 4 to 5 mice per group per experiment. Significant differences between neutrophil-depleted mice and nondepleted mice are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, P > 0.05. Significant differences between neutrophil-depleted mice infected with S. pneumoniae only and neutrophil-depleted mice coinfected with influenza for 6 days followed by S. pneumoniae as well as differences between neutrophil-depleted mice coinfected with influenza for 3 days followed by S. pneumoniae and neutrophil-depleted mice coinfected with influenza for 6 days followed by S. pneumoniae are indicated as follows: #, P < 0.05; ##, P < 0.01. Horizontal lines represent median values for each infection. Data were analyzed using Mann-Whitney U test.
In the BALF of S. pneumoniae-only-infected mice, neutrophil depletion resulted in lung neutrophil reductions of 83.3% and 69.4% at 12 and 24 h, respectively, after S. pneumoniae infection (P = 0.0317 and P = 0.0952, respectively) (Fig. 2B and data not shown). Although neutrophil-depleted mice had significantly fewer BALF neutrophils after 12 h of S. pneumoniae infection, a significant reduction in BALF neutrophils was not achieved in this infection group after 24 h of S. pneumoniae infection because one mouse was not infected to the same level as the other mice in the group and therefore had fewer neutrophils than the other mice within the group. This mouse had 1.7 × 104 lung CFU, while the other four mice in the group averaged 3.5 × 105 ± 1.6 × 105 lung CFU (Fig. 2C). Neutrophil-depleted mice that were infected with influenza for 3 days had lung neutrophil reductions of 87.7% and 90.3% at 12 and 24 h, respectively, after S. pneumoniae infection (P = 0.0079) (Fig. 2B and data not shown). For mice coinfected for 6 days, lung neutrophils were reduced in the depleted mice by 81.3% and 89.2% at 12 and 24 h after S. pneumoniae infection (P = 0.0159) (Fig. 2B and data not shown). In addition, there were no significant differences in neutrophil numbers in nondepleted, S. pneumoniae-infected mice whether or not they had a previous influenza infection, indicating that a prior influenza infection of 3 or 6 days does not affect neutrophil migration into the lungs (P > 0.05) (Fig. 2B). Together, these data indicate that our RB6-8C5 neutrophil depletion model can be used to study neutrophil-dependent and -independent mechanisms of increased susceptibility to S. pneumoniae infection after an influenza infection.
Neutrophil-dependent mechanisms of increased susceptibility to S. pneumoniae infection.
To determine whether neutrophil depletion affects susceptibility to S. pneumoniae infection, we compared S. pneumoniae CFU in the lungs of depleted mice to nondepleted mice with the same infection(s). Neutrophil-depleted mice infected with S. pneumoniae only had 10.5- and 384.5-fold higher lung S. pneumoniae CFU at 12 and 24 h, respectively, after S. pneumoniae infection than nondepleted mice (P = 0.0159 and 0.0079, respectively) (Fig. 2C and data not shown). In mice coinfected for 3 days, depleted mice had 26.8- and 108.9-fold more lung S. pneumoniae CFU at 12 and 24 h, respectively, after S. pneumoniae infection than nondepleted mice (P = 0.0079 for both; Fig. 2C and data not shown). However, after 6 days of influenza infection, there was no significant difference in susceptibility to S. pneumoniae in the lungs of mice infected with S. pneumoniae for 12 or 24 h whether or not they were neutrophil depleted (P = 0.7302 and 0.0635, respectively) (Fig. 2C and data not shown).
In summary, our depletion studies indicate that neutrophils play an important role in resistance to an S. pneumoniae infection in both noninfected mice and mice with an influenza infection for 3 days. When neutrophils were depleted in these groups, susceptibility was increased. However, in mice infected with influenza for 6 days, neutrophil depletion did not affect resistance to S. pneumoniae, indicating that neutrophil function was compromised by the influenza infection to a point similar to that caused by neutrophil depletion. These results suggest the possibility that influenza-induced effects have compromised the neutrophils so much by day 6 after an influenza infection (but not at day 3) that they are no longer a significant factor in protection.
Influenza-induced changes in neutrophil-independent mechanisms increase susceptibility to S. pneumoniae infection.
To determine whether a prior influenza infection affected susceptibility to S. pneumoniae independent of neutrophil function, we compared S. pneumoniae lung CFU of neutrophil-depleted mice infected with S. pneumoniae only to depleted mice infected with influenza for 3 or 6 days. After 12 h of S. pneumoniae infection, mice infected with influenza for both 3 and 6 days had 5.3- and 46.9-fold more lung S. pneumoniae CFU, respectively, than S. pneumoniae-only-infected mice depleted of neutrophils (P = 0.0159 for both; data not shown). In addition, depleted mice coinfected for 6 days had 8.8-fold more S. pneumoniae CFU than depleted mice coinfected for 3 days (P = 0.0159; data not shown). After 24 h of S. pneumoniae infection, depleted mice coinfected for 3 days had only 1.9-fold more S. pneumoniae CFU (P = 0.4206), while depleted mice coinfected for 6 days had 10.5-fold more S. pneumoniae CFU than S. pneumoniae-only-infected depleted mice (P = 0.0079) (Fig. 2C). In addition, depleted mice infected with influenza for 6 days had 5.6-fold higher S. pneumoniae CFU than depleted mice coinfected for 3 days after 24 h of S. pneumoniae infection (P = 0.0159) (Fig. 2C). Thus, in mice depleted of neutrophils, an influenza infection still caused increased susceptibility to a secondary S. pneumoniae infection. Together, these data suggest that a prior influenza infection of 6 days increases susceptibility to an S. pneumoniae infection by neutrophil-independent mechanisms.
Neutrophil function assays.
To determine whether influenza virus causes defects in neutrophil function in vivo, thereby increasing susceptibility to a secondary S. pneumoniae infection, we measured the amount of S. pneumoniae associated (either attached or phagocytosed) with either BALF or bone marrow neutrophils and ROS production by neutrophils from the lungs and bone marrow of noninfected, LPS-stimulated and mice infected with influenza for 3 or 6 days using flow cytometry. In a separate experiment, we stimulated influenza-infected mice with LPS to determine if any changes in neutrophil function were due to the effects of LPS or influenza.
Lung and bone marrow neutrophils were analyzed at 0 and 60 min after the addition of S. pneumoniae to compare the resting and activated levels of S. pneumoniae association with neutrophils and ROS generation by neutrophils. Since normal, healthy lungs contain few, if any, neutrophils and were therefore not able to be used for comparison to influenza-infected lungs in these studies, it was necessary to induce neutrophil migration to compare lung neutrophils from noninfected mice to those from influenza-infected mice. LPS was aerosolized for 20 min to induce a strong neutrophil influx into the lungs of noninfected mice, and BALF was collected 8 h later when neutrophil accumulation was at its peak (data not shown). For the bone marrow neutrophils, noninfected mice were also used in the analysis.
In the lungs, there were no significant differences in the total numbers of neutrophils between LPS-stimulated mice, mice infected with influenza for 3 days, and mice infected with influenza for 6 days (Fig. 3A). Mice infected with influenza for 3 or 6 days and stimulated with LPS had 2.4- and 2.5-fold more lung neutrophils, respectively, than mice stimulated with LPS only (P < 0.0001 for both) (Fig. 3B), indicating that the neutrophil reservoir was not depleted during the influenza infection. In the bone marrow, there were no significant differences in neutrophil numbers between any of the groups, except there was a 1.6-fold increase in neutrophils in the mice infected with influenza for 3 days compared to the LPS-stimulated mice (P = 0.0096) (Fig. 3C), although this difference was not seen in a repeat of the experiment (Fig. 3D). For mice infected with influenza for 3 or 6 days followed by LPS stimulation, there were no significant differences in the numbers of bone marrow neutrophils between any of the groups (Fig. 3D).
FIG. 3.
Neutrophil counts for BALF (A and B) and bone marrow (C and D) samples from noninfected mice, LPS-stimulated mice, or mice infected with influenza for 3 or 6 days with (B and D) or without (A and C) LPS stimulation. Data represent results from two independent experiments with 3 sets of 2 mice per group. Significant differences between LPS-stimulated mice and mice with both influenza infection and LPS stimulation are indicated as follows: 

 , P < 0.001. Data are expressed as means ± standard deviations. Data were analyzed using unpaired t test.
, P < 0.001. Data are expressed as means ± standard deviations. Data were analyzed using unpaired t test.
Influenza infection reduces S. pneumoniae association with neutrophils.
The association of S. pneumoniae with neutrophils was measured by incubating neutrophils with DiI-labeled S. pneumoniae for 0 or 60 min. The mean fluorescence in the FL-2 channel was then measured to determine the relative number of S. pneumoniae bacteria associated with neutrophils, either bound to the surface or phagocytosed. In the lungs, there were 68.2% and 71.2% reductions in the initial (0 min) S. pneumoniae association with neutrophils from mice infected with influenza for 3 or 6 days compared to those from LPS-stimulated mice (P = 0.0057 and 0.0049, respectively; data not shown). After 60 min of incubation with S. pneumoniae, for lung neutrophils from mice infected with influenza for 3 and 6 days, the percentages of associated S. pneumoniae bacteria were 70.6% and 79.8%, respectively, of that seen with neutrophils from LPS-stimulated mice (P < 0.0001 and P = 0.0001, respectively; data not shown). After 60 min of incubation with S. pneumoniae, the level of S. pneumoniae association with influenza-infected neutrophils only reached levels comparable to those seen initially with neutrophils from LPS-stimulated mice at 0 min incubation (data not shown).
Studies using trypan blue to quench the fluorescence of extracellular S. pneumoniae indicated that most of the S. pneumoniae association with lung neutrophils after 60 min of incubation with S. pneumoniae was the result of phagocytosis of S. pneumoniae rather than adherence to the neutrophils (Table 1).
TABLE 1.
Trypan blue staining of BALF neutrophils to determine the percentage of neutrophils that were phagocytic and the percentage of phagocytic neutrophils that were producing ROSa
| Influenza infection or LPS treatment | % of neutrophils that were phagocytic after time (min):
|
% of phagocytic neutrophils that were producing ROS after time (min):
|
||
|---|---|---|---|---|
| 0 | 60 | 0 | 60 | |
| LPS, 8 h | 43.3 ± 4.2 | 67.7 ± 17.8 | 56.5 ± 1.5 | 80.6 ± 1.5 |
| Influenza, 3 days | 95.1 ± 19.9* | 82.2 ± 5.7 | 31.3 ± 3.8* | 62.8 ± 5.2 |
| Influenza, 6 days | 78.1 ± 24.7 | 91.1 ± 8.4 | 10.4 ± 9.0*** | 35.9 ± 16.8***† |
Values represent means ± standard deviations (3 mice/group). Neutrophils were collected from mice and incubated with DiI-labeled S. pneumoniae for 0 or 60 min to measure phagocytosis and ROS generation. Statistical differences between groups were calculated using one-way analysis of variance, followed by the Bonferroni post test. Differences between groups with the same incubation time are indicated as follows: *, P < 0.05; ***, P < 0.001 compared to neutrophils from LPS-challenged mice; †, P < 0.05 compared to neutrophils from mice infected with influenza for 3 days.
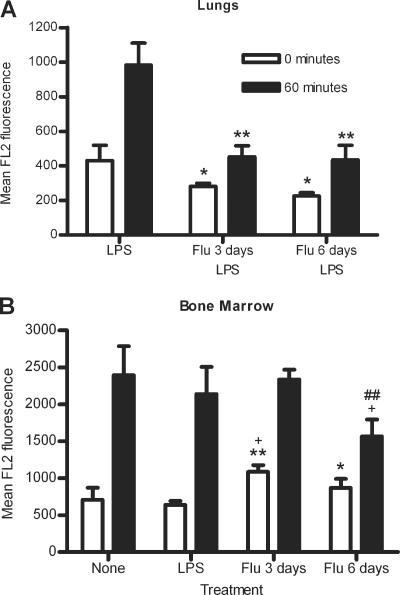
In the lungs of mice infected with influenza for 3 or 6 days followed by LPS stimulation, there was a significant decrease in initial S. pneumoniae association with neutrophils compared to mice stimulated with LPS only (P = 0.0464 and 0.0175, respectively) (Fig. 4A). A similar decrease was seen after 60 min of incubation (P = 0.0031 and 0.0035, respectively) (Fig. 4A). These data indicate that the differences seen in S. pneumoniae association are due to the effects of influenza infection and not LPS stimulation. Thus, a prior influenza infection of 3 or 6 days decreases the ability of lung neutrophils to associate with S. pneumoniae.
FIG. 4.
S. pneumoniae association after 0 or 60 min incubation with lung (A) or bone marrow (B) neutrophils from noninfected mice, LPS-stimulated mice, or mice infected with influenza for 3 or 6 days with (A) or without (B) subsequent LPS stimulation. Data represent results from one (A) or two (B) independent experiments with 3 sets of 2 mice per group. Significance is indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to LPS-stimulated mice; +, P < 0.05 compared to noninfected (none) mice; ##, P < 0.01 compared to mice infected with influenza for 3 days. Data are expressed as means ± standard deviations. Data were analyzed using unpaired t test.
In the bone marrow, there were significantly more S. pneumoniae initially associated with bone marrow neutrophils from mice infected with influenza for 3 days compared to bone marrow neutrophils from noninfected or LPS-stimulated mice, with increases of 53.7% and 69.9%, respectively (P = 0.0261 and 0.0020, respectively) (Fig. 4B). However, by 60 min, there were no significant differences in S. pneumoniae association with bone marrow neutrophils after 3 days of influenza infection compared to noninfected or LPS-stimulated mice (P = 0.8293 and 0.4234, respectively) (Fig. 4B). Bone marrow neutrophils from mice infected with influenza for 6 days showed 35.2% greater initial S. pneumoniae association than LPS-stimulated bone marrow neutrophils (P = 0.0419) (Fig. 4B). By 60 min of incubation with S. pneumoniae, there were significantly fewer S. pneumoniae bacteria associated with bone marrow neutrophils from mice infected with influenza for 6 days than those from noninfected mice or mice infected with influenza for 3 days, with reductions of 34.7% and 33.1% (P = 0.0343 and 0.0071, respectively) (Fig. 4B). There was no significant difference in S. pneumoniae association at 60 min between mice infected with influenza for 6 days or LPS-stimulated mice, although there was a 26.8% reduction in S. pneumoniae association (P = 0.0855) (Fig. 4B). Similar results were seen when influenza-infected mice were stimulated with LPS (data not shown).
Trypan blue quenching showed that initially, 43.2% ± 6.9% of bone marrow neutrophils associated with S. pneumoniae from all groups studied had phagocytosed S. pneumoniae (Table 2). By 60 min, the level of phagocytosis had increased to 79.9% ± 10.5% (Table 2). After 60 min of incubation with S. pneumoniae, bone marrow neutrophils from mice infected with influenza for 6 days had a lower percentage of phagocytic neutrophils than those from LPS-stimulated mice (P < 0.01) (Table 2).
TABLE 2.
Trypan blue staining of bone marrow neutrophils to determine the percentage of neutrophils that were phagocytic and the percentage of phagocytic neutrophils that were producing ROSa
| Influenza infection or LPS treatment | % of neutrophils that were phagocytic after time (min):
|
% of phagocytic neutrophils that were producing ROS after time (min):
|
||
|---|---|---|---|---|
| 0 | 60 | 0 | 60 | |
| None | 38.5 ± 4.3 | 76.3 ± 5.5 | 41.1 ± 16.0* | 93.7 ± 3.4 |
| LPS, 8 h | 36.7 ± 2.6 | 92.9 ± 5.5 | 64.4 ± 4.4 | 95.9 ± 1.8 |
| Influenza, 3 days | 50.7 ± 4.3 | 78.5 ± 6.9 | 55.5 ± 13.9 | 94.7 ± 1.3 |
| Influenza, 6 days | 46.9 ± 3.9 | 71.8 ± 8.4** | 23.6 ± 6.4*† | 88.3 ± 2.4 |
Values represent means ± standard deviations (3 mice/group). Neutrophils were collected from mice and incubated with DiI-labeled S. pneumoniae for 0 or 60 min to measure phagocytosis and ROS generation. Statistical differences between groups were calculated using one-way analysis of variance, followed by the Bonferroni post test. Differences between groups with the same incubation time are indicated as follows: *, P < 0.05; **, P < 0.01 compared to neutrophils from LPS-challenged mice; †, P < 0.05 compared to neutrophils from mice infected with influenza for 3 days.
Thus, an influenza infection of 6 days' duration compromised bone marrow neutrophil function but not to the extent of suppression seen in lung neutrophil function. These data show that an influenza infection of 6 days can have slight systemic effects on the ability of bone marrow neutrophils to associate with S. pneumoniae.
Influenza infection reduces ROS production by neutrophils.
ROS production by neutrophils after 0 or 60 min of incubation with S. pneumoniae was measured using 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, which fluoresces in the FL-1 channel. As a positive control, cells were incubated with PMA, which activates neutrophils to produce ROS. The initial amount of ROS produced after S. pneumoniae stimulation was significantly reduced by 69.2% and 85.2% in lung neutrophils from mice infected with influenza for 3 and 6 days compared to those from LPS-stimulated mice (P < 0.0001; data not shown). Similar results were seen with lung neutrophils incubated with PMA, with reductions of 63.0% and 80.2% for mice infected with influenza for 3 or 6 days (P < 0.0001; data not shown). After 60 min of incubation with S. pneumoniae, lung neutrophils from mice infected with influenza for both 3 and 6 days produced significantly less ROS than those from LPS-stimulated mice, with reductions of 68.1% and 82.9% (P = 0.0002 and < 0.0001, respectively; data not shown). With PMA stimulation, similar results were seen, with reductions of 73.2% and 85.4% for mice infected with influenza for 3 and 6 days, respectively (P = 0.0002 and 0.0001, respectively; data not shown). As seen with S. pneumoniae association, the levels of ROS produced by lung neutrophils from influenza-infected mice after 60 min of incubation with either S. pneumoniae or PMA were comparable to that seen initially at 0 min by neutrophils from LPS-stimulated mice (data not shown).
Trypan blue quenching of extracellular fluorescence showed that a lower percentage of phagocytic lung neutrophils from mice infected with influenza for 3 or 6 days were initially producing ROS than from LPS-stimulated mice (P < 0.05 and 0.001, respectively) (Table 1). In addition, after 60 min of incubation with S. pneumoniae, a lower percentage of phagocytic lung neutrophils from mice infected with influenza for 6 days were producing ROS than from mice infected with influenza for 3 days and LPS-stimulated mice (P < 0.05 and 0.001, respectively) (Table 1). Thus, in the lungs of mice infected with influenza for 6 days, fewer neutrophils were associated with S. pneumoniae, and although most of these neutrophils were phagocytic, they were not able to produce ROS in response to S. pneumoniae as readily as those neutrophils from LPS-stimulated mice or mice infected with influenza for 3 days.
Influenza-infected mice stimulated with LPS showed similar results. Initial ROS production by neutrophils after 0 min of incubation with S. pneumoniae was significantly reduced in mice infected with influenza for 3 or 6 days followed by LPS simulation compared to mice stimulated with LPS only (P = 0.0024 and 0.0009, respectively) (Fig. 5A). Similar decreases were seen initially with PMA (P = 0.0038 and 0.0013, respectively) (Fig. 5B). After 60 min of incubation with S. pneumoniae, lung neutrophils from mice infected with influenza for 3 or 6 days followed by LPS stimulation produced significantly less ROS than neutrophils from mice stimulated with LPS only (P = 0.0016 and 0.0004, respectively) (Fig. 5A). Similar results were seen after 60 min of incubation with PMA (P < 0.0001 for both) (Fig. 5B). These data show that the changes in ROS production are due to influenza-induced effects and not the effects of LPS stimulation. In summary, a prior influenza infection of 3 or 6 days reduces the ability of lung neutrophils to produce ROS after stimulation with either S. pneumoniae or PMA.
FIG. 5.
ROS generation by lung (A and B) and bone marrow (C and D) neutrophils from noninfected mice, LPS-stimulated mice, or mice infected with influenza for 3 or 6 days with (A and B) or without (C and D) subsequent LPS stimulation. Neutrophils were incubated with S. pneumoniae (A and C) or PMA (B and D) for 0 or 60 min. Data represent results from one (A and B) or two (C and D) independent experiments with 3 sets of 2 mice per group. Significance is indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to LPS-stimulated mice; ##, P < 0.01 compared to mice infected with influenza for 3 days. Data are expressed as means ± standard deviations. Data were analyzed using unpaired t test.
In the bone marrow, initial ROS production induced by S. pneumoniae stimulation was reduced by 52.6% and 60.0% in bone marrow neutrophils from mice infected with influenza for 6 days compared to those from LPS-stimulated mice or mice infected with influenza for 3 days (P = 0.0069 and 0.0019, respectively) (Fig. 5C). After 60 min of incubation with S. pneumoniae, bone marrow neutrophils from mice infected with influenza for 6 days still produced less ROS than LPS-stimulated mice or mice infected with influenza for 3 days, with reductions of 43.4% and 43.9%, respectively (P = 0.0212 and 0.0034, respectively) (Fig. 5C). Bone marrow neutrophils from mice infected with influenza for 6 days also showed reductions of 54.5% and 46.7% in initial ROS production after incubation with PMA compared to LPS-stimulated mice and mice infected with influenza for 3 days (P = 0.0007 and 0.0068, respectively) (Fig. 5D). However, unlike the results seen after 60 min of incubation with S. pneumoniae, there were no significant differences in ROS production after 60 min of incubation with PMA between bone marrow neutrophils from mice infected with influenza for 6 days and LPS-stimulated mice or mice infected with influenza for 3 days (P = 0.0536 and 0.1389, respectively) (Fig. 5D). There were no significant differences in ROS production by bone marrow neutrophils from mice infected with influenza for 3 days compared to noninfected or LPS-stimulated neutrophils after 0 or 60 min of incubation with S. pneumoniae or PMA (Fig. 5C and D).
Trypan blue quenching of extracellular fluorescence associated with S. pneumoniae bound to the surface of bone marrow neutrophils showed that initially there was some variation between groups in the percentages of phagocytic neutrophils which were producing ROS (Table 2). However, after 60 min of incubation with S. pneumoniae, no differences between any of the groups were seen (Table 2).
Similar results were seen when influenza-infected mice were stimulated with LPS (data not shown). These results indicate that an influenza infection of 6 days has systemic effects on the ability of bone marrow neutrophils to produce ROS initially and after stimulation with S. pneumoniae for 60 min. However, with PMA stimulation, any differences in initial ROS production are overcome by 60 min of incubation with PMA. In summary, an influenza infection of 6 days affects ROS production by bone marrow neutrophils, indicating that the effects of an influenza infection are systemic.
Effects of influenza and/or S. pneumoniae infection(s) on lung cytokine production.
Since our neutrophil depletion studies indicated that influenza-induced changes in neutrophil-independent mechanisms also contributed to the increased susceptibility to S. pneumoniae infection, we measured cytokine levels in the lungs of noninfected mice, mice infected with S. pneumoniae only, mice infected with influenza for 3 or 6 days, and mice infected with influenza for 3 or 6 days followed by S. pneumoniae for 24 h to determine whether changes in cytokine expression levels were responsible for this increase in susceptibility. In addition, we measured cytokine levels in the lungs of neutrophil-depleted mice in each of the infection groups to determine whether the increases in susceptibility to S. pneumoniae seen in depleted mice were due to increased cytokine production.
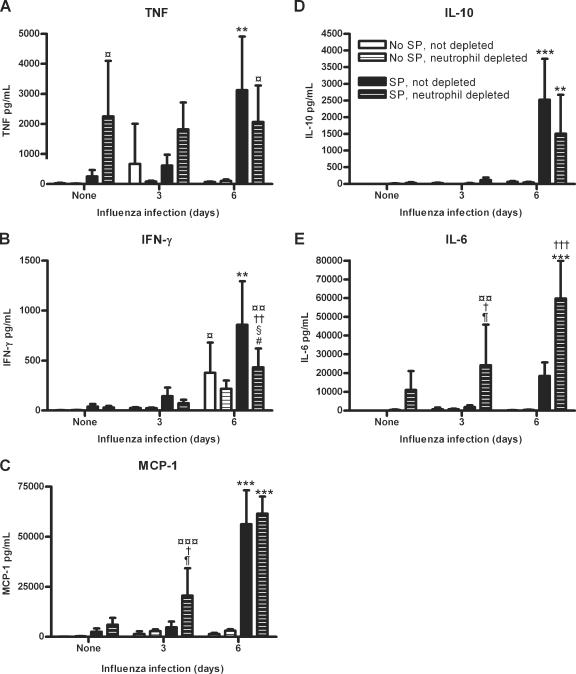
No significant differences in IL-12 p70 levels were seen between any of the groups examined (P > 0.05; data not shown). Mice infected with S. pneumoniae only for 24 h had elevated levels of tumor necrosis factor (TNF), gamma interferon (IFN-γ), monocyte chemoattractant protein 1 (MCP-1), IL-10, and IL-6 compared to noninfected mice, although none of these increases were statistically significant (P > 0.05) (Fig. 6A to E). Mice infected with influenza only for 3 or 6 days had elevated levels of TNF, IFN-γ, MCP-1, IL-10, and IL-6 compared to noninfected mice; however, only IFN-γ was significantly increased after 6 days of influenza infection compared to noninfected mice (P < 0.05) (Fig. 6A to E). Together, these data indicate that an influenza infection of 3 or 6 days or an S. pneumoniae infection of 24 h slightly increase lung TNF, IFN-γ, MCP-1, IL-10, and IL-6 levels.
FIG. 6.
Lung cytokine levels in neutrophil-depleted and nondepleted mice with or without S. pneumoniae for 24 h and/or influenza virus for 3 or 6 days. TNF (A), IFN-γ (B), MCP-1, IL-10, and IL-6 levels were measured in nondepleted and neutrophil-depleted mice which were not infected, infected with S. pneumoniae only for 24 h, infected with influenza only for 3 or 6 days, or infected with influenza for 3 or 6 days followed by S. pneumoniae for 24 h. Significance is indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001 for mice infected with influenza virus for 6 days followed by S. pneumoniae compared to noninfected mice, mice infected with S. pneumoniae only, mice infected with influenza virus only, and mice infected with influenza virus for 3 days followed by S. pneumoniae with the same depletion status; ¶, P < 0.05 for mice with the same influenza infection and depletion status to determine the effects of a S. pneumoniae infection; #, P < 0.05 for mice with the same S. pneumoniae infection and depletion status to determine the effects of an influenza infection; †, P < 0.05; ††, P < 0.01; †††, P < 0.001 for mice with the same influenza and S. pneumoniae infections to determine the effects of neutrophil depletion; §, P < 0.05 for mice with the same S. pneumoniae infection and depletion status to determine the effects of 3 days versus 6 days of influenza infection; ¤, P < 0.05; ¤¤, P < 0.01; ¤¤¤, P < 0.001 for mice with the same depletion status to determine the effects of a coinfection or S. pneumoniae infection versus no infection. Data are expressed as means ± standard deviations. Data were analyzed using one-way analysis of variance, followed by the Bonferroni post test.
A previous influenza infection of 6 days followed by an S. pneumoniae infection increased lung cytokine production. Nondepleted mice coinfected with influenza for 6 days followed by S. pneumoniae had significantly more TNF (P < 0.001), IFN-γ (P < 0.01), MCP-1 (P < 0.001), and IL-10 (P < 0.001) than nondepleted mice infected only with influenza for 6 days (Fig. 6A to D). These coinfected mice also had significantly more TNF (P < 0.001), IFN-γ (P < 0.001), MCP-1 (P < 0.001), and IL-10 (P < 0.001) than nondepleted mice infected with S. pneumoniae only and noninfected mice. Compared to nondepleted mice coinfected with influenza for 3 days followed by S. pneumoniae, these coinfected mice had significantly more TNF (P < 0.01), IFN-γ (P < 0.001), MCP-1 (P < 0.001), and IL-10 (P < 0.001) (Fig. 6A to D). In addition, IL-6 was slightly increased, although not significantly (P > 0.05), in these coinfected mice compared to nondepleted groups of mice which were not infected, infected with S. pneumoniae only, coinfected with influenza for 3 days followed by S. pneumoniae, or infected with influenza only for 3 or 6 days (Fig. 6E). Together, these data indicate that an influenza infection of 6 days followed by an S. pneumoniae infection increases lung cytokine production compared to noninfected mice, mice infected with influenza for 3 or 6 days, and mice coinfected with influenza for 3 days followed by an S. pneumoniae infection.
Neutrophil depletion also affected the amounts of lung cytokines. After neutrophil depletion, the concentration of TNF was increased, although not significantly, for mice coinfected with influenza for 3 days followed by S. pneumoniae (Fig. 6A). Depleted mice infected with S. pneumoniae only had significantly more TNF than depleted noninfected mice (P < 0.05). There were no significant differences in TNF between all depleted groups which were infected with S. pneumoniae only or coinfected with influenza for 3 or 6 days followed by S. pneumoniae infection (P > 0.05) (Fig. 6A). In addition, depleted mice coinfected with influenza for 6 days followed by S. pneumoniae had significantly more TNF than depleted noninfected mice (P < 0.05) (Fig. 6A).
Neutrophil depletion tended to slightly decrease the amount of IFN-γ compared to nondepleted mice, although insignificantly except for mice which were coinfected with influenza for 6 days followed by S. pneumoniae (P < 0.01) (Fig. 6B). In addition, these coinfected mice had significantly more IFN-γ than depleted mice which were not infected (P < 0.01), infected with S. pneumoniae only (P < 0.05), or coinfected with influenza for 3 days followed by S. pneumoniae (P < 0.05) (Fig. 6B). Neutrophil depletion also affected the levels of MCP-1. Depleted mice infected with S. pneumoniae only had a slight, though insignificant, increase in MCP-1 compared to nondepleted mice (P > 0.05) (Fig. 6C). In depleted mice coinfected with influenza for 3 days followed by S. pneumoniae, there was significantly more MCP-1 than in nondepleted mice coinfected with influenza for 3 days followed by S. pneumoniae (P < 0.05), depleted mice infected with influenza only for 3 days (P < 0.05), and depleted noninfected mice (P < 0.001) (Fig. 6C). Depleted mice coinfected with influenza for 6 days followed by S. pneumoniae had significantly more MCP-1 and IL-6 than depleted groups of mice infected with S. pneumoniae only (P < 0.001), coinfected with influenza for 3 days followed by S. pneumoniae (P < 0.001), infected with influenza only for 6 days (P < 0.001), and noninfected (P < 0.001) (Fig. 6C and E). Neutrophil depletion significantly increased the amount of IL-6 in mice coinfected with influenza for 3 days (P < 0.05) or 6 days (P < 0.001) followed by S. pneumoniae compared to nondepleted mice (Fig. 6E). Depleted mice infected with S. pneumoniae only had a slight, though insignificant, increase in IL-6 production compared to nondepleted mice. In addition, depleted mice coinfected with influenza for 3 days followed by S. pneumoniae had significantly more IL-6 than depleted mice which were not infected (P < 0.01) or infected with influenza only for 3 days (P < 0.05) (Fig. 6E). Depleted mice coinfected with influenza for 6 days followed by S. pneumoniae had significantly more IL-10 than depleted groups of mice infected with S. pneumoniae only (P < 0.001), coinfected with influenza for 3 days followed by S. pneumoniae (P < 0.01), infected with influenza only for 6 days (P < 0.001), and not infected (P < 0.001) (Fig. 6E). In summary, neutrophil depletion affected the cytokine production in infected lungs, with elevated levels of TNF, MCP-1, and IL-6 in depleted mice infected with S. pneumoniae only or coinfected with influenza for 3 days followed by S. pneumoniae compared to mice with the same infection treatment which were not depleted of neutrophils.
DISCUSSION
To establish a time course of susceptibility to S. pneumoniae infection after influenza infection, mice infected with influenza virus for 3 or 6 days were challenged with S. pneumoniae. We found that susceptibility to S. pneumoniae was the greatest at 6 days after influenza infection and was not increased at 3 days after influenza infection. Several other groups have found similar results, and clinical evidence supports this timeframe for maximum susceptibility (14, 24, 42, 59). Influenza-induced tissue damage is greatest after 6 days of influenza infection (44), and Plotkowski et al. found that adherence of S. pneumoniae to influenza-infected tracheas was greatest after 6 days of influenza infection (48). However, influenza-induced neutrophil dysfunction has also been shown to be greatest around 6 days after influenza infection (1, 2). We found that when influenza titers within the lungs were at their peak 3 days after infection, there was no significant difference in susceptibility to S. pneumoniae with or without a prior influenza infection. Gerone et al. showed similar results, with no increased susceptibility to S. pneumoniae infection until after 3 days of influenza infection (24). McCullers and Rehg found that mortality to S. pneumoniae infection was greatest after 7 days of influenza infection, with all mice dying within 24 h, but at 3 days after influenza infection, the rate of mortality was decreased, with all mice dying 3.3 days after S. pneumoniae infection (42). Although our own data of the kinetics of influenza infection and susceptibility to S. pneumoniae infection agree with previously published results, none of these studies determined the relative contributions of both neutrophil-dependent and -independent mechanisms to increased susceptibility to S. pneumoniae during an influenza infection.
Earlier neutrophil depletion studies done in our laboratory showed that neutrophils play an important role in resistance to S. pneumoniae infection in the lungs (23). By using similar neutrophil depletion models, other studies have shown that neutrophils play an important role in controlling infections such as herpes simplex virus type I, Chlamydophila abortus, Toxoplasma gondii, and Listeria monocytogenes (20-22, 49, 62). We used neutrophil depletions to determine whether neutrophils play a role in resistance to S. pneumoniae infection in mice infected with S. pneumoniae only or mice previously infected with influenza for 3 or 6 days. Our data confirmed our previous studies that neutrophils are important to S. pneumoniae resistance in non-influenza-infected mice (23). In addition, we showed that they are important in S. pneumoniae resistance in mice infected with influenza for 3 days. However, although neutrophils accumulate in the lungs of mice infected with influenza for 6 days, they do not function in resistance to S. pneumoniae. It is likely that in the influenza-infected, nondepleted mice, lung neutrophil bactericidal functions were suppressed, making the mice as susceptible to S. pneumoniae as those which had been neutrophil depleted.
Effective phagocytosis and killing of S. pneumoniae is necessary for elimination of S. pneumoniae from the lungs (33). Studies have found that neutrophils are affected within 30 min of an in vitro influenza infection (4, 5, 7, 8, 13, 15, 31), with some effects seen as rapidly as 5 min after incubation with influenza virus (5). Such influenza-induced changes in neutrophils include decreased protein phosphorylation, accelerated apoptosis, decreased respiratory burst activity, altered cytoskeleton, depressed bactericidal capacity, decreased chemotactic ability, decreased adherence, decreased release of lactoferrin into phagosomes, and inhibition of lysosome-phagosome fusion (4, 5, 7, 8, 13, 15, 31). Changes seen in blood neutrophils from mice infected with influenza in vivo include decreased chemotactic, chemiluminescent, and bactericidal activities (1, 2). These studies, along with the results from our studies, show that the effects of influenza virus on neutrophil function are not limited to the lungs, indicating that the effects are systemic despite a lack of viremia (1, 2). Studies examining the function of lung neutrophils infected with influenza in vivo have measured the amount of superoxide and myeloperoxidase produced after infection with S. pneumoniae (37, 51). While these previous neutrophil studies provide evidence of influenza-induced neutrophil dysfunction, they did not examine the effects of an in vivo influenza infection on lung neutrophil function independently of an S. pneumoniae infection. In addition, the previous studies did not examine neutrophil function directly, since they did not select for neutrophils and, therefore, were measuring the responses of other lung cells such as macrophages and lymphocytes, whose function may also be affected during an influenza infection.
In the present study, the function of neutrophils that had accumulated in the lungs and were exposed to influenza virus in the lungs was investigated. The function of BALF neutrophils as opposed to BALF macrophages was determined by flow cytometry using neutrophil-specific staining to gate the neutrophil population. The effects of influenza on these neutrophils in response to an S. pneumoniae infection could be measured independently of other changes to the host response, since the S. pneumoniae was added in vitro. Since we were interested in determining whether influenza was affecting neutrophil function locally at the site of infection and/or systemically, we measured the ability of neutrophils isolated from the lungs and bone marrow of noninfected (LPS stimulated) and influenza-infected (3 or 6 days) mice to associate with S. pneumoniae (either through binding or phagocytosis) and produce reactive oxygen species. Our method allows for a direct measurement of any changes in neutrophil function in the lungs during an in vivo influenza infection prior to an S. pneumoniae infection, since the neutrophils were exposed to influenza virus in vivo and S. pneumoniae in vitro.
Lungs from normal, healthy mice contain primarily alveolar macrophages and few to no neutrophils. To compare lung neutrophils from healthy mice to those from influenza-infected mice, we used LPS to elicit a rapid neutrophil migration into the lungs of healthy mice. The peak of neutrophil migration and accumulation in the lungs was seen 8 h after LPS stimulation. We compared these neutrophils to those from mice infected with influenza for 3 or 6 days to determine whether an influenza infection alters neutrophil function, and if so, whether this alteration was greatest at 6 days after influenza, resulting in increased susceptibility to S. pneumoniae infection at 6 days, but not 3 days, after influenza infection. We found that both S. pneumoniae association and ROS production by lung neutrophils were significantly decreased at both 3 and 6 days after influenza infection compared to LPS-stimulated mice. This decrease was seen after initial contact with S. pneumoniae and after 60 min incubation with S. pneumoniae. In addition, lung neutrophils from mice infected with influenza for 6 days which were phagocytosing bacteria were not producing ROS as well as phagocytic neutrophils from LPS-stimulated mice. These data indicate that lung neutrophil function is affected at both 3 and 6 days after influenza infection. However, there may be neutrophil functions which we did not examine that are altered more at 6 days than 3 days after influenza, resulting in the difference in susceptibility seen between 3 and 6 days after influenza infection.
In the bone marrow of mice infected with influenza for 6 days, we saw an initial decrease in ROS production after initial contact with both S. pneumoniae and PMA. This decrease was seen after 60 min of incubation with S. pneumoniae. However, after 60 min of incubation with PMA, there was no decrease in ROS production by neutrophils. S. pneumoniae phagocytosis by bone marrow neutrophils was also decreased after 60 min of incubation with S. pneumoniae but not initially. The decrease in bone marrow neutrophil function may be due to systemic viral effects. Another possible explanation is that the proportion of immature neutrophils may be greater in mice infected with influenza for 6 days than in LPS-stimulated mice or mice infected with influenza for 3 days, since neutrophils are recruited from the bone marrow during an influenza infection. However, Boxio et al. found that murine bone marrow neutrophils function relatively homogenously and that there is a large population of competent neutrophils which can be recruited during infections (12).
Since the LPS challenge may have altered the neutrophil function in the lungs of otherwise healthy mice, we challenged influenza-infected mice with LPS to determine whether the changes in neutrophil function seen previously were due to influenza or LPS effects. In the lungs, there was a significant increase in the number of neutrophils after LPS stimulation, indicating that the neutrophil reservoir had not been depleted by the influenza infection. The LPS challenge is similar to a secondary bacterial infection and therefore indicates that fresh neutrophils can enter the lungs in response to a secondary infection. After LPS stimulation in influenza-infected mice, we found reductions in S. pneumoniae association with and ROS production by lung neutrophils similar to those seen with mice infected with influenza only, indicating that the effects on neutrophil function seen were due to the effects of influenza infection and not LPS stimulation. Additionally, the new neutrophils recruited into the lungs by LPS were not able to overcome the previous deficit in neutrophil function caused by the influenza infection. This may be due to the relatively short period of time in contact with influenza virus (5 to 30 min) necessary to induce neutrophil dysfunction, as seen by others after an in vitro influenza infection (4, 5, 7, 8, 13, 15, 31). The neutrophils recruited to the lungs by LPS may be inhibited by influenza just as rapidly as those in in vitro infections, since infectious influenza particles were seen in the lungs at both 3 and 6 days after influenza infection, indicating that influenza infection affects neutrophil function locally, possibly by binding directly to the freshly recruited neutrophils. In the bone marrow, the effects of influenza infection and LPS stimulation on neutrophil function do not appear to be as significant as those seen in the lungs.
Our results indicate that an influenza infection of either 3 or 6 days in neutrophil-depleted mice increased susceptibility to S. pneumoniae infection compared to depleted mice infected with S. pneumoniae only, with the greatest increase at 6 days after influenza infection. These data indicate that an influenza infection also increases susceptibility to S. pneumoniae by a mechanism other than neutrophil dysfunction, such as elevated cytokine production in the lungs. We found that mice which were infected with influenza for 6 days followed by S. pneumoniae for 24 h had elevated levels of TNF, IFN-γ, MCP-1, IL-10, and IL-6. Previous studies have shown that IFN-γ, TNF-α, and IL-6 levels are increased when staphylococcal enterotoxin B or LPS are added to influenza-infected mice, indicating that a prior influenza infection primed influenza-infected cells for rapid cytokine production upon secondary challenge with bacterial components (26, 68). In addition, LeVine et al. showed that TNF-α and IL-1 are significantly increased in mice infected with influenza for 7 days followed by S. pneumoniae for 48 h (37). Influenza infection is well known to lyse epithelial cells, leading to tissue damage, and this elevation in both pro- and anti-inflammatory cytokines may lead to increased tissue damage and pathogenesis during influenza virus and S. pneumoniae infections.
Studies by McCullers and Bartmess have shown that viral influenza neuraminidase increases S. pneumoniae adherence in the lungs by cleaving sialic acid residues, exposing receptors to which S. pneumoniae adhere (41). Hirano et al. examined the changes in carbohydrates exposed after sialic acid residues are removed by neuraminidase during an influenza infection, which may allow for increased S. pneumoniae adherence (32). Plotkowski et al. documented the influenza-induced changes in the respiratory epithelium throughout the course of an influenza infection and adherence of S. pneumoniae to influenza-infected tissue using scanning electron microscopy (48). Throughout the course of an influenza infection, different cell types and surface molecules are exposed to which S. pneumoniae may adhere more readily. As shown previously, influenza-induced tissue damage is greatest around 6 days after influenza infection (44), which correlates with our data showing increased susceptibility to S. pneumoniae infection at 6 days after influenza, indicating that tissue damage likely plays a role in increased susceptibility.
IL-10 has recently been shown to be elevated in postinfluenza pneumococcal pneumonia, leading to increased susceptibility to S. pneumoniae long after an influenza infection has been resolved (63). This increase in susceptibility 14 days after a primary influenza infection is believed to be due to IL-10 inhibiting neutrophil function, which results in increased bacterial growth in the lungs and increased mortality (63). Our data indicate that the onset of this increase in IL-10 production is as early as 6 days after an influenza infection, which also correlates with the peak of susceptibility shown in our study. IL-10 levels were not significantly elevated at 3 days after an influenza infection when we did not see an increase in susceptibility to S. pneumoniae infection.
Since depleted mice infected with S. pneumoniae only or influenza for 3 days followed by S. pneumoniae were more susceptible to S. pneumoniae than nondepleted mice, we measured the cytokine levels in these mice to determine whether an elevated cytokine response may be responsible for this increase in susceptibility. We found that TNF, MCP-1, and IL-6 all showed slight increases in depleted mice compared to nondepleted mice. In addition, TNF levels were similar between depleted groups which were infected with S. pneumoniae only or influenza for 3 or 6 days followed by S. pneumoniae. These data indicate that elevated cytokine production in depleted mice may increase susceptibility to S. pneumoniae infection. Further investigation into the role of these cytokines in secondary bacterial pneumonia is needed to determine the contributions of these cytokines to increased susceptibility to S. pneumoniae after influenza.
Another possible explanation for the mechanism of increased susceptibility to S. pneumoniae infection after influenza infection is alterations in alveolar macrophage function. Several studies have demonstrated that influenza infection decreases the functions of alveolar macrophages, such as bacterial phagocytosis and killing (11, 65). Astry and Jakab found the suppression of alveolar macrophage phagocytosis by influenza was greatest after 7 to 10 days of influenza infection (11), which correlates with our data for increased susceptibility to S. pneumoniae infection after 6 days of influenza infection. Alveolar macrophages have been shown to have a protective anti-inflammatory role during S. pneumoniae infections through the elimination of apoptotic neutrophils (36). If apoptotic neutrophils are not effectively cleared from the lungs, their cytotoxic components eventually leak into the alveolar space, leading to increased tissue damage (28, 36). Since alveolar macrophages are important in the defense against bacterial infections, a change in their ability to eliminate S. pneumoniae and apoptotic neutrophils could alter the severity of the infection.
In summary, our neutrophil depletion studies indicated that both neutrophil-dependent and -independent mechanisms are responsible for increased susceptibility to a secondary S. pneumoniae infection after an influenza infection. While an influenza infection may cause other changes to the host immune response which we did not examine, our data indicate that both neutrophil dysfunction and elevated cytokine production contribute to the increased susceptibility to a secondary S. pneumoniae infection. These effects are cumulative, with the greatest susceptibility to S. pneumoniae infection at 6 days after influenza infection. At 3 days after influenza infection, viral replication is at its peak and tissue damage is minimal. At this time, lung neutrophil function was depressed, yet there was no significant increase in susceptibility to S. pneumoniae infection compared to mice infected with S. pneumoniae only. In addition, neutrophil depletion studies showed that after 3 days of influenza infection, neutrophil depletion increased susceptibility to S. pneumoniae infection, indicating that neutrophils from mice infected with influenza for 3 days can still eliminate S. pneumoniae. However, even at 3 days after influenza infection, neutrophil depletion studies showed evidence of neutrophil-independent mechanisms for increased susceptibility. After 6 days of influenza infection, when susceptibility to S. pneumoniae infection is at its peak, viral replication is decreased and tissue damage is at its greatest (44, 48). Neutrophil function was also suppressed after 6 days of influenza infection. Neutrophils in mice infected with influenza for 6 days, which already have a decreased phagocytic capability, may not be able to eliminate S. pneumoniae in vivo as readily as neutrophils from mice infected with influenza for 3 days due to the increased burden of phagocytosing cellular debris from influenza-induced tissue damage and apoptotic neutrophils. In addition, there may be more influenza-induced changes in neutrophil function at 6 days than 3 days, which we did not examine. Cytokine levels were also increased in mice coinfected with influenza for 6 days followed by S. pneumoniae, indicating that an elevated cytokine response may be responsible for the increased morbidity and mortality seen during influenza and S. pneumoniae infections. By better understanding how the influenza virus affects both tissue damage and host bacterial responses, such as neutrophil function and cytokine expression, therapies can rationally be developed that will prevent secondary S. pneumoniae infections and the increased morbidity and mortality associated with them.
Acknowledgments
This work was supported by INBRE-BRIN grant P20 RR16455-04 and NIH grant COBRE 1 P20 RR020185-01.
We have no financial conflicts of interest.
We thank Gayle Callis, Sara Erickson, Ann Harmsen, Quinton King, Melanie Rutkowski, Mike Tighe, and Jim Wiley for expert technical assistance. In particular, we thank Steve Swain for expert technical assistance and critical reading of the manuscript.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 18 September 2006.
REFERENCES
- 1.Abramson, J. S., G. S. Giebink, E. L. Mills, and P. G. Quie. 1981. Polymorphonuclear leukocyte dysfunction during influenza virus infection in chinchillas. J. Infect. Dis. 143:836-845. [DOI] [PubMed] [Google Scholar]
- 2.Abramson, J. S., G. S. Giebink, and P. G. Quie. 1982. Influenza A virus-induced polymorphonuclear leukocyte dysfunction in the pathogenesis of experimental pneumococcal otitis media. Infect. Immun. 36:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramson, J. S., and H. R. Hudnor. 1994. Effect of priming polymorphonuclear leukocytes with cytokines (granulocyte-macrophage colony-stimulating factor [GM-CSF] and G-CSF) on the host resistance to Streptococcus pneumoniae in chinchillas infected with influenza A virus. Blood 83:1929-1934. [PubMed] [Google Scholar]
- 4.Abramson, J. S., J. C. Lewis, D. S. Lyles, K. A. Heller, E. L. Mills, and D. A. Bass. 1982. Inhibition of neutrophil lysosome-phagosome fusion associated with influenza virus infection in vitro. Role in depressed bactericidal activity. J. Clin. Investig. 69:1393-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abramson, J. S., D. S. Lyles, K. A. Heller, and D. A. Bass. 1982. Influenza A virus-induced polymorphonuclear leukocyte dysfunction. Infect. Immun. 37:794-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramson, J. S., and E. L. Mills. 1988. Depression of neutrophil function induced by viruses and its role in secondary microbial infections. Rev. Infect. Dis. 10:326-341. [DOI] [PubMed] [Google Scholar]
- 7.Abramson, J. S., E. L. Mills, G. S. Giebink, and P. G. Quie. 1982. Depression of monocyte and polymorphonuclear leukocyte oxidative metabolism and bactericidal capacity by influenza A virus. Infect. Immun. 35:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abramson, J. S., J. W. Parce, J. C. Lewis, D. S. Lyles, E. L. Mills, R. D. Nelson, and D. A. Bass. 1984. Characterization of the effect of influenza virus on polymorphonuclear leukocyte membrane responses. Blood 64:131-138. [PubMed] [Google Scholar]
- 9.Abramson, J. S., and J. G. Wheeler. 1994. Virus-induced neutrophil dysfunction: role in the pathogenesis of bacterial infections. Pediatr. Infect. Dis. J. 13:643-652. [PubMed] [Google Scholar]
- 10.Anderson, R. N., and B. L. Smith. 2005. Deaths: leading causes for 2002. Natl. Vital Stat. Rep. 53:1-89. [PubMed] [Google Scholar]
- 11.Astry, C. L., and G. J. Jakab. 1984. Influenza virus-induced immune complexes suppress alveolar macrophage phagocytosis. J. Virol. 50:287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boxio, R., C. Bossenmeyer-Pourie, N. Steinckwich, C. Dournon, and O. Nusse. 2004. Mouse bone marrow contains large numbers of functionally competent neutrophils. J. Leukoc. Biol. 75:604-611. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell, S. E., L. F. Cassidy, and J. S. Abramson. 1988. Alterations in cell protein phosphorylation in human neutrophils exposed to influenza A virus. A possible mechanism for depressed cellular end-stage functions. J. Immunol. 140:3560-3567. [PubMed] [Google Scholar]
- 14.Cate, T. R. 1998. Impact of influenza and other community-acquired viruses. Semin. Respir. Infect. 13:17-23. [PubMed] [Google Scholar]
- 15.Colamussi, M. L., M. R. White, E. Crouch, and K. L. Hartshorn. 1999. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood 93:2395-2403. [PubMed] [Google Scholar]
- 16.Cotter, M. J., K. E. Norman, P. G. Hellewell, and V. C. Ridger. 2001. A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am. J. Pathol. 159:473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cundell, D., H. R. Masure, and E. I. Tuomanen. 1995. The molecular basis of pneumococcal infection: a hypothesis. Clin. Infect. Dis. 21(Suppl. 3):S204-S211. [DOI] [PubMed] [Google Scholar]
- 18.Cundell, D. R., C. Gerard, I. Idanpaan-Heikkila, E. I. Tuomanen, and N. P. Gerard. 1996. PAf receptor anchors Streptococcus pneumoniae to activated human endothelial cells. Adv. Exp. Med. Biol. 416:89-94. [DOI] [PubMed] [Google Scholar]
- 19.Cundell, D. R., N. P. Gerard, C. Gerard, I. Idanpaan-Heikkila, and E. I. Tuomanen. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435-438. [DOI] [PubMed] [Google Scholar]
- 20.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836-1846. [PubMed] [Google Scholar]
- 21.Czuprynski, C. J., C. Theisen, and J. F. Brown. 1996. Treatment with the antigranulocyte monoclonal antibody RB6-8C5 impairs resistance of mice to gastrointestinal infection with Listeria monocytogenes. Infect. Immun. 64:3946-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Oca, R. M., A. J. Buendia, L. Del Rio, J. Sanchez, J. Salinas, and J. A. Navarro. 2000. Polymorphonuclear neutrophils are necessary for the recruitment of CD8(+) T cells in the liver in a pregnant mouse model of Chlamydophila abortus (Chlamydia psittaci serotype 1) infection. Infect. Immun. 68:1746-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garvy, B. A., and A. G. Harmsen. 1996. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation 20:499-512. [DOI] [PubMed] [Google Scholar]
- 24.Gerone, P. J., T. G. Ward, and W. A. Chappell. 1957. Combined infections in mice with influenza virus and Diplococcus pneumoniae. Am. J. Hyg. 66:331-341. [DOI] [PubMed] [Google Scholar]
- 25.Glezen, W. P., A. A. Payne, D. N. Snyder, and T. D. Downs. 1982. Mortality and influenza. J. Infect. Dis. 146:313-321. [DOI] [PubMed] [Google Scholar]
- 26.Gong, J. H., H. Sprenger, F. Hinder, A. Bender, A. Schmidt, S. Horch, M. Nain, and D. Gemsa. 1991. Influenza A virus infection of macrophages. Enhanced tumor necrosis factor-alpha (TNF-alpha) gene expression and lipopolysaccharide-triggered TNF-alpha release. J. Immunol. 147:3507-3513. [PubMed] [Google Scholar]
- 27.Hament, J. M., J. L. Kimpen, A. Fleer, and T. F. Wolfs. 1999. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol. Med. Microbiol. 26:189-195. [DOI] [PubMed] [Google Scholar]
- 28.Haslett, C. 1999. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respir. Crit. Care Med. 160:S5-S11. [DOI] [PubMed] [Google Scholar]
- 29.Herigstad, B., M. Hamilton, and J. Heersink. 2001. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 44:121-129. [DOI] [PubMed] [Google Scholar]
- 30.Hestdal, K., F. W. Ruscetti, J. N. Ihle, S. E. Jacobsen, C. M. Dubois, W. C. Kopp, D. L. Longo, and J. R. Keller. 1991. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 147:22-28. [PubMed] [Google Scholar]
- 31.Hinshaw, V. S., C. W. Olsen, N. Dybdahl-Sissoko, and D. Evans. 1994. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J. Virol. 68:3667-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirano, T., Y. Kurono, I. Ichimiya, M. Suzuki, and G. Mogi. 1999. Effects of influenza A virus on lectin-binding patterns in murine nasopharyngeal mucosa and on bacterial colonization. Otolaryngol. Head Neck Surg. 121:616-621. [DOI] [PubMed] [Google Scholar]
- 33.Johnston, R. B., Jr. 1981. The host response to invasion by Streptococcus pneumoniae: protection and the pathogenesis to tissue damage. Rev. Infect. Dis. 3:282-288. [DOI] [PubMed] [Google Scholar]
- 34.Kim, P. E., D. M. Musher, W. P. Glezen, M. C. Rodriguez-Barradas, W. K. Nahm, and C. E. Wright. 1996. Association of invasive pneumococcal disease with season, atmospheric conditions, air pollution, and the isolation of respiratory viruses. Clin. Infect. Dis. 22:100-106. [DOI] [PubMed] [Google Scholar]
- 35.Klimov, A., L. Simonsen, K. Fukuda, and N. Cox. 1999. Surveillance and impact of influenza in the United States. Vaccine 17(Suppl. 1):S42-S46. [DOI] [PubMed] [Google Scholar]
- 36.Knapp, S., J. C. Leemans, S. Florquin, J. Branger, N. A. Maris, J. Pater, N. van Rooijen, and T. van der Poll. 2003. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167:171-179. [DOI] [PubMed] [Google Scholar]
- 37.LeVine, A. M., V. Koeningsknecht, and J. M. Stark. 2001. Decreased pulmonary clearance of S. pneumoniae following influenza A infection in mice. J. Virol. Methods 94:173-186. [DOI] [PubMed] [Google Scholar]
- 38.Linder, T. E., R. L. Daniels, D. J. Lim, and T. F. DeMaria. 1994. Effect of intranasal inoculation of Streptococcus pneumoniae on the structure of the surface carbohydrates of the chinchilla eustachian tube and middle ear mucosa. Microb. Pathog. 16:435-441. [DOI] [PubMed] [Google Scholar]
- 39.Martin, R. R., R. B. Couch, S. B. Greenberg, T. R. Cate, and G. A. Warr. 1981. Effects of infection with influenza virus on the function of polymorphonuclear leukocytes. J. Infect. Dis. 144:279-280. [DOI] [PubMed] [Google Scholar]
- 40.McCullers, J. A. 2004. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J. Infect. Dis. 190:519-526. [DOI] [PubMed] [Google Scholar]
- 41.McCullers, J. A., and K. C. Bartmess. 2003. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 187:1000-1009. [DOI] [PubMed] [Google Scholar]
- 42.McCullers, J. A., and J. E. Rehg. 2002. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 186:341-350. [DOI] [PubMed] [Google Scholar]
- 43.McCullers, J. A., and E. I. Tuomanen. 2001. Molecular pathogenesis of pneumococcal pneumonia. Front. Biosci. 6:D877-D889. [DOI] [PubMed] [Google Scholar]
- 44.Nugent, K. M., and E. L. Pesanti. 1983. Tracheal function during influenza infections. Infect. Immun. 42:1102-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Brien, K. L., M. I. Walters, J. Sellman, P. Quinlisk, H. Regnery, B. Schwartz, and S. F. Dowell. 2000. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin. Infect. Dis. 30:784-789. [DOI] [PubMed] [Google Scholar]
- 46.Peltola, V. T., and J. A. McCullers. 2004. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr. Infect. Dis. J. 23:S87-S97. [DOI] [PubMed] [Google Scholar]
- 47.Peltola, V. T., K. G. Murti, and J. A. McCullers. 2005. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J. Infect. Dis. 192:249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plotkowski, M. C., E. Puchelle, G. Beck, J. Jacquot, and C. Hannoun. 1986. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am. Rev. Respir. Dis. 134:1040-1044. [DOI] [PubMed] [Google Scholar]
- 49.Sayles, P. C., and L. L. Johnson. 1996. Exacerbation of toxoplasmosis in neutrophil-depleted mice. Nat. Immun. 15:249-258. [PubMed] [Google Scholar]
- 50.Schwarzmann, S. W., J. L. Adler, R. J. Sullivan, Jr., and W. M. Marine. 1971. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968-1969. Arch. Intern. Med. 127:1037-1041. [PubMed] [Google Scholar]
- 51.Seki, M., Y. Higashiyama, K. Tomono, K. Yanagihara, H. Ohno, Y. Kaneko, K. Izumikawa, Y. Miyazaki, Y. Hirakata, Y. Mizuta, T. Tashiro, and S. Kohno. 2004. Acute infection with influenza virus enhances susceptibility to fatal pneumonia following Streptococcus pneumoniae infection in mice with chronic pulmonary colonization with Pseudomonas aeruginosa. Clin. Exp. Immunol. 137:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seki, M., K. Yanagihara, Y. Higashiyama, Y. Fukuda, Y. Kaneko, H. Ohno, Y. Miyazaki, Y. Hirakata, K. Tomono, J. Kadota, T. Tashiro, and S. Kohno. 2004. Immunokinetics in severe pneumonia due to influenza virus and bacteria coinfection in mice. Eur. Respir. J. 24:143-149. [DOI] [PubMed] [Google Scholar]
- 53.Simonsen, L. 1999. The global impact of influenza on morbidity and mortality. Vaccine 17(Suppl. 1):S3-S10. [DOI] [PubMed] [Google Scholar]
- 54.Simonsen, L., M. J. Clarke, G. D. Williamson, D. F. Stroup, N. H. Arden, and L. B. Schonberger. 1997. The impact of influenza epidemics on mortality: introducing a severity index. Am. J. Public Health 87:1944-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simonsen, L., K. Fukuda, L. B. Schonberger, and N. J. Cox. 2000. The impact of influenza epidemics on hospitalizations. J. Infect. Dis. 181:831-837. [DOI] [PubMed] [Google Scholar]
- 56.Simonsen, L., L. B. Schonberger, D. F. Stroup, N. H. Arden, and N. J. Cox. 1996. The impact of influenza on mortality in the USA, p. 26-33. In A. W. Hampston and R. G. Webster (ed.), Options for the control of influenza. Elsevier, New York, N.Y.
- 57.Smith, C. B., C. Golden, M. R. Klauber, R. Kanner, and A. Renzetti. 1976. Interactions between viruses and bacteria in patients with chronic bronchitis. J. Infect. Dis. 134:552-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swain, S. D., T. W. Wright, P. M. Degel, F. Gigliotti, and A. G. Harmsen. 2004. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect. Immun. 72:5722-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takase, H., H. Nitanai, E. Yamamura, and T. Otani. 1999. Facilitated expansion of pneumococcal colonization from the nose to the lower respiratory tract in mice preinfected with influenza virus. Microbiol. Immunol. 43:905-907. [DOI] [PubMed] [Google Scholar]
- 60.Tong, H. H., I. Grants, X. Liu, and T. F. DeMaria. 2002. Comparison of alteration of cell surface carbohydrates of the chinchilla tubotympanum and colonial opacity phenotype of Streptococcus pneumoniae during experimental pneumococcal otitis media with or without an antecedent influenza A virus infection. Infect. Immun. 70:4292-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tong, H. H., M. A. McIver, L. M. Fisher, and T. F. DeMaria. 1999. Effect of lacto-N-neotetraose, asialoganglioside-GM1 and neuraminidase on adherence of otitis media-associated serotypes of Streptococcus pneumoniae to chinchilla tracheal epithelium. Microb. Pathog. 26:111-119. [DOI] [PubMed] [Google Scholar]
- 62.Tumpey, T. M., S. H. Chen, J. E. Oakes, and R. N. Lausch. 1996. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J. Virol. 70:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Sluijs, K. F., L. J. van Elden, M. Nijhuis, R. Schuurman, J. M. Pater, S. Florquin, M. Goldman, H. M. Jansen, R. Lutter, and T. van der Poll. 2004. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J. Immunol. 172:7603-7609. [DOI] [PubMed] [Google Scholar]
- 64.Voyich, J. M., and F. R. DeLeo. 2002. Host-pathogen interactions: leukocyte phagocytosis and associated sequelae. Methods Cell Sci. 24:79-90. [DOI] [PubMed] [Google Scholar]
- 65.Warshauer, D., E. Goldstein, T. Akers, W. Lippert, and M. Kim. 1977. Effect of influenza viral infection on the ingestion and killing of bacteria by alveolar macrophages. Am. Rev. Respir. Dis. 115:269-277. [DOI] [PubMed] [Google Scholar]
- 66.Wheeler, J. G., L. S. Winkler, M. Seeds, D. Bass, and J. S. Abramson. 1990. Influenza A virus alters structural and biochemical functions of the neutrophil cytoskeleton. J. Leukoc. Biol. 47:332-343. [DOI] [PubMed] [Google Scholar]
- 67.Wiley, J. A., R. J. Hogan, D. L. Woodland, and A. G. Harmsen. 2001. Antigen-specific CD8(+) T cells persist in the upper respiratory tract following influenza virus infection. J. Immunol. 167:3293-3299. [DOI] [PubMed] [Google Scholar]
- 68.Zhang, W. J., S. Sarawar, P. Nguyen, K. Daly, J. E. Rehg, P. C. Doherty, D. L. Woodland, and M. A. Blackman. 1996. Lethal synergism between influenza infection and staphylococcal enterotoxin B in mice. J. Immunol. 157:5049-5060. [PubMed] [Google Scholar]