Abstract
The identification of novel markers and therapeutic targets in advanced cancer is critical for improving diagnosis and therapy. Six-transmembrane epithelial antigen of the prostate (STEAP) is expressed predominantly in human prostate tissue and in other common malignancies including prostate, bladder, colon, and ovarian carcinomas, and in Ewing's sarcoma, suggesting that it could function as an almost universal tumor antigen. We have used MHC peptide binding algorithms to predict potential STEAP sequences capable of stimulating in vitro naïve HLA-A2–restricted CTLs. Four of six peptides predicted by these algorithms were able to induce antigen-specific CTLs that killed peptide-pulsed HLA-A2 target cells. Two of these peptides, STEAP-292 (MIAVFLPIV) and a modification of this peptide STEAP-292.2L (MLAVFLPIV), were the most efficient in the induction of primary CTL responses. More importantly, these CTLs were able to respond to tumor cells that express HLA-A2 and STEAP (colon, bladder, prostate, Ewing's sarcoma, and melanoma). Our results provide strong evidence that STEAP-292 is naturally processed by many tumor types and is presented in the context of HLA-A2 in sufficient amounts to allow recognition by CTLs. Also because STEAP-292.2L is a more immunogenic peptide able to induce CTL recognition of these STEAP-containing tumors and may have potential as an antitumor peptide vaccine.
As knowledge about the immune response has evolved, expectations have been raised that immunotherapy for cancer may now be feasible (1, 2). Tumor rejection via immunotherapy is primarily mediated by T-lymphocytes recognizing unique tumor-associated antigens (TAA) resulting in antitumor responses. T-lymphocytes recognize these tumor antigens as small peptides bound to cell surface molecules encoded by the MHC (2). CTLs are characterized by expression of CD8 cell-surface molecules and recognize peptides bound to MHC class I molecules.
T-cell–based immunotherapy has been seriously considered as a promising novel noninvasive treatment option for cancer that could be used to treat minimal residual disease, to prevent metastatic spread, or to delay recurrences without compromising quality of life. However, the existence of appropriate tumor-associated antigen capable of initiating effective antitumor T-cell responses remains one of the major obstacles for developing effective immunotherapies. For many tumor types such as melanoma and ovarian, breast, and colorectal adenocarcinomas, there is clear evidence that peptide epitopes derived from conventional tumor markers (e.g., gp100, carcinoembryonic antigen, and HER2/neu) can be effectively recognized by tumor-reactive CTLs in the context of MHC class I molecules (3). In addition, the in vitro induction of naïve T-lymphocytes to tumor peptide antigens resulting in antitumor activity has been described (4-6). The antigenic peptide epitopes identified in these studies are usually selected by computer algorithms that predict the capacity of peptide sequences to bind to specific MHC alleles (7-9).
Utilizing microarray analysis, a gene encoding a serpentine transmembrane protein, named six-transmembrane epithelial antigen of the prostate (STEAP), was recently identified (10, 11). STEAP is expressed predominantly in human prostate tissue and also multiple cancers including prostate, bladder, colon, ovarian, and Ewing sarcoma. Its high levels in prostate cancer and other tumors, its cell surface location, and its lack of expression on normal tissue, except for prostate and very low levels in bladder, suggest that STEAP may be an ideal target for tumor immunotherapy.
This report presents the first identification of antigenic peptide epitopes within the STEAP protein. Our results provide strong evidence that STEAP-292 (peptide sequence MIAVFLPIV) is naturally processed by various tumor cell lines from different tumor types (colon, prostate, melanoma, and Ewing's sarcoma) and is presented in the context of HLA-A2 in sufficient amounts to allow recognition by CTLs. The results also indicate that a modification of STEAP-292, by the replacement of I at position 2 to L in the second amino acid position to create STEAP-292.2L (peptide MLAVFLPIV), was more effective at inducing naïve CTLs to recognize STEAP-expressing cancers. This antigenic peptide may therefore be useful to induce an effective in vivo T-cell–mediated antitumor response for patients with a variety of STEAP-expressing tumors.
Materials and Methods
Peptides
We used a combination of MHC binding algorithms to evaluate the STEAP protein for potential antigenic epitopes (8, 12). The amino acid sequence of STEAP was analyzed for the existence of nine-amino-acid peptides predicted to bind to HLA-A2. Peptides identified as potential antigens were then synthesized according to standard solid-phase synthesis methods using Applied Biosystems apparatus and purified by high-performance liquid chromatography. The purity (>95%) and identity of peptides were determined by analytic high-performance liquid chromatography and mass spectrometry analysis. Peptides were dissolved at 10 mg/mL in DMSO containing 0.1% trifluoroacetyl or trifluoroacetic acid and were aliquoted in small volumes to be maintained frozen at −20°C until further use.
Cell lines
The T2 (ATCC CRL-1992) B-lymphoblast;T-lymphoblast hybrid cell line is a variant of the T1 (ATCC CRL-1991) cell line. The cells do not express HLA DR and are MHC class II antigen negative; however, they do express HLA-A2. This makes the T2 cell line useful for studying antigen processing and T-cell recognition of MHC class I antigens. The HLA-A2 tumor cell lines used in this study were TTC1105 (alveolar rhabdomyosarcoma); UM-UC3 (transitional cell carcinoma bladder); COLO205 and CACO2 (colorectal adenocarcinoma); J82 (transitional cell carcinoma bladder, STEAP negative); A673 and TC32 (Ewing's sarcoma); and HLA-A2–transfected PC-3, LAPC4, and DU145 (prostate tumors). STEAP expression was determined by PCR analysis or from previous description (10). Cell lines were kept in RPMI 1640 supplemented with 10% fetal bovine serum (v/v), l-glutamine, nonessential amino acids, sodium pyruvate, and gentamicin (complete RPMI medium). All of the culture materials were purchased from Life Technologies, Inc. (Rockville, MD). To increase the level of MHC class I expression, tumor cell lines (except for T2) were treated with 5,000 units/mL IFN-γ for 48 hours before assays.
Generation of dendritic cells and peptide pulsing
Dendritic cells were generated using peripheral blood mononuclear cells from normal volunteers that had been purified using Ficoll gradient centrifugation as previously described (12). The Institutional Review Board on Human Subjects (Mayo Foundation) approved this research, and informed consent for blood donation was obtained from all volunteers. Monocytes were isolated and incubated with Ex vivo medium containing 280 units/mL of granulocyte macrophage colony-stimulating factor and 50 ng/mL of interleukin (IL)-4. On days 3 and 5, additional media plus IL-4 and granulocyte macrophage colony-stimulating factor was added. The dendritic cells were then ready for CTL induction on day 7. The tissue culture-generated dendritic cells (1 × 106-2 × 106 cells/mL) were pulsed with 10 μg/mL of peptide and 3 μg/mL β2-microglobulin at room temperature for 4 hours and then irradiated (4,200 rad). The dendritic cells were seeded to a 48-well plate at 2.5 × 104/well (1 × 105/mL) in 0.25 mL RPMI 5% horse serum containing 10 ng/mL IL-7.
Induction of antigen-specific T-cell responses
The methodology of T-cell induction and functional assays using in vitro methods have been well established in this lab (12, 13). In brief, CD8+ T-cells are purified using negative selection with MACS immunomagnetic beads. The CTL immunization cultures were done in 48-well plates, where each well contained 0.25 × 105 dendritic cells and 5 × 105 CD8+ T-cells in 0.5 mL RPMI 5% horse serum containing 10 ng/mL IL-7. The day after CTL induction, 0.5 mL of RPMI 5% horse serum containing 10 ng/mL IL-10 was added to the wells. On days 7 and 14, the T-cell cultures were individually restimulated with peptide-pulsed irradiated autologous antigen-presenting cells (adherent monocytes) as described, adding IL-10 on the following day. Starting on day 9, the T-cell cultures were fed with fresh medium containing 10 units/mL IL-2 every 2 to 3 days. The first screening cytotoxicity assay was done after three rounds of peptide stimulation.
Culture medium for all procedures consisted of RPMI 1640 supplemented with 5% human male AB serum, 0.1 mmol/L MEM, nonessential amino acids, 1 mmol/L sodium pyruvate, 2 mmol/L l-glutamine, and 50 μg/mL gentamicin.
Evaluation of CTL activity by 51Cr-release assays
The cytotoxic activity of the CTL cultures was determined by 51Cr-release assay (6). Peptide-pulsed (5 μg/mL) T2 (HLA-A2 positive) target cells and STEAP-containing tumor cells that express HLA-A2 were labeled with 300 μCi of [51Cr]sodium chromate (Amersham Pharmacia Biotech, Piscataway, NJ) for 2 hours at 37°C in a water bath. The target cells are then mixed with the effector cells (CTL lines and clones) at ratios of 1:3 up to 1:100 in a final volume of 0.2 mL. After 4 hours of incubation at 37°C, supernatant was collected from each well and percentage of specific lysis was determined by the release of 51Cr from the target cells and measured using a γ-counter. Percent specific lysis is calculated as follows: (cpmtest sample − cpmspontaneous release) / (cpmmaximal release − cpmspontaneous release) × 100. Dose-response curves were done to determine the overall activity of the T-lymphocytes. CTLs showing high lytic activity were then tested in a 51Cr-release cold target inhibition test to determine specificity of activity (6).
Evaluation of T-lymphocyte activity by IFN-γ production
The production of IFN-γ was evaluated to show activity of CTL clones and cell lines against target cells (14). The concentration of IFN-γ secreted into the culture media by the T-lymphocytes was measured using a standard human IFN-γ ELISA kit (BD PharMingen, San Diego, CA). IFN-γ production by CTL lines or clones in response to target cells was also evaluated using ELISPOT analysis. ELISPOT plates (Millipore, Billerica, MA) were coated with IFN-γ capture antibody (MabTech USA, Mariemont, OH) and incubated overnight. The culture plates were then washed after 24 hours with PBS containing 0.05% Tween 20. A biotinylated secondary antibody for IFN-γ (MabTech USA) was added and the plates were incubated for 2 hours, followed by another wash cycle. Next, streptavidin-horseradish peroxidase (BD PharMingen) was added and the plates were incubated for 1 hour. For the final wash, plates were first washed with PBS containing 0.05% Tween 20, followed by washing with PBS. Plates were developed using a 3-amino-9-ethyl-carbazole substrate (Sigma-Aldrich, St. Louis, MO) and the reaction was stopped with water.
Semiquantitative reverse transcription-PCR to detect STEAP mRNA expression
Total RNA was extracted from the indicated cell lines (Qiagen, Valencia, CA) and quantified with a spectrophotometer (Beckman, Fullerton, CA). A total of 3 μg total RNA was reverse transcribed using M-MLV Reverse Transcriptase (Invitrogen Corporation, Carlsbad, CA). A portion of the cDNA was amplified by PCR using 94°C denaturation, 59°C annealing, and 72°C extension temperatures for STEAP, and 94°C denaturation, 55°C annealing, and 72°C extension temperatures for β-actin, for a total of 35 cycles. Positive and negative strand primers used for amplification were as follows: STEAP, CTTCAGAACTTCAGCACACACAGG and CGCCTCATTGGGTAAGACAGAC; and β-actin, CCAAGGCCAACCGCGAGAAGATGAC and AGGGTACATGGTGGTGCCGCCAGAC, respectively. Amplified products were visualized under UV illumination following electrophoresis on ethidium bromide–stained agarose gels. Amplification of the appropriate gene fragments was assured by comparison with molecular weight markers run on the same gel.
Results
Selection of potential STEAP CTL epitopes
Because T-lymphocytes are only able to recognize peptide antigens presented by specific MHC class I molecules, we first evaluated the sequence of the STEAP protein for the presence of peptides containing motifs predicted to bind to the HLA-A2 allele. This allele was chosen because it is one of the most common in the general population (∼40% of the population determined by the National Marrow Donor Registry). The highest ranking sequences (STEAP-86, STEAP-165, STEAP-262, and STEAP-292), scoring high to both MHC/peptide binding algorithms, were selected for further evaluation (Table 1). An approach of modifying the MHC binding primary anchors to optimal binding residues has been used to increase MHC binding while still maintaining recognition of the native peptide sequence in tumor cells (15, 16). Therefore, the potential CTL epitope STEAP-292 was modified to STEAP-292.2L, where the binding anchor at the second amino acid position was exchanged from I to L to potentially improve its binding to HLA-A2 (Table 1). With this modification, both the BIMAS and SYFPEITHI algorithm binding scores for STEAP-292.2L dramatically improved. Thus, the results from the MHC/peptide binding algorithms suggest there were several potential CTL epitopes contained within the STEAP protein.
Table 1.
Effectiveness of the STEAP peptide epitopes at induction of naïve CD8+ into peptide-reactive CTLs
| Tumor-associated antigen | Start position | Sequence | BIMAS | SYFPEITHI | Positive wells at induction (% of total wells tested) |
|---|---|---|---|---|---|
| STEAP | 86 | FLYTLLREV | 471 | 29 | 5 (5%) |
| 165 | GLLSFFFAV | 10,776 | 25 | 1 (0.7%) | |
| 262 | LLLGTIHAL | 309 | 32 | 0 | |
| 292 | MIAVFLPIV | 58 | 26 | 14 (10%) | |
| 292.2L | MLAVFLPIV | 424 | 28 | 80 (24%) | |
| PSMA | 27 | VLAGGFFLL | 400 | 27 | NT |
NOTE: The table shows the starting amino acid position for the peptide epitope, the sequence of the epitope, the scores from the 2 MHC binding algorithms, and the number of wells positive after induction. PSMA-27 is shown as a known antigenic HLA-A2 – restricted epitope that was not tested in these experiments (14). A positive well was defined as a well with greater than 10% specific lysis of peptide STEAP-292.2L – loaded T2 cells at an effector/target ratio of approximately 30:1. The predictive algorithm, BIMAS, ranks potential MHC binders according to the half time disassociation of peptide/MHC complexes. The second algorithm, SYFPEITHI, ranks the peptides according to a score that takes into account the presence of primary and secondary MHC-binding anchor residues.
Abbreviations: PSMA, prostate-specific membrane antigen; NT, not tested.
CTL induction to the HLA-A2 peptide epitopes
Synthetic peptides corresponding to the potential STEAP CTL epitopes shown in Table 1 were prepared and used to elicit primary in vitro CTL responses from HLA-A2 naïve normal donors. Each peptide was tested at least twice with different donors. Four of the peptides were able to induce CTL responses against peptide-pulsed T2 target cells (Table 1). The most effective peptide was STEAP-292.2L, which was able to induce a large percentage of CTLs with cytolytic activity against peptide-loaded target cells. To directly compare STEAP-292 with the modified STEAP-292.2L, parallel inductions were done using two separate donors. STEAP-292 in both inductions had fewer positive wells than STEAP-292.2L (14/192 versus 33/192 wells, respectively).
Positive CTL microcultures were expanded for further analysis. However, on further restimulation and expansion, only STEAP-292 and STEAP-292.2L were able to maintain peptide specificity. Of these, the modified STEAP-292.2L–induced CTLs were more likely to maintain specificity and proliferate well. This is shown by the observation that the percentage of STEAP-292–induced CTL lines that maintained peptide specificity decreased from 30% at induction to 16% after expansion.
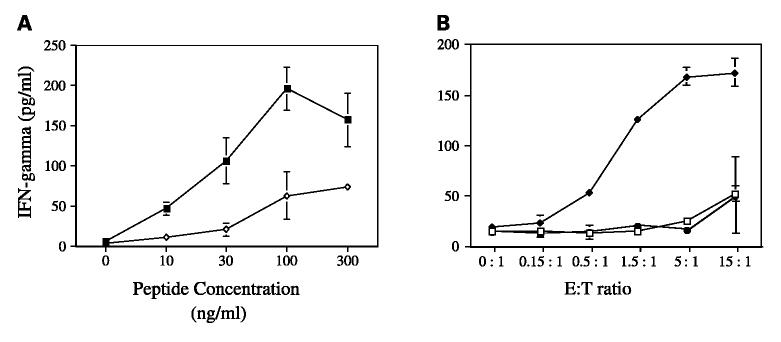
The efficacy and affinity of representative CTL lines and clones to recognize the STEAP peptide were evaluated by performing peptide dose-response titration curves utilizing IFN-γ production as the indicator for CTL recognition and activity. CTLs had a relatively high avidity for their peptide ligands because half maximal production of IFN-γ could be achieved at peptide concentrations as low as 10 to 30 ng/mL (Fig. 1A). CTLs that had been induced with STEAP-292.2L were able to recognize both the modified STEAP-292.2L and, to a lesser extent, the unmodified STEAP-292 (Fig. 1A). This finding of increased activity towards the modified STEAP-292.2L peptide would be consistent with improved MHC binding as predicted by the algorithm scores for the modified peptide. In addition, CTLs did not recognize targets pulsed with an irrelevant HLA-A2 binding peptide, thus demonstrating antigen specificity (Fig. 1B). Similar results were obtained for CTLs induced with the STEAP-292 peptide (data not shown).
Fig. 1.
Peptide STEAP-292.2L – induced CTL response to peptide-pulsed targets. A, CTLs were tested for their capacity to recognize T2 target cells in the presence of various concentrations of peptide. T2 target cells were incubated with multiple concentrations of STEAP-292.2L (■)or STEAP-292(◇) for 2 hours at 37°C, after which CTLs were added to the target cells at an effector/target (E/T) ratio of 5:1. IFN-γ production was determined after incubation for 18 hours at 37°C by ELISA. B, CTL specificity to the STEAP-292.2L peptide was evaluated. T2 cells were incubated with either no peptide (□), STEAP-292.2L (◆), or an irrelevant HLA-A2–restricted peptide (QLMAFNHLV) from rhabdomyosarcoma (●) for 2 hours at 37°C. The peptide concentration was kept constant (5 μg/mL) and the effector/target ratio was varied. IFN-γ production after 6 hours of incubation at 37°C was evaluated by ELISA. These graphs are from a single CTL line that is representative of experiments from three CTL lines.
These initial results indicated that several of the selected STEAP peptides were immunogenic and able to induce a CTL response to peptide-loaded target cells. This response was both specific and sensitive. Because the CTLs induced by STEAP-292.2L were able to recognize, to some extent, the native STEAP-292, this indicates that these CTLs may be able to recognize and respond to STEAP-expressing tumor cells if STEAP-292 is naturally processed and presented.
Antitumor reactivity of STEAP-specific CTLs
The STEAP peptide–reactive CTLs were then tested for their ability to recognize tumor cells that naturally express and process STEAP. The expression of both HLA-A2 molecules and STEAP will dictate whether the CTLs will be able to interact with the tumor cells. This interaction is antigen dependent and would show that the STEAP-292 peptide epitope is naturally processed and expressed by the tumor cells. Previous work has described several cell lines from prostate, colon, bladder, ovarian, and Ewing sarcoma tumors that express STEAP (10). To determine if other cancer types express STEAP, PCR analysis was done on several other classes of tumor cell lines. STEAP was identified in all three melanoma cell lines, two Ewing sarcoma cell lines, and one of two embryonal rhabdomyosarcoma lines, but was not present in the alveolar rhabdomyosarcoma line tested (Fig. 2A). The identification of STEAP in melanoma and embryonal rhabdomyosarcoma has not been described. This further indicates the generalized nature of STEAP expression across multiple types of cancers, making STEAP an even more promising vaccine candidate.
Fig. 2.
Description of tumor target cells. A, semiquantitative reverse transcription-PCR to detect STEAP mRNA expression in rhabdomyosarcoma, Ewing's sarcoma, and melanoma tumor cell lines.Total RNA was extracted from the indicated cell lines. A total of 1 μg total RNA was reverse transcribed. A portion of the cDNA was amplified by PCR for STEAP and β-actin for a total of 35 cycles. Amplified products were visualized under UV illumination following electrophoresis on ethidium bromide – stained agarose gels. Amplification of the appropriate gene fragments was assured by comparison with molecular weight markers run on the same gel. B, expression of surface HLA-A2 on tumor cells to be used as targets for CTL assays.The tumor lines were analyzed after 48 hours of incubation with IFN-γ (5,000 IU/mL) by flow cytometry for their levels of cell-surface HLA-A2 using FITC-labeled anti – HLA-A2 monoclonal antibody (BB7.2). Shaded area, isotype control; solid line, HLA-A2 staining, except for PC3 where the shaded area represents HLA-A2 staining for PC3 and the solid line represents transfected PC3-A2.
Subsequently, tumors that expressed STEAP were evaluated by flow cytometric analysis for cell-surface HLA-A2 using an anti–HLA-A2 specific monoclonal antibody after pretreatment of tumor cells with IFN-γ to increase MHC expression (5,000 IU/mL for 48 hours). As clearly shown in Fig. 2B, multiple STEAP-expressing tumors displayed good levels of HLA-A2. Three prostate tumor cell lines (PC3, DU145, and LAPC4), which do not normally express HLA-A2 but have good levels of STEAP, were transfected with HLA-A2 (designated PC3-A2, DU145-A2, and LAPC4-A2). Flow data showed good HLA-A2 expression for the transfected cells but not the native (untransfected) cells. The data also supported J82 as an appropriate negative control for these assays because these HLA-A2–positive tumor cells do not express STEAP (10).
By the completion of these preliminary studies, multiple tumor cell lines had been identified that expressed both HLA-A2 and STEAP. Standard> 51Cr-release assays were done to determine if STEAP-292.2L–induced CTLs recognized STEAP-expressing tumor cells. These CTLs were quite effective in lysing the STEAP tumor cells in a dose-dependent manner. Cytotoxicity was shown against multiple tumor lines that contain the STEAP protein including CACO2, COLO205, DU145-A2, TC32, A673, Mel526, and G361 (Fig. 3). The cytotoxicity was specific because J82 cells (STEAP-negative bladder cancer) and Rh36 (STEAP-negative embryonal rhabdomyosarcoma) were not recognized. In addition, Du145 tumor cells that did not express HLA-A2 were not recognized, thus underscoring the HLA-A2 restriction of CTL activity (data not shown). The CTLs induced by the native peptide STEAP-292 were also able to kill tumor target cells (Fig. 4). These results illustrate the ability of these STEAP peptide–induced CTLs to recognize and lyse STEAP-expressing tumor cells.
Fig. 3.
Antitumor activity of STEAP-292.2L peptide–induced CTL lines and clones. A, peptide-reactive CTL lines were tested for their capability to recognize and lyse HLA-A2–expressing STEAP-containing tumor cells. Representative graphs from two different donors (1 and 2). Several tumor lines were evaluated including COLO205 (●), CACO2 (◇), DU145-A2 (▲), and, as a negative control, J82 (○). In addition,T2 cells with no peptide (■) and with STEAP-292.2L (□) were evaluated as negative and positive controls, respectively. Antitumor cytolytic activity was measured using a 4-hour 51Cr-release cytolysis assay at various effector/target ratios. For clarity, the error bars were deleted, however, the SDs were less than 10% of the mean. B, cytolysis of newly identified STEAP-expressing tumor cell lines by STEAP-292.2L – induced CTLs. Representative graph from experiments done using CTLs from two different donors. Several tumor lines were evaluated including the embryonal rhabdomyosarcoma Rh36 (■), the Ewing sarcomas TC32 (○)and A673 (▲), the melanomas G361 (●)and Mel526 (◆), and, as a negative control, J82 (△). Antitumor cytolytic activity was measured using a 4-hour 51Cr-release cytolysis assay at various effector/target ratios. For clarity the error bars were deleted; however, the SDs were less than 10% of the mean.
Fig. 4.

Antitumor reactivity of STEAP-292 peptide–induced CTL lines. Tumor lines were evaluated including COLO205 (●) and, as a negative control, J82 (○). In addition, T2 cells with no peptide (■)and with STEAP-292(□) were evaluated as negative and positive controls, respectively. Antitumor cytolytic activity was measured using a 4-hour 51Cr-release cytolysis assay at various effector/target ratios.
Another function of CTLs is their ability to secrete lymphokines on antigen stimulation. IFN-γ production was evaluated by both ELISA and ELISPOT assays. The STEAP-292.2L–specific CTLs secreted significant amounts of IFN-γ when challenged with peptide-pulsed T2 cells or with STEAP-containing tumor cells (Fig. 5). On the other hand, the production of IFN-γ by CTLs was significantly reduced when peptide-negative T2 cells, J82 and Rh36 tumor cells, were used as target cells.
Fig. 5.
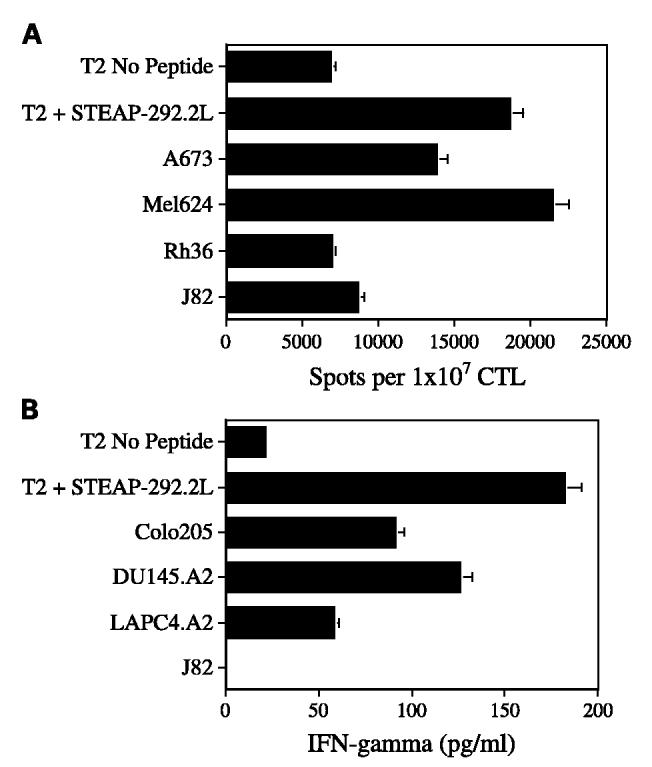
Antigen-induced production of IFN-γ by the STEAP-292.2L – induced CTLs. These CTL lines secreted IFN-γ as a result of recognizing antigen in either peptide-pulsed T2 targets or STEAP-containing tumor cells. A, the frequency of IFN-γ secreting cells is expressed as the detected spot number per 107 CTLs. Mean of duplicate wells that contain a 1:1 ratio of target cells to STEAP-292.2L – induced CTLs. The following target cells were evaluated for IFN-γ production:T2 cells loaded with STEAP-292.2L, T2 cells with no peptide, and the STEAP-positive tumor cells from Ewing sarcoma A673 and melanoma 624, with embryonal rhabdomyosarcoma Rh36 and J82 as negative controls. B, IFN-γ production against a broader range of tumor lines was evaluated. Representative graph from a CTL line for which the conditions of the experiment were an effector/target ratio of 5:1 and 12 hours of incubation at 37°C. The concentration of IFN-γ secretion was measured using an ELISA kit. Both graphs are representative of experiments repeated using CTL lines and clones from two donors.
Specificity of the CTL cytolysis was confirmed using a cold target inhibition assay. In this assay, unlabeled T2 cells pulsed with peptide were included in the cytolysis assay wells at a 15-fold excess above the number of labeled target tumor cells. The CTLs should therefore react and lyse the “cold” peptide–pulsed T2 cells and diminish the amount of 51Cr released from the “hot” tumor targets. The amount of 51Cr released from COLO205 cells was significantly inhibited with the addition of the peptide-pulsed T2 cells but not by unpulsed T2 cells (Fig. 6A). Similar results were obtained with STEAP-292–induced CTLs (data not shown). Cold target inhibition of STEAP-292.2L–induced CTL cytolysis was also observed using the Ewing sarcoma cell lines TC32 and A673, but not with the negative control J82 as targets (data not shown). The results of these experiments, combined with the lack of CTL reactivity to Du145 (STEAP positive, HLA-A2 negative), indicate that the CTLs respond to the tumor cells on the basis of their STEAP-292 peptide presentation by MHC class I molecules.
Fig. 6.
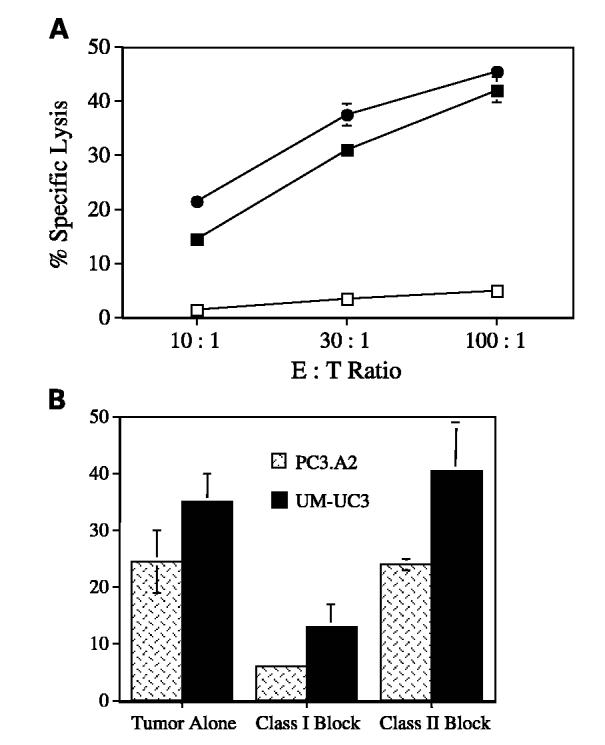
Specificity of CTL recognition and response to tumor targets. A, CTL specificity to antigen as determined by a cold target inhibition assay. In this assay, 51Cr-loaded hot target COLO205 (●) cells, alone or mixed with a 15-fold greater number of cold T2 cells, were loaded with peptide STEAP-292.2L (□) or no peptide (■). CTLs were then added and incubated at 37°C. A standard 4-hour 51Cr-release cytolysis assay at various effector/target ratios was then done. Representative graph from two different donors. B, tumor-reactive CTLs induced by peptide STEAP-292.2L are restricted to MHC class I molecules. Monoclonal antibodies specific for MHC class I (W6/32), but not antibodies reactive with MHC class II molecules (9.3F10), inhibited the lysis of the prostate cancer cell line PC3-A2 (hatched bar) and bladder cancer cell line UM-UC3 (solid bar). Both antibodies were tested at 10 μg/mL, and a standard 4-hour 51Cr-release cytolysis assay was done at an effector/target ratio of 30:1. Both graphs are representative of experiments repeated using CTL lines and clones from two donors.
Antibody blocking experiments were used to confirm that the CTL recognition of the STEAP-containing tumor cells was MHC class I restricted. Thus, we tested the capacity of anti–MHC class I and class II monoclonal antibodies to inhibit the cytolytic activity of CTLs. The cytotoxicity of STEAP tumor cells by CTLs was significantly decreased by the anti–MHC class I but not by the anti–MHC class II antibodies (Fig. 6B). These results show that the CTL epitope represented by peptide STEAP-292 is processed and presented by MHC class I molecules on tumor cells.
Discussion
The purpose of this study was to evaluate STEAP for possible antigenic peptide epitopes that could be used for an anticancer peptide vaccine. Ideal targets for the immuno-therapy of cancer should be antigens that are exclusively expressed in nonvital tissues, highly expressed in metastatic disease, and accessible to therapeutic modalities. The identification of such peptide epitopes capable of triggering antitumor T-cell responses is important for the development of vaccines against cancer. Although significant advances have been made in this area for tumor types such as melanoma, colon, breast, ovarian, and cervical carcinomas, progress for other cancers has been more modest. For the present study, we selected STEAP as a potential source of T-cell epitopes for HLA-A2–restricted CTLs.
STEAP was chosen for further study because of its strong expression in multiple cancer types, restricted expression in normal tissues, and cell surface localization. STEAP shows no homology to any other known proteins, but biochemical analysis and secondary structure prediction suggest that it is a cell-surface molecule with six transmembrane domains (10, 11). It seems possible that STEAP may function as an ion channel in tight junctions, in gap junctions, or in cell adhesion. STEAP meets the criteria for a good immunotherapeutic target, as it is strongly expressed in many cancers such as prostate, colon, pancreas, bladder, Ewing's sarcoma, and ovarian (10). Its expression in normal tissues is restricted to the prostate, a dispensable organ, and the bladder, a tissue with high regenerative capacity. In addition to these previously described malignancies that express STEAP, two other major categories of cancer were identified as STEAP positive. The first was rhabdomyosarcoma, which is the most common sarcoma and the fourth most common solid tumor during childhood and adolescence (17). There are two main types of rhabdomyosarcoma: embryonal (60% of all sarcomas) and alveolar (20%). The other newly identified STEAP-expressing tumor was melanoma. This is one of the most aggressive tumors in the world and its incidence is dramatically increasing (18). In addition, the death rate for melanoma has doubled in the last 35 years. These statistics for melanoma have been the driving force for the identification of antigenic peptide epitopes derived from melanoma-specific tumor-associated antigen (19). Some of these epitopes have shown promise as melanoma vaccines; however, with STEAP we have identified a tumor-associated antigen that is active not only against melanoma but also against several other tumors.
Our lab has previously had good results using MHC binding algorithms for the prediction of peptide antigens for several different types of tumors (5, 12, 14, 20, 21). Following the same strategy that has allowed us to identify T-cell epitopes for other tumor markers, we selected four potential CTL epitopes from the STEAP sequence using a combination of algorithm analysis that predicts immunogenic MHC-binding peptides. Induction of CD8+ cells with peptide STEAP-262 did not elicit any responsive CTLs, whereas peptides STEAP-86 and STEAP-165 were initially successful at eliciting a CTL response; however, this response was not sustainable. All three of these peptides scored very well in the MHC binding algorithms and therefore had the potential to be good antigenic epitopes. It has been hypothesized that thymocytes which recognize self-peptides with high or low affinities for MHC class I antigens expressed by the thymic stromal cells are negatively selected, whereas peptides with moderate affinities for MHC class I antigen complexes are positively selected and passed on to the periphery (22, 23). This would explain why CTLs reactive towards self-peptides are most effective against peptides with moderate binding affinities for the MHC class I antigens (24). Another possibility is that because STEAP-86, -262, and -165 scored so highly in the algorithms, and therefore should have been the most immunogenic antigens, these peptides induce CTL apoptosis through activation-induced cell death due to the repeated antigen stimulation that occurs during in vitro CTL induction (25-27).
Our results indicate that the other peptides, STEAP-292 and STEAP-292.2L, were capable of inducing naϊve CD8+ precursors into CTLs that recognized peptide-loaded target cells. CTLs induced with the modified STEAP-292.2L showed better CTL induction rates than the native STEAP-292. This may be due to improved HLA-A2 binding resulting from the change of an amino acid at the HLA-A2 binding anchor site. This strategy of modifying the peptide to improve immunogenicity has been shown to be effective in other peptide vaccine studies (15, 16, 28). It is important to note that the modification of STEAP-292 to STEAP-292.2L did not adversely affect the ability of the STEAP-292.2L – induced CTLs to recognize the native STEAP-292 in either peptide-pulsed target cells or tumor cells.
The peptide dose-response titration experiments, which indirectly reflect the affinity of the CTLs for their antigenic peptide epitope, illustrate that the STEAP-292 and STEAP-292.2L peptides both have good affinity because half of maximal IFN-γ production was achieved at 30 ng/mL of peptide. This level of peptide affinity should allow CTLs to recognize tumors that express relatively low levels of antigen (29).
STEAP-292.2L–induced CTLs recognized not only peptide-loaded target cells but also multiple STEAP-expressing tumor cells including prostate, colon, bladder, Ewing's sarcoma, melanoma, and embryonal rhabdomyosarcoma. This STEAP tumor cell recognition was HLA class I dependent and resulted in both CTL-mediated tumor cytolysis and secretion of IFN-γ. Because STEAP is present in such a variety of tumors, this offers the possibility that a single peptide vaccine could be effective against multiple cancer types. Given the better CTL induction rates with STEAP-292.2L compared with STEAP-292, and the effective tumor recognition of these CTLs, we propose that STEAP-292.2L is the better antigen to be pursued for further vaccine therapy experiments.
This report presents the first identification of antigenic peptide epitopes within the STEAP protein. Our results provide strong evidence that STEAP-292 is naturally processed by many tumor types (colon, prostate, bladder, melanoma, and sarcoma) and is presented in the context of HLA-A2 in sufficient amounts to allow recognition by CTLs. The present results also indicate that the CTL epitope represented by peptide STEAP-292.2L should be considered for developing epitope-based T-cell–based immunotherapy for HLA-A2 cancer patients with STEAP-containing cancers.
Footnotes
Grant support: NIH grants P50CA91956 and R01CA80782.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Note: E. Celis is currently at Louisiana State University Health Sciences Center, New Orleans, LA.
References
- 1.Rosenberg S. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll DM. Therapeutic vaccination for cancer. Clin Immunol. 2000;95:S44–62. doi: 10.1006/clim.1999.4819. [DOI] [PubMed] [Google Scholar]
- 3.Renkvist N, Castelli C, Robbins P, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50:3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celis E, Tsai V, Crimi C, et al. Induction of antitumor cytotoxic t lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc Natl Acad Sci U S A. 1994;91:2105–9. doi: 10.1073/pnas.91.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi H, Wood M, Song Y, Appella E, Celis E. Defining promiscuous MHC class II helper T-cell epitopes for the HER2/Neu tumor antigen. Cancer Res. 2000;60:5228–36. [PubMed] [Google Scholar]
- 6.Lu J, Wettstein PJ, Higashimoto Y, Appella E, Celis E. TAP-independent presentation of CTL epitopes by Trojan antigens. J Immunol. 2001;166:7063–71. doi: 10.4049/jimmunol.166.12.7063. [DOI] [PubMed] [Google Scholar]
- 7.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side chains. J Immunol. 1994;152:163–75. [PubMed] [Google Scholar]
- 8.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stenanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–9. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 9.Ruppert J, Sidney J, Celis E, Kubo R, Grey H, Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993;74:929–37. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 10.Hubert RS, Vivanco I, Chen E, et al. STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci U S A. 1999;96:14523–8. doi: 10.1073/pnas.96.25.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang D, Holt GE, Velders MP, Kwon ED, Kast WM. Murine six-transmembrane epithelial antigen of the prostate, prostate stem cell antigen, and prostate-specific membrane antigen: prostate-specific cell-surface antigens highly expressed in prostate cancer of transgenic adenocarcinoma mouse prostate mice. Cancer Res. 2001;61:5857–60. [PubMed] [Google Scholar]
- 12.Lu J, Celis E. Use of two predictive algorithms of the world wide web for the identification of tumor-reactive T-cell epitopes. Cancer Res. 2000;60:5223–7. [PubMed] [Google Scholar]
- 13.Tsai V, Kawashima I, Keogh E, Daly K, Sette A, Celis E. In vitro immunization and expansion of antigen-specific cytotoxic T lymphocytes for adoptive immunotherapy using peptide-pulsed dendritic cells. Crit Rev Immunol. 1998;18:65–75. doi: 10.1615/critrevimmunol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Celis E. Recognition of prostate tumor cells by cytotoxic T lymphocytes specific for prostate-specific membrane antigen. Cancer Res. 2002;62:5807. [PubMed] [Google Scholar]
- 15.Smith JW, II, Walker EB, Fox BA, et al. Adjuvant immunization of HLA-A2-positive melanoma patients with a modified gp100 peptide induces peptide-specific CD8+ T-cell responses. J Clin Oncol. 2003;21:1562–73. doi: 10.1200/JCO.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Huarte E, Sarobe P, Lu J, et al. Enhancing immunogenicity of a CTL epitope from carcinoembryonic antigen by selective amino acid replacements. Clin Cancer Res. 2002;8:2336–44. [PubMed] [Google Scholar]
- 17.Arndt CAS, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–52. doi: 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- 18.Elwood JM, Koh HK. Etiology, epidemiology, risk factors, and public health issues of melanoma. Curr Opin Oncol. 1994;6:179–87. doi: 10.1097/00001622-199403000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Komenaka I, Hoerig H, Kaufman H. Immunotherapy for melanoma. Clin Dermatol. 2004;22:251–65. doi: 10.1016/j.clindermatol.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi H, Song Y, Hoon D, Appella E, Celis E. Tumor-reactive T helper lymphocytes recognize a promiscuous MAGE-3 epitope presented by various major histocompatibility complex class II alleles. Cancer Res. 2001;61:4773–8. [PubMed] [Google Scholar]
- 21.Kobayashi H, Omiya R, Ruiz M, et al. Identification of an antigenic epitope for helper T lymphocytes from carcinoembryonic antigen. Clin Cancer Res. 2002;8:3219–25. [PubMed] [Google Scholar]
- 22.Schwartz RH. Acquisition of immunologic self-tolerance. Cell. 1989;57:1073–81. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 23.Sebzda E, Wallace VA, Mayer J, Yeung RSM, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994;263:1615–8. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 24.Andersen MLM, Ruhwald M, Nissen MH, Buus S, Claesson MH. Self-peptides with intermediate capacity to bind and stabilize MHC class I molecules may be immunogenic. Scand J Immunol. 2003;57:21–7. doi: 10.1046/j.1365-3083.2003.01182.x. [DOI] [PubMed] [Google Scholar]
- 25.Budd RC. Activation-induced cell death. Curr Opin Immunol. 2001;13:356–62. doi: 10.1016/s0952-7915(00)00227-2. [DOI] [PubMed] [Google Scholar]
- 26.Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003;4:410–5. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- 27.Ju S-T, Panka DJ, Cui H, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 28.Tsuboi A, Oka Y, Udaka K, et al. Enhanced induction of human WT1-specific cytotoxic T lymphocytes with a 9-mer WT1 peptide modified at HLA-A*2402-binding residues. Cancer Immunol Immunother. 2002;51:614–20. doi: 10.1007/s00262-002-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francini G, Scardino A, Kosmatopoulos K, et al. High-affinity HLAA (*) 02.01 Peptides from parathyroid hormone-related protein generate in vitro and in vivo antitumor CTL response without autoimmune side effects. J Immunol. 2002;169:4840–9. doi: 10.4049/jimmunol.169.9.4840. [DOI] [PubMed] [Google Scholar]