Abstract
σ28 RNA polymerase is an alternative RNA polymerase that has been proposed to have a role in late developmental gene regulation in Chlamydia, but only a single target gene has been identified. To discover additional σ28-dependent genes in the Chlamydia trachomatis genome, we applied bioinformatic methods using a probability weight matrix based on known σ28 promoters in other bacteria and a second matrix based on a functional analysis of the σ28 promoter. We tested 16 candidate σ28 promoters predicted with these algorithms and found that 5 were active in a chlamydial σ28 in vitro transcription assay. hctB, the known σ28-regulated gene, is only expressed late in the chlamydial developmental cycle only, and two of the newly identified σ28 target genes (tsp and tlyC_1) also have late expression profiles, providing support for σ28 as a regulator of late gene expression. One of the other novel σ28-regulated genes is dnaK, a known heat shock-responsive gene, suggesting that σ28 RNA polymerase may be involved in the response to cellular stress. Our σ28 prediction algorithm can be applied to other bacteria, and by performing a similar analysis on the Escherichia coli genome, we have predicted and functionally identified five previously unknown σ28-regulated genes in E. coli.
Genome sequencing has indicated that all Chlamydia species encode two alternative sigma factors, suggesting a role for alternative forms of RNA polymerase in chlamydial gene regulation. We have demonstrated that one of these alternative RNA polymerases, σ28 RNA polymerase, transcribes hctB (24), a gene whose transcript is detectable only at late time points in the chlamydial developmental cycle (6, 16). hctB encodes Hc2, one of two histone-like proteins in Chlamydia that have been shown to be responsible for the condensation of DNA during conversion of the metabolically active form of chlamydiae, known as a reticulate body, to the infectious extracellular form, the elementary body (8). To date, hctB is the only σ28-regulated gene that has been identified in Chlamydia, and it is not known whether the role of σ28 RNA polymerase is confined to the regulation of late gene expression in the developmental cycle.
To identify additional σ28-regulated genes in Chlamydia, we have combined the use of bioinformatics, to predict σ28-regulated promoters in the chlamydial genome, with testing of promoter activity in a chlamydial σ28 in vitro transcription assay. We used two in silico approaches, identifying candidate promoters on the basis of sequences that either resemble the consensus bacterial σ28 promoter (9, 10) or are predicted to be highly transcribed by σ28 RNA polymerase based on functional studies (25). Using information from both approaches, we have a developed a computer algorithm to identify candidate σ28 promoters in the chlamydial genome and have shown that five promoters are transcribed by chlamydial σ28 RNA polymerase. This method can be applied to other bacterial genomes, and we have also identified five new σ28-regulated genes in Escherichia coli.
MATERIALS AND METHODS
Development of a program for extracting sequences.
We developed a program called SequenceExtractor to extract user-defined DNA sequences from a genome. The program requires two input files, consisting of a genome sequence file and a file containing the start and stop coordinates for each gene within the DNA sequence being examined. We applied this program to extract two files in fasta format from each of the genomes of C. trachomatis serovar D, E. coli K-12, and Salmonella enterica serovar Typhimurium using sequences obtained from TIGR (http://www.tigr.org). For each organism, the first output file contained 200 bp of sequence upstream for each gene (“200 bp upstream”). The second output file was more restrictive and contained up to 200 bp of upstream sequence for each gene, provided that these sequences were in the intergenic region and not within the coding region of the nearest upstream gene (“200-bp nonoverlap”).
Development of a program for predicting promoters.
We also developed a program, called PromoterMatcher, that uses a probability weight matrix to predict promoters in a genome. We generated two probability weight matrices, each based on complementary information about σ28 promoter structure, and applied them to an input file consisting of extracted sequences in fasta format. The frequency matrix was based on a set of 21 known bacterial σ28 promoters and takes into account the frequency of occurrence of each nucleotide at each position within this promoter set. The activity matrix used functional data in the form of the relative promoter strength attributable to each nucleotide at each promoter position and was derived from a comprehensive mutational analysis of a σ28 promoter (25). For each promoter position, the algorithm assigned a probability value to the four possible nucleotides, with a total probability of 1. Both matrices also contained probability-weighted models for the length of the spacer between the two promoter elements based on either nucleotide frequency or relative promoter activity. The final score for each candidate promoter was determined by summing the log of the probability at each position (which is the mathematical equivalent of multiplying the probabilities). Only the highest-scoring promoter was recorded per upstream region. The predicted promoters were sorted by score, from best to worst.
Generation of the sequence logo.
All sequence logos were derived using SEQLOGO, which is available online at http://ep.ebi.ac.uk/EP/SEQLOGO/. The format for data input into this site is a series of numbers representing either nucleotide frequency or relative promoter activity. The resulting sequence logo consists of stacks of letters at each position. The height of the stack indicates the importance of a particular position for promoter activity. The height of an individual letter within a stack indicates the relative preference for that nucleotide based on transcriptional activity or frequency (with a maximum height defined as 2 bits).
Cloning of transcription plasmids.
Each candidate σ28-regulated promoter to be tested was cloned into a plasmid so that promoter activity could be measured with a σ28 in vitro transcription assay. The promoter insert, consisting of sequence from approximately 300 bp upstream of the transcription start site to the +5 position, was amplified by PCR using either C. trachomatis serovar D or E. coli K-12 genomic DNA and respective primers (see Table S1 in supplemental material). This PCR insert was cloned upstream of a synthetic G-less cassette transcription template in plasmid pMT1125 (23). Transcription from the predicted promoter by σ28 RNA polymerase was expected to produce a 130-nt transcript.
Overexpression and purification of σ28.
C. trachomatis serovar L2 His6-σ28 and E. coli His6-σ28 were individually overexpressed in E. coli BL21(DE3) and purified, as previously described (24, 25), to a concentration of 35.7 μg/ml and 115.8 μg/ml, respectively.
In vitro transcription reactions.
Transcription reactions were performed as previously described (24, 25). C. trachomatis σ28 RNA polymerase was reconstituted by mixing 1 μl C. trachomatis recombinant His6-σ28 with 1 μl heparin-agarose-purified C. trachomatis RNA polymerase at 4°C for 15 min, immediately prior to the transcription reaction. E. coli σ28 RNA polymerase was reconstituted from 1 μl E. coli recombinant His6-σ28 and 0.03 units E. coli core enzyme (Epicenter, Madison, Wis.). For antibody inhibition reactions, 8 μg of rabbit polyclonal antichlamydial σ28 antibodies (24) was preincubated with the RNA polymerase for 20 min at room temperature prior to transcription.
Purification of C. trachomatis RNA polymerase from chlamydiae grown in tissue culture.
C. trachomatis serovar L2 was grown in mouse L929 cells and harvested at 18 h postinfection (hpi). RNA polymerase was partially purified by heparin-agarose chromatography as previously described (21).
Purification of reticulate body RNA.
L929 cells grown in suspension and infected with C. trachomatis serovar D were recovered by centrifugation and lysed by Dounce homogenization as previously described, with slight modifications (21). A second centrifugation step separated chlamydiae from host cellular debris. Chlamydial RNA was extracted using RNA STAT-60 (Teltest, Inc., Friendswood, TX).
Mapping transcription start sites by primer extension.
The primer was prepared from 100 ng of a DNA oligonucleotide that was labeled with 50 μCi of [γ-32P]ATP in the presence of T4 polynucleotide kinase at 37°C for 1 h. Unincorporated free nucleotides were removed with a DNA mini-Quick Spin DNA column (Roche Diagnostics, Indianapolis, Ind.). Radioactive samples were counted with a scintillation counter. Fifty micrograms of reticulate body RNA and 5 × 106 cpm labeled primer were preheated at 65°C for 10 min and chilled on ice. cDNA was synthesized by adding Superscript II reverse transcriptase (Invitrogen, Carlsbad, Calif.) and 10 mM deoxynucleoside triphosphates, followed by incubation at 42°C for 50 min. The reaction was stopped by the addition of distilled water and a 1/10 volume of 3 M sodium acetate to a total volume of 100 μl, followed by phenol-chloroform extraction and chloroform extraction. cDNA was recovered by ethanol precipitation, dried, and resuspended in 9 μl formamide stop solution (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol). The primer extension products were electrophoresed on a 6% acrylamide-urea sequencing gel together with a single-stranded M13mp18 DNA sequence ladder and exposed to X-ray film.
RESULTS
Development of computer algorithms to identify σ28 promoters.
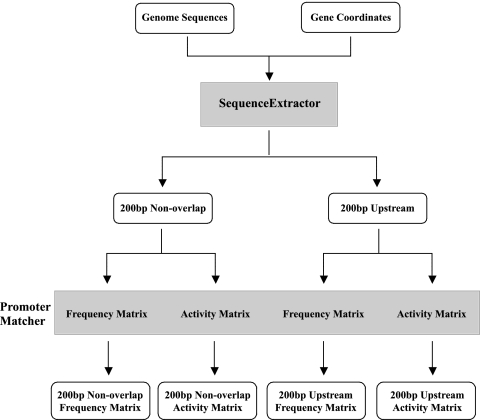
We developed two computer algorithms, which we used in parallel to identify candidate σ28 promoters within a genome (Fig. 1). The first program, called SequenceExtractor, selects sequences from a genome for analysis by the second program, PromoterMatcher, which makes predictions on the basis of the σ28 promoter structure and sequence. We used the structure of the extended bacterial σ28 promoter (12) with eight positions in the −35 element and another eight positions in the −10 element separated by a spacer of variable length.
FIG. 1.
Flow chart showing the use of computer algorithms for promoter prediction. The SequenceExtractor algorithm was used to extract two sequence files for each open reading frame (ORF) that were then analyzed with a promoter prediction algorithm (PromoterMatcher) using either of two probability weight matrices (see the text). In total, this scheme produced four lists of predicted promoters ranked by scores. Details are provided in Materials and Methods and Results.
We focused our search on the intergenic region upstream of each predicted gene where promoters were most likely to be present. SequenceExtractor was used to select sequences up to 200 bp upstream of each gene, provided that they were not in a coding region (200-bp nonoverlap region). In Chlamydia, however, many intergenic regions are short, and promoters have been located within the upstream gene (13). Thus, we also separately examined all sequences in the region 200 bp upstream of each gene even if they were beyond the intergenic region (200-bp upstream region).
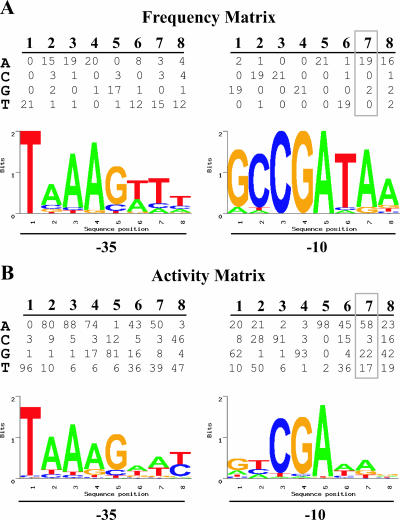
To identify candidate σ28 promoters within these upstream sequences, we used the PromoterMatcher algorithm to apply a weighted matrix and assign probability scores for the 16 promoter positions and the spacer length. To increase the likelihood of identifying σ28 promoters, we used two weighted matrices, each based on a different measure of the contribution of sequence to promoter activity. The first matrix, called the frequency matrix, was based on the occurrence of a given nucleotide at each promoter position within a compilation of 21 known σ28 promoters, including 20 promoters from E. coli and Salmonella, and the C. trachomatis hctB promoter. For example, at the seventh position in the −10 element (Fig. 2A), an A was present in 19 of the 21 promoters, and the remaining two promoters had a G at this position. Accordingly, an A was given a strong weighting of 19/21, while the weighting for a G was 2/21. As all known σ28 promoters, with the exception of the chlamydial promoter, have a spacer of 11 nt, this spacer length was also heavily weighted.
FIG. 2.
Probability weight matrices and sequence logos for predicting promoters in the chlamydial genome. (A) Frequency matrix for the 16 positions in the −35 and −10 promoter elements and the four possible nucleotides at each position (see Materials and Methods). Each value in the matrix indicates how many of the 21 known σ28 promoters in the training set contained the given nucleotide at that promoter position. A sequence logo depicting the relative nucleotide preference at each position in the promoter is shown below the matrix (25). (B) Activity matrix for the −35 and −10 promoter elements with values derived from a mutational analysis of the C. trachomatis hctB promoter as described in the text (25). At each promoter position, the values are proportional to the relative promoter activity attributable to that nucleotide for a total of 100%. The sequence logo for the promoter is shown below the matrix. Details of the sequence logo format are presented in Materials and Methods and Results. All sequence logos were derived using SEQLOGO, which is available online at http://ep.ebi.ac.uk/EP/SEQLOGO/.
A second weighted matrix, called the activity matrix, assigned a weighting to each of the four possible nucleotides for every position based on the promoter activity attributed to that nucleotide in a mutational analysis of the hctB promoter (25). For example, at the seventh position of the −10 element, hctB promoter activity with C. trachomatis σ28 RNA polymerase was greatest when an A was present and was reduced by 2.6-, 3.4-, and 19.3-fold with a G, T, or C, respectively (25). We thus assigned probability scores for A (58/100), G (22/100), T (17/100), or C (3/100) that were proportional to these promoter activities (Fig. 2B). The probability weighting for the spacer length was based on the measured effect of a spacer length from 9 to 13 nt on hctB promoter activity (25).
By applying these two weighted matrices to the two sets of upstream sequences, we generated four lists of candidate σ28 promoters using PromoterMatcher. hctB, the known C. trachomatis σ28-regulated promoter, was the highest-scoring promoter sequence in all four lists. The top 30 predictions for each list are shown in Table S2 (frequency matrix), and Table S3 (activity matrix) in the supplemental material.
Five candidate chlamydial promoters were transcribed by σ28 RNA polymerase.
We chose 16 of the top candidate promoters (Table 1) for functional testing with our chlamydial σ28 in vitro transcription assay. In general, these promoters were among the top-50-scoring promoters in at least two of the four prediction lists. Since the source of our core enzyme contains chlamydial σ66 RNA polymerase activity (24), we tested for transcription in the absence and presence of recombinant chlamydial σ28 as a measure of σ66-specific and σ28-specific activity, respectively. We also assayed for σ28-dependent activity by testing for inhibition of transcription by anti-σ28antibodies.
TABLE 1.
Predicted chlamydial σ28 promoters tested for in vitro activity
| Gene ID | Gene name | Function | Location from ORF (nt) | −35 sequence | Spacer length (nt) | −10 sequence | Rankingsa | σ28 activity |
|---|---|---|---|---|---|---|---|---|
| CT047 | Possible outer membrane protein I | −96 | TTTTGTAT | 11 | GTCGAAAT | 36, *, 51, 24 | No | |
| CT099 | trxB | NADPH thioredoxin reductase | −104 | TTAGTTTT | 11 | GTCGAAAC | *, 32, 6, 3 | No |
| CT181 | Hypothetical protein | −193 | TAATGTAT | 12 | CACGAATG | *, *, 17, 8 | No | |
| CT249 | Hypothetical protein | −188 | TCAACATT | 12 | ATCGAAAT | *, *, 9, 42 | No | |
| CT256 | tlyC_1 | Hypothetical protein, possible hemolysin | −104 | TACAGTTG | 11 | GCCGAAGA | 4, 3, 45, 20 | Yes |
| CT359 | bioY | Probable biotin synthase | −48 | TAAAGGCC | 12 | GTCGATTC | *, *, 8, 4 | Yes |
| CT396 | dnaK | Dnak protein (Hsp70) | −63 | TAAAGGAA | 11 | AACGAAGA | 28, *, 27, * | Yes |
| CT415 | yebL | ABC transporter | −89 | TAAAGAAT | 11 | GAAGACAA | 14, 9, 14, 6 | Weak |
| CT418 | yhbZ | GTP-binding protein | −196 | TAAACGTT | 12 | GACGATAC | 10, *, 4, * | Weak |
| CT425 | Hypothetical protein | −42 | TAAAGGAC | 10 | CTCGAACT | *, *, 54, 28 | Weak | |
| CT441 | tsp | Probable tail-specific protease | −61 | TCAAGTTT | 11 | GTCGAAGA | 3, 2, 2, 2 | Yes |
| CT553 | fmu | FMU/SUN-related methyltransferase | −37 | TAAAGAGC | 13 | CTCGAAGG | *, *, 23, 12 | No |
| CT631 | Hypothetical protein | −41 | TCTGCTTT | 12 | ATCGAAAA | *, *, 41, 18 | No | |
| CT693 | pgk | Phosphoglycerate kinase | −56 | TGAGTTTT | 11 | GCCTATAA | 13, 8, *, * | Yes |
| CT789 | Hypothetical protein | −45 | TTAATATT | 11 | AACGAAAG | 21, 12, 21, 10 | No | |
| CT798 | glgA | Glycogen synthase | −41 | TTAATGAG | 12 | AACGAAAC | *, *, 28, 13 | No |
The rankings for each of four promoter prediction lists are shown in the following order: 200-bp upstream frequency matrix, 200-bp nonoverlap frequency matrix, 200-bp upstream activity matrix, and 200-bp nonoverlap activity matrix. *, ranking is not in the top 50.
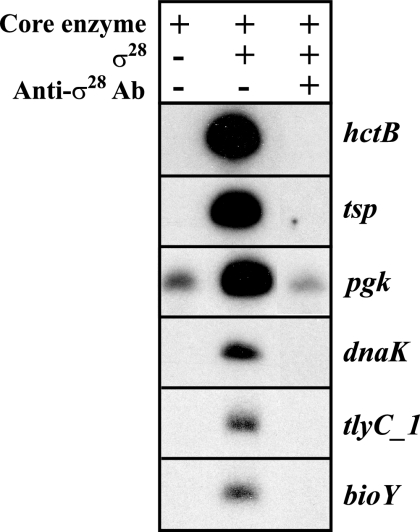
Five of the 16 candidate promoters tested showed σ28-specific activity (Fig. 3), and an additional three promoters (yebL, yhbZ, and CT425) were weakly transcribed (data not shown). Four of the strongly transcribed promoters (tsp, dnaK, tlyC_1, and bioY) produced a transcript only when recombinant chlamydial σ28 was added, as was the case with the hctB positive control promoter. Transcription of these four promoters was also abrogated by rabbit polyclonal anti-σ28 antibodies (Fig. 3, lane 3). The results were less clear-cut with the pgk promoter, because although there was a large increase in transcription when σ28 was added, there was baseline transcription in the absence of σ28, raising the possibility of some σ66-dependent activity. Anti-σ28 antibodies decreased transcription of the pgk promoter, but there was still residual transcript present. These results provide evidence that the promoters for tsp, dnaK, tlyC_1, and bioY are transcribed by σ28 RNA polymerase and suggest that the pgk promoter is recognized by both σ28 and σ66 RNA polymerases.
FIG. 3.
In vitro transcription of predicted C. trachomatis σ28-dependent promoters using chlamydial σ28 RNA polymerase. Lane 1, transcription by a C. trachomatis core enzyme preparation containing σ66 transcriptional activity but no σ28 activity; lane 2, addition of recombinant chlamydial σ28 to the core enzyme; lane 3, transcription by core enzyme plus recombinant chlamydial σ28 in the presence of anti-chlamydial σ28 antibodies (Ab).
For in vivo validation of these results, we used primer extension to map the transcription start sites for the three strongest promoters, hctB, tsp, and pgk, to within 6 nt of the predicted σ28 −10 promoter element, at a location consistent with the predicted promoter (Fig. 4). A previously mapped transcription start site for dnaK (5) was located within 8 nt of the σ28 promoter that we have predicted for this gene.
FIG. 4.
Mapped transcription start sites. The sequence immediately upstream of the ATG start codon (underlined) is shown for each C. trachomatis gene. Transcription start sites mapped by primer extension are shown in capital letters. Predicted σ28 promoters are underlined and in boldface type. The sequences for hctB, tsp, and pgk are from C. trachomatis serovar D. The sequence for dnaK is from the C. trachomatis strain MoPn (C. muridarum), and this transcription start site was mapped previously by Engel et al. (5).
pgk is regulated by two overlapping promoters.
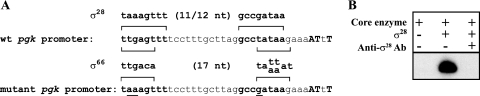
Analysis of the sequence in the pgk promoter revealed a possible σ66 promoter overlapping the predicted σ28 promoter. To confirm the presence of an active σ28 promoter, without the confounding effect of a second promoter, we introduced substitutions predicted to disrupt the putative σ66 promoter but not the σ28 promoter (Fig. 5A). With this mutant promoter, there was no baseline σ66-dependent activity, and all transcription was dependent on the addition of chlamydial σ28 (Fig. 5B, lanes 1 and 2). Transcription was specifically inhibited by anti-σ28 antibodies (Fig. 5B, lane 3). These results provide good experimental support for the predicted σ28-dependent pgk promoter and an overlapping σ66 promoter.
FIG. 5.
Transcription of a mutated pgk promoter. (A) The sequence upstream of the C. trachomatis pgk promoter is shown with the predicted σ28 promoter in boldface type, and the mapped transcription start sites are in boldface capital letters. For reference, the consensus bacterial σ28 promoter (10, 12) is shown above the sequence, and the chlamydial σ66 promoter (17, 22) is shown below the sequence. The bottom sequence shows a mutant pgk promoter with two nucleotide changes in the −35 element and one nucleotide substitution in the −10 element (changes are underlined). wt, wild type. (B) In vitro transcription of the mutant pgk promoter by chlamydial σ28 RNA polymerase. Lane 1, C. trachomatis core enzyme preparation alone; lane 2, core enzyme with recombinant chlamydial σ28 protein; lane 3, transcription of the mutant pgk promoter by core enzyme plus recombinant chlamydial σ28 in the presence of anti-chlamydial σ28 antibodies (Ab).
Five predicted E. coli σ28 promoters were transcriptionally active.
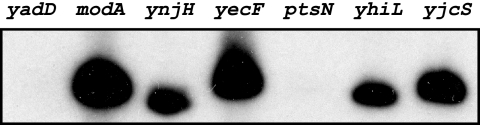
As we also had functional data for promoter recognition by E. coli σ28 RNA polymerase (25), we were able to apply our promoter-finding algorithm to the genomes of E. coli and the closely related bacterium Salmonella. Lists of the top 30 candidate promoters are shown in Tables S4 and S5 for E. coli and Tables S6 and S7 for Salmonella (see the supplemental material). Many of these promoters are known σ28 promoters in E. coli and Salmonella. We tested seven candidate σ28 E. coli promoters that had not been previously studied (Table 2) and found that five (modA, ynjH, yecF, yhiL, and yjcS) were functionally active in an E. coli in vitro σ28 transcription assay (Fig. 6).
TABLE 2.
Predicted E. coli σ28 promoters tested for in vitro activity
| Gene ID | Gene name | Function | Location from ORF (nt) | −35 sequence | Spacer length (nt) | −10 sequence | Rankingsa | σ28 activity |
|---|---|---|---|---|---|---|---|---|
| b0132 | yadD | Hypothetical protein | −82 | TAAACAAT | 12 | GACGATAG | *, 25, 8, 6 | No |
| b0763 | modA | Molybdate ABC transporter | −129 | TCAACTTC | 11 | GCCGATAT | 10, 9, 29, 18 | Yes |
| b1760 | ynjH | NADPH thioredoxin reductase, predicted protein | −62 | TAAAGTTT | 12 | ACCGTTAT | *, *, 13, 9 | Yes |
| b1915 | yecF | Hypothetical protein | −134 | TAACGTAT | 11 | GCCGATAT | 20, 14, 11, 7 | Yes |
| b3204 | ptsN | Enzyme IIANtr | −63 | TAAACAAC | 12 | ACCGATAA | 29, 19, 24, 15 | No |
| b3490 | yhiL | Hypothetical protein | −118 | TCAAGAAT | 11 | GCCGACAA | 11, 10, 12, 8 | Yes |
| b4083 | yjcS | Hypothetical protein | −190 | TAAAGTTT | 11 | GACGATTA | 16, 12, 5, 5 | Yes |
The rankings for each of four promoter prediction lists are shown in the following order: 200-bp upstream frequency matrix, 200-bp nonoverlap frequency matrix, 200-bp upstream activity matrix, and 200-bp nonoverlap activity matrix. *, ranking is not in the top 30.
FIG. 6.
In vitro transcription of predicted E. coli σ28-dependent promoters. Promoters were transcribed with E. coli σ28 RNA polymerase reconstituted from E. coli core enzyme and recombinant E. coli σ28 as described in the text.
DISCUSSION
This study demonstrates how a combination of a bioinformatic analysis and functional validation can be used to identify previously unrecognized target genes of an alternative RNA polymerase. From a genome-wide search for sequences resembling known σ28 promoters and sequences that have been shown to be highly transcribed by σ28 RNA polymerase, we identified five novel σ28-regulated genes in Chlamydia and another five new σ28-regulated genes in E. coli. Although we did not test any of the predicted Salmonella σ28 promoters, our list of top-scoring promoters includes three (STM3152, STM3216, and STM2314) of four newly identified σ28 target genes in S. enterica serovar Typhimurium (7). These results demonstrate that our promoter prediction algorithm can successfully identify σ28 promoters, and it is likely that additional σ28-regulated genes remain to be discovered in many bacterial genomes.
Our results show that the combination of a frequency matrix, derived from known σ28 promoter sequences, and an activity matrix, based on a mutational analysis of a chlamydial σ28-dependent promoter, increased our chances of identifying additional σ28 promoters. hctB and tsp had the two strongest promoters in terms of transcriptional activity and sequence conservation with the bacterial σ28 consensus promoter, and both ranked equally high with the two matrices (see Tables S2 and S3 in the supplemental material). For promoters with weaker sequence conservation, such as bioY, the activity matrix may be a better predictor. For instance, bioY ranked in the top 10 using the activity matrix (Table 1) but was not in the top 50 with the frequency matrix.
In general, we found that a strict pattern-matching algorithm based only on the bacterial σ28 consensus sequence would not be very sensitive as a means of identifying σ28-dependent promoters in Chlamydia. With the exception of the hctB promoter, the other chlamydial σ28 promoters identified in this study are not well conserved with the bacterial consensus promoter. For example, while the dnaK promoter (TAAAGGAA-N11-AACGAAGA) contains the signature TAAA of the −35 promoter element, the −10 promoter element has only a 4/8 match with the consensus sequence. The CGA motif was the only recognizable sequence in the dnaK −10 promoter element, highlighting the importance of this motif for σ28 promoter activity (25). In all, the CGA motif of the −10 element was present in five of the six transcriptionally active chlamydial σ28 promoters.
In Chlamydia, two of the five newly identified σ28-regulated genes have been shown to be expressed late in the developmental cycle in a fashion similar to that of the original σ28 target gene, hctB. Transcripts for hctB, tsp, and tlyC_1 were each first observed at 16 hpi by microarray expression analysis (3). These late expression profiles support a role for σ28 RNA polymerase in late gene expression in Chlamydia. In contrast, mRNA from pgk and bioY were detectable earlier, at 8 hpi, while the dnaK transcript was present by 3 hpi (3). It is worth noting, however, that this microarray analysis measures only steady-state transcript levels and would not be able to distinguish between the temporal activity of multiple promoters, such as transcription of pgk by both σ28 and σ66 RNA polymerases. Thus, it is entirely possible that σ28-regulated expression of these target genes may also be restricted to late time points, and as yet, there is no definitive evidence that σ28 RNA polymerase is transcriptionally active at earlier times in the chlamydial developmental cycle. In summary, there is accumulating evidence for σ28-dependent regulation of a subset of late genes in Chlamydia, distinct from the late genes transcribed by σ66 RNA polymerase (6).
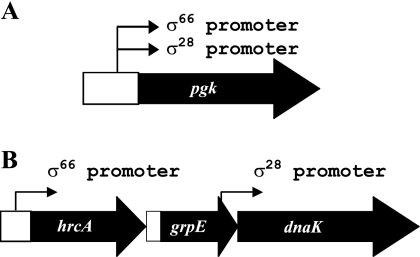
pgk and dnaK are the first examples of genes in Chlamydia that can be transcribed by more than one form of RNA polymerase. With pgk, the promoters for σ28 RNA polymerase and σ66 RNA polymerase overlap and appear to have the same transcription start site (Fig. 5 and 7A), which raises the question of how promoter occupancy by the two forms of RNA polymerase is regulated. dnaK is known to be transcribed as part of the hrcA-grpE-dnaK operon by σ66 RNA polymerase (21) under the control of the HrcA repressor (23). We now show that dnaK has an independent promoter that is transcribed by σ28 RNA polymerase (Fig. 4 and 7B), and we predict that this promoter is responsive to heat shock. We base this prediction on the observation that elevated temperatures have been shown to upregulate levels of the dnaK transcript by greater than 10-fold, while hrcA and grpE mRNA levels were not similarly increased (5).
FIG. 7.
Diagram of the C. trachomatis pgk gene and hrcA-grpE-dnaK operon. Arrows indicate the approximate locations of transcription start sites. (A) Upstream of the pgk gene are two overlapping promoters recognized by σ66 and σ28 RNA polymerases, respectively. (B) The σ66 promoter for the hrcA-grpE-dnaK operon is located upstream of hrcA, while a σ28 promoter is located upstream of dnaK within the coding region of grpE. The figure is not drawn to scale.
While we have identified a total of six σ28-regulated genes in Chlamydia, it is not clear whether these genes belong to a specific functional group. hctB encodes Hc2, a histone-like protein that causes DNA condensation (1, 2, 4, 14, 15). Tsp is a predicted protease with similarity to CPAF, a secreted chlamydial protease that cleaves host transcription factors involved in major histocompatibility complex class I and class II antigen expression (18, 26). tlyC_1 encodes a hypothetical protein, which may be involved in hemolysis (20). Of the remaining three target genes, dnaK encodes a heat shock chaperone, pgk encodes a phosphoglycerate kinase, and bioY encodes a hypothetical protein with homology to a predicted biotin synthase in Bacillus subtilis and Treponema pallidum. Thus, unlike other bacteria, where σ28 RNA polymerase regulates particular classes of genes involved in chemotaxis, motility, and flagellum synthesis, it is not clear how these σ28 target genes in Chlamydia are related.
Our studies support a role for σ28 as a developmental regulator of late gene expression in Chlamydia, but little is known about how σ28 activity is itself regulated. Although Chlamydia encodes a predicted anti-sigma factor, RsbW, as part of a partner-switching mechanism, doubts have been raised about its ability to regulate σ28 (11). The discovery that one of the target genes of σ28, dnaK, is a known heat shock gene is intriguing and may provide clues about the signal for σ28-dependent transcription. Perhaps σ28 RNA polymerase is involved in the general stress response in Chlamydia, as supported by the finding that σ28 transcript levels were increased under conditions of heat shock (19). By extension, σ28-regulated transcription late in the developmental cycle may be triggered in response to cellular stress, such as nutrient deprivation or other conditions within the chlamydial inclusion, although the details remain to be elucidated.
Our promoter search algorithm is versatile and can be applied to predict σ28 promoters in other bacteria or promoters for other forms of RNA polymerase. σ28 promoter recognition appears to be conserved among bacteria (25), and thus, our existing frequency- and activity-weighted matrices can be readily used for other prokaryotic genomes. With the appropriate probability weight matrix, the algorithm can also be used to identify promoters recognized by different forms of RNA polymerase. More generally, this same algorithm could be applied to any DNA sequence, such as a protein-binding site, as long as examples are available to build the weighted matrix. As our results have shown, however, an essential component of this bioinformatic approach is the validation of the in silico predictions with functional testing.
Acknowledgments
We thank Eike Niehus, Johnny Akers, Elizabeth Di Russo, Allan Chen, and Narae Park for their support and G. Wesley Hatfield and Marian Waterman for critical review of the manuscript.
This work was supported by a grant from the NIH (AI 44198). M.T. is supported by an NIH Independent Scientist Award (AI 057563), and H.H.Y.Y. was supported by a predoctoral training grant from the NIH (National Research Service Award 1 T15 LM007443 from the National Library of Medicine).
Footnotes
Published ahead of print on 22 September 2006.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Barry, C. E., III, S. Hayes, and T. Hackstadt. 1992. Nucleoid condensation in Escherichia coli that express a chlamydial histone homolog. Science 256:377-379. [DOI] [PubMed] [Google Scholar]
- 2.Barry, C. E., III, T. J. Brickman, and T. Hackstadt. 1993. Hc1-mediated effects on DNA structure: a potential regulator of chlamydial development. Mol. Microbiol. 9:273-283. [DOI] [PubMed] [Google Scholar]
- 3.Belland, R. J., G. Zhong, D. D. Crane, D. Hogan, D. Sturdevant, J. Sharma, W. L. Beatty, and H. D. Caldwell. 2003. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 100:8478-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brickman, T. J., C. E. Barry III, and T. Hackstadt. 1993. Molecular cloning and expression of hctB encoding a strain-variant chlamydial histone-like protein with DNA-binding activity. J. Bacteriol. 175:4274-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engel, J. N., J. Pollack, E. Perara, and D. Ganem. 1990. Heat shock response of murine Chlamydia trachomatis. J. Bacteriol. 172:6959-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fahr, M. J., A. L. Douglas, W. Xia, and T. P. Hatch. 1995. Characterization of late gene promoters of Chlamydia trachomatis. J. Bacteriol. 177:4252-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frye, J., J. E. Karlinsey, H. R. Felise, B. Marzolf, N. Dowidar, M. McClelland, and K. T. Hughes. 2006. Identification of new flagellar genes of Salmonella enterica serovar Typhimurium. J. Bacteriol. 188:2233-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackstadt, T. 1999. Cell biology, p. 321. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. ASM Press, Washington, D.C.
- 9.Haldenwang, W. G. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmann, J. D. 1991. Alternative sigma factors and the regulation of flagellar gene expression. Mol. Microbiol. 5:2875-2882. [DOI] [PubMed] [Google Scholar]
- 11.Hua, L., P. S. Hefty, Y. J. Lee, Y. M. Lee, R. S. Stephens, and C. W. Price. 2006. Core of the partner switching signalling mechanism is conserved in the obligate intracellular pathogen Chlamydia trachomatis. Mol. Microbiol. 59:623-636. [DOI] [PubMed] [Google Scholar]
- 12.Ide, N., T. Ikebe, and K. Kutsukake. 1999. Reevaluation of the promoter structure of the class 3 flagellar operons of Escherichia coli and Salmonella. Genes Genet. Syst. 74:113-116. [DOI] [PubMed] [Google Scholar]
- 13.Mathews, S., and P. Timms. In silico identification of chlamydial promoters and their role in regulation of development. In P. Bavoil and P. Wyrick (ed.), Chlamydia: genomics, pathogenesis and implications for control, in press. Horizon Scientific Press, Wymondham, United Kingdom.
- 14.Pedersen, L. B., S. Birkelund, and G. Christiansen. 1994. Interaction of the Chlamydia trachomatis histone H1-like protein (Hc1) with DNA and RNA causes repression of transcription and translation in vitro. Mol. Microbiol. 11:1085-1098. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen, L. B., S. Birkelund, and G. Christiansen. 1996. Purification of recombinant Chlamydia trachomatis histone H1-like protein Hc2, and comparative functional analysis of Hc2 and Hc1. Mol. Microbiol. 20:295-311. [DOI] [PubMed] [Google Scholar]
- 16.Perara, E., D. Ganem, and J. Engel. 1992. A developmentally regulated chlamydial gene with apparent homology to eukaryotic histone H1. Proc. Natl. Acad. Sci. USA 89:2125-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaumburg, C. S., and M. Tan. 2003. Mutational analysis of the Chlamydia trachomatis dnaK promoter defines the optimal −35 promoter element. Nucleic Acids Res. 31:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw, A. C., B. B. Vandahl, M. R. Larsen, P. Roepstorff, K. Gevaert, J. Vandekerckhove, G. Christiansen, and S. Birkelund. 2002. Characterization of a secreted Chlamydia protease. Cell. Microbiol. 4:411-424. [DOI] [PubMed] [Google Scholar]
- 19.Shen, L., M. Li, and Y. X. Zhang. 2004. Chlamydia trachomatis sigma28 recognizes the fliC promoter of Escherichia coli and responds to heat shock in chlamydiae. Microbiology 150:205-215. [DOI] [PubMed] [Google Scholar]
- 20.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 21.Tan, M., and J. N. Engel. 1996. Identification of sequences necessary for transcription in vitro from the Chlamydia trachomatis rRNA P1 promoter. J. Bacteriol. 178:6975-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan, M., T. Gaal, R. L. Gourse, and J. N. Engel. 1998. Mutational analysis of the Chlamydia trachomatis rRNA P1 promoter defines four regions important for transcription in vitro. J. Bacteriol. 180:2359-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson, A. C., and M. Tan. 2002. Functional analysis of the heat shock regulator HrcA of Chlamydia trachomatis. J. Bacteriol. 184:6566-6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu, H., and M. Tan. 2003. Sigma 28 RNA polymerase regulates hctB, a late developmental gene in Chlamydia. Mol. Microbiol. 50:577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu, H. H. Y., E. G. Di Russo, M. A. Rounds, and M. Tan. 2006. Mutational analysis of the promoter recognized by Chlamydia and Escherichia coli σ28 RNA polymerase. J. Bacteriol. 188:5524-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong, G., P. Fan, H. Ji, F. Dong, and Y. Huang. 2001. Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors. J. Exp. Med. 193:935-942. [DOI] [PMC free article] [PubMed] [Google Scholar]