Abstract
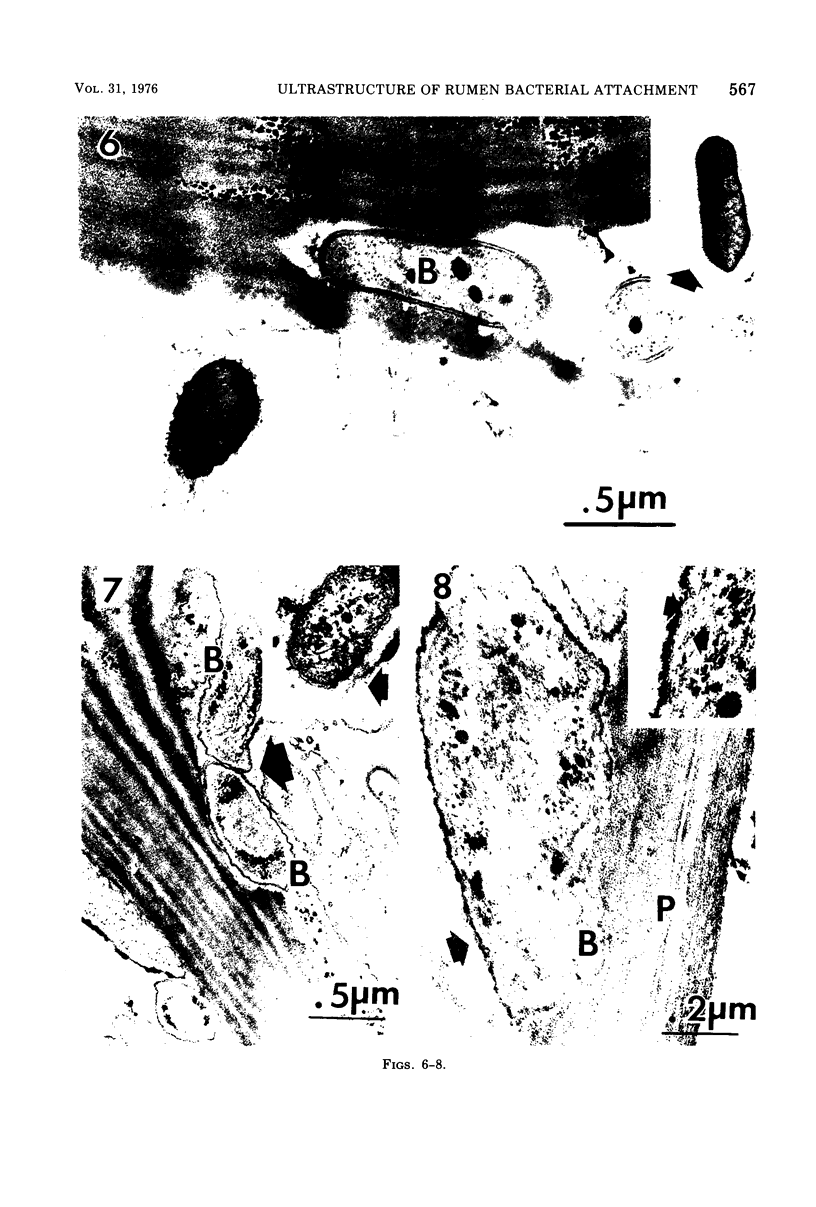
The degradation of forage cell walls by rumen bacteria was investigated with critical-point drying/scanning electron microscopy and ruthenium red staining/transmission electron microscopy. Differences were observed in the manner of attachment of different morphological types of rumen bacteria to plant cell walls during degradation. Cocci, constituting about 22% of the attached bacteria, appeared to be attached to degraded plant walls via capsule-like substances averaging 58 nm in width (range, 21 to 84 nm). Many bacilli appeared to adhere to forage substrates without distinct capsule-like material, although unattached bacteria with capsules were observed occasionally. Certain bacili appeared to be attached to degraded tissue via small amounts of extracellular material, but others apparently had no extracellular material. Bacilli with a distinct morphology due to an irregularly folded, electron-dense outer layer or layers (about 15 nm thick) and without fibrous extracellular material consituted about 37% of the attached bacteria and were observed to adhere so closely to degraded plant walls that the bacterial shape conformed to the shape of the degraded zone. In the rumen ecosystem, bacteria appeared to adhere to plant substrates during degradation by capsule-like material and by small amounts of extracellular material, as well as by the other means not observable by electron microscopy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Amos H. E. Rumen bacterial degradation of forage cell walls investigated by electron microscopy. Appl Microbiol. 1975 May;29(5):692–701. doi: 10.1128/am.29.5.692-701.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg B., von Hofsten B., Pettersson G. Electronmicroscopie observations on the degradation of cellulose fibres by Cellvibrio fulvus and Sporocytophaga myxococcoides. J Appl Bacteriol. 1972 Jun;35(2):215–219. doi: 10.1111/j.1365-2672.1972.tb03692.x. [DOI] [PubMed] [Google Scholar]
- Cagle G. D. Critical-point drying: rapid method for the determination of bacterial extracellular polymer and surface structures. Appl Microbiol. 1974 Aug;28(2):312–316. doi: 10.1128/am.28.2.312-316.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle G. D., Pfister R. M., Vela G. R. Improved staining of extracellular polymer for electron microscopy: examination of Azotobacter, Zoogloea, Leuconostoc, and Bacillus. Appl Microbiol. 1972 Sep;24(3):477–487. doi: 10.1128/am.24.3.477-487.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Ultrastructure of cell envelopes of bacteria of the bovine rumen. Appl Microbiol. 1975 Jun;29(6):841–849. doi: 10.1128/am.29.6.841-849.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Damgaard H. N., Cheng K. J. Cell envelope morphology of rumen bacteria. J Bacteriol. 1974 Jun;118(3):1132–1143. doi: 10.1128/jb.118.3.1132-1143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherwood J. M. Cellulose degradation by Ruminococcus. Fed Proc. 1973 Jul;32(7):1814–1818. [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- McDougall E. I. Studies on ruminant saliva. 1. The composition and output of sheep's saliva. Biochem J. 1948;43(1):99–109. [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. The ultrastructure of the capsules of Diplococcus pneumoniae and Klebsiella pneumoniae stained with ruthenium red. J Gen Microbiol. 1973 Jan;74(1):21–31. doi: 10.1099/00221287-74-1-21. [DOI] [PubMed] [Google Scholar]
- WILKINSON J. F. The extracellualr polysaccharides of bacteria. Bacteriol Rev. 1958 Mar;22(1):46–73. doi: 10.1128/br.22.1.46-73.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]










