Abstract
Legionella pneumophila, a causative agent of bacterial pneumonia, survives inside phagocytic cells by avoiding rapid targeting to the lysosome. This bacterium utilizes a type IVB secretion system, encoded by the dot/icm genes, to replicate inside host cells. DotL, a critical component of the Dot/Icm secretion apparatus, functions as the type IV coupling protein. In contrast to most dot/icm genes, which are dispensable for growth on bacteriological media, dotL is required for the viability of wild-type L. pneumophila. Previously we reported that ΔdotL lethality could be suppressed by inactivation of the Dot/Icm complex via mutations in other dot/icm genes. Here we report the isolation of non-dot/icm suppressors of this phenotype. These ΔdotL suppressors include insertions that disrupt the function of the L. pneumophila homologs of cpxR, djlA, lysS, and two novel open reading frames, lpg0742 and lpg1594, that we have named ldsA and ldsB for lethality of ΔdotL suppressor. In addition to suppressing ΔdotL lethality, inactivation of these genes in a wild-type strain background causes a range of defects in L. pneumophila virulence traits, including intracellular growth, implicating these factors in the proper function of the Dot/Icm complex. Consistent with previous data showing a role for the cpx system in regulating expression of several dot/icm genes, the cpxR insertion mutant produced decreased levels of three Dot/Icm proteins, DotA, IcmV, and IcmW. The remaining four suppressors did not affect the steady-state levels of any Dot/Icm protein and are likely to represent the first identified factors necessary for assembly and/or activation of the Dot/Icm secretion complex.
Legionella pneumophila is a gram-negative, facultative intracellular parasite of freshwater protozoa. Although 48 species of Legionella have been described, L. pneumophila is the most common human pathogen in the genus Legionella and is the primary causative agent of Legionnaires' disease (15). Humans can become infected with L. pneumophila when they are exposed to aerosols from contaminated water sources (15). Upon internalization by alveolar macrophages, L. pneumophila prevents the acidification and lysosomal fusion of its phagosomal compartment and co-opts secretory vesicles from the endoplasmic reticulum (18-20, 36). After these early steps, the phagosome matures into a unique intracellular compartment, the replicative phagosome, where the organism multiplies (17). After the conclusion of a replicative cycle, L. pneumophila lyses its host cell and infects surrounding phagocytic cells.
Numerous cell biological processes have been implicated in the intracellular survival and replication of L. pneumophila, including modulation of both apoptosis and autophagy (1, 2). Although the role of these processes in L. pneumophila virulence is not fully understood, it is clear that alteration of the host cell endocytic pathway is critical to bacterial multiplication, as most avirulent L. pneumophila mutants are unable to replicate inside host cells because they mistarget and fail to form a replicative phagosome (5, 38). Complementation experiments utilizing these mutants revealed that L. pneumophila employs a specialized secretion system encoded by 26 “dot” or “icm” genes to prevent nascent L. pneumophila-containing phagosomes from entering into the endocytic pathway (39, 49).
The dot/icm genes encode an adapted conjugation apparatus that has been classified as a type IVB secretion system (T4SS) (10, 45). The L. pneumophila Dot/Icm T4SS is responsible for injecting a dozen or more bacterial proteins into the cytoplasm of host cells (4, 13, 14, 26, 30, 32). Although the specific biological functions of most Dot/Icm substrates have yet to be identified, many of these secreted proteins are expressed only in the early stationary phase of growth (4, 26). This may explain why the pathogen is not infectious during exponential growth and must differentiate into a transmissive form prior to infecting a new host cell (7).
Although critical to intracellular replication, most of the L. pneumophila dot/icm genes are not required for in vitro growth on bacteriological media (6, 40). However, it was recently reported that three dot genes, dotL, dotM, and dotN, are essential for the viability of L. pneumophila strain Lp02 (6). The dotL gene is also essential for the viability of an unrelated L. pneumophila strain, AA100, suggesting that this phenotype may be a conserved trait (C. D. Vincent and J. P. Vogel, unpublished data). Interestingly, it was discovered that the ΔdotL lethality phenotype could be suppressed by mutations in the majority of the dot/icm genes (6). These observations led to the model that loss of dotL is lethal to L. pneumophila due to the accumulation of a toxic complex, perhaps an unregulated secretion pore, in the envelope of the bacterium (6). According to this model, suppression of ΔdotL lethality could be achieved either directly or indirectly. For example, mutation of a component of the T4SS would directly disrupt the toxic secretion channel and would thus restore viability. Alternatively, the mechanism of suppression could be indirect, for example, by inactivation of genes needed for the proper assembly and/or activity of the secretion channel.
To test this, we performed a screen for transposon mutants that were able to survive in the absence of dotL (6). As predicted, the screen yielded a number of insertions in known dot/icm genes, including four in dotA, two in dotG, one in dotI, five in dotO, three in icmF, and one in icmX (6) and three in a new dot gene, dotV (J. A. Sexton et al., unpublished data). We report here the identification of non-dot/icm suppressor mutations, including disruptions of the L. pneumophila homologs of cpxR, lysS, djlA, and two novel genes, ldsA and ldsB. Although inactivation of each of these genes suppresses ΔdotL lethality, their inactivation in the presence of wild-type DotL results in varying effects on L. pneumophila virulence. These findings indicate that multiple genes are likely to play key roles in regulating the assembly and/or activation of the Dot/Icm secretion complex.
MATERIALS AND METHODS
Bacterial strains and media.
Strains used in this study are listed in Table 1. All L. pneumophila strains used are derived from Lp02 (hsdR rpsL thyA), a wild-type strain derived from the clinical isolate Philadelphia 1 (5). L. pneumophila strains were grown on charcoal yeast extract agar (CYE) buffered with N-(2-acetamido)-2-aminoethanesulfonic acid (ACES) or in ACES-buffered yeast extract broth (AYE). Antibiotics (kanamycin, 30 μg/ml; chloramphenicol, 5 μg/ml; streptomycin, 50 μg/ml; and gentamicin, 7.5 μg/ml), sucrose (5%), and thymidine (100 μg/ml) were added as necessary. Escherichia coli strains were grown in Luria-Bertani (LB) liquid medium or on LB agar plates. Antibiotics (ampicillin, 100 μg/ml; chloramphenicol, 5 μg/ml, and kanamycin, 20 μg/ml) were added as necessary.
TABLE 1.
Strains, plasmids and primers used
| Strain, plasmid, or primer | Relevant genotype or sequence | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH5αλpir | DH5α(λpir) tet::Mu | 24 |
| ER1821 | F−glnV44 e14− (McrA−) endA1 thi-1 Δ(mcrC-mrr)114::IS10 | New England Biolabs (Beverly, MA) |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| L. pneumophila strains | ||
| Philadelphia 1 | Wild-type strain | 5, 27 |
| Lp02 | Philadelphia-1 rpsL hsdR thyA | 5 |
| Lp03 | Lp02 dotA | 5 |
| JV1003 | Lp02 dotL+/ΔdotL::Cmr | 6 |
| JV1005 | Lp02 dotA dotL+/ΔdotL::Cmr | 6 |
| JV1139 | Lp02 + pJB908 | 4 |
| JV1141 | Lp03 + pJB908 | 4 |
| JV1372 | Lp02 djlA dotL+/ΔdotL::Cmr | |
| JV1484 | Lp02 ldsB::Kanr | This study |
| JV1485 | Lp02 yitW::Kanr | This study |
| JV1807 | Lp02 cpxR::Kanr | This study |
| JV1811 | Lp02 djlA::Kanr | This study |
| JV1817 | Lp02 djlA::Kanr | This study |
| JV1835 | Lp02 ldsA::Kanr | This study |
| JV1837 | Lp02 ldsA::Kanr | This study |
| JV4044 | Lp02 lacking all 26 dot/icm genes | This study |
| JV4070 | JV1484 + pJB908 | This study |
| JV4073 | JV1485 + pJB908 | This study |
| JV4075 | JV1807 + pJB908 | This study |
| JV4077 | JV1811 + pJB908 | This study |
| JV4078 | JV1811 + pJB3230 | This study |
| JV4157 | Lp02 + pKB5 | This study |
| JV4158 | Lp03 + pKB5 | This study |
| JV4163 | JV1484 + pKB5 | This study |
| JV4164 | JV1485 + pKB5 | This study |
| JV4165 | JV1807 + pKB5 | This study |
| JV4166 | JV1811 + pKB5 | This study |
| JV4172 | JV1835 + pKB5 | This study |
| JV4196 | JV1807 + pJB3243 | This study |
| JV4313 | JV1811 + pJB3192 | This study |
| JV4321 | JV1485 + pJB3197 | This study |
| JV4322 | JV1485 + pJB3198 | This study |
| JV4358 | JV1835 + pJB908 | This study |
| JV4359 | JV1835 + pJB3411 | This study |
| Plasmids | ||
| pJB908 | pKB5 ΔoriT | 43 |
| pJB1005 | ΔdotL::Cmr in pSR47S | 6 |
| pJB1627 | pKB5 + Cmr | This study |
| pJB3192 | pJB1627 + djlA+ | This study |
| pJB3197 | pJB908 + yitW+lysS+ | This study |
| pJB3198 | pJB908 + lysS+ | This study |
| pJB3230 | pJB908 + djlA+ | This study |
| pJB3243 | pJB908 + cpxR+cpxA+ | This study |
| pJB3411 | pJB908 + ldsA+ | This study |
| pJK211-2 | Mini-Tn10 transposon | 6 |
| pKB5 | RSF1010 cloning vector | 5 |
| pRK600 | RP4 helper plasmid | 22 |
| pSR47S | oriR6K oriTRP4 kan sacB | 29 |
| Primers | ||
| JVP1147 | CCCGGATCCAGGATAAGTAATTCATGAGCAGC | |
| JVP1148 | CCCGTCGACTAGTCAATAAGGAGGAACTCG | |
| JVP1149 | CCCGGATCCAGGTAATTTCTGATAATGAACTTACG | |
| JVP1150 | CCCGTCGACAACAAATTGGACTACCAACCC | |
| JVP1159 | CCCGGATCCGGCAATAGTGGCAGAGCTCG | |
| JVP1217 | CCCGGATCCGGGAATATTTTATTGATCATGTCTGATAACTCCTGG | |
| JVP1218 | CCCGTCGACCAGGATTTAGTGATTATGTCTGC | |
| JVP1229 | CCCGGATCCGCGAGTGATTGAGCATTTGG | |
| JVP1230 | CCCGTCGACGAACCCTCATGTCATTTTAGAAGC |
Assay to determine suppression of ΔdotL lethality.
The assay used to screen for ΔdotL lethality suppression has been described previously (6). Plasmid pJB1005 was transferred to L. pneumophila by using the RP4 conjugation system encoded on plasmid pRK600 (22). L. pneumophila strains that had integrated the plasmid onto the chromosome were selected by plating on medium containing streptomycin and kanamycin. The resulting dotL/ΔdotL::Cmr merodiploid strains were plated on medium containing sucrose to select for resolution of the merodiploid to either dotL or ΔdotL::Cmr. Sucrose-resistant colonies were streaked on medium containing kanamycin to confirm that the integrated plasmid had been lost and on medium containing chloramphenicol to screen for dotL or ΔdotL::Cmr.
Plasmid construction.
All complementing clones were made in the RSF1010 cloning vector pJB908 (43). The cpxR cpxA clone (pJB3243) was generated by amplifying the cpxR-cpxA operon, using primers JVP1147 and JVP1148. The yitW lysS clone (pJB3197) was generated by amplifying the yitW-lysS operon, using primers JVP1159 and JVP1218. The lysS clone (pJB3198) was generated by amplifying lysS, using primers JVP1217 and JVP1218. The ldsA clone (pJB3411) was generated by amplifying ldsA, using primers JVP1229 and JVP1230. The djlA clone (pJB3230) was generated by amplifying djlA, using primers JVP1149 and JVP1150. To generate a djlA-complementing clone in a plasmid that could be transferred by the Dot/Icm complex, the djlA gene from pJB3230 was cloned into pJB1627, generating plasmid pJB3192. All constructs were sequenced to confirm that no errors were introduced during PCR amplification.
Growth of L. pneumophila strains in mouse bone marrow-derived macrophages.
Mouse bone marrow-derived macrophages were isolated from the femurs of female A/J mice as described previously (47). Intracellular growth of L. pneumophila strains was assayed as described previously (4, 47).
Western analysis.
Western blotting was performed using standard techniques (4). Whole-cell extracts were generated from L. pneumophila cells grown to early stationary phase in AYE. Western blotting was performed using antibodies specific to DotL (diluted 1:10,000), DotB (diluted 1:500), DotG (diluted 1:1,000), DotO (diluted 1:3,000), IcmR (diluted 1:3,000), IcmX (diluted 1:10,000), RalF (diluted 1:3,000), SdeC (diluted 1:3,000), or isocitrate dehydrogenase (ICDH) (diluted 1:10,000). Generation of DotL-, DotB-, and SdeC-specific antibodies has been described previously (4, 6, 43). Antibodies to DotG, IcmX, IcmR, and RalF were generated against purified amino-terminal His6 fusions injected into rabbits (Cocalico, Inc.). Antibody specific to ICDH was kindly provided by L. Sonenshein. Antibody specific to DotO was generously provided by R. Isberg. All antibodies were detected using goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (Sigma) diluted 1:10,000 and an ECL detection kit (Amersham Biosciences).
Conjugation assay.
Conjugation assays were performed as described previously (42). L. pneumophila strains were grown to early stationary phase, and 1 × 109 L. pneumophila donor cells were mixed with 1 × 1010 E. coli (strain ER1821) recipient cells. Cell mixtures were incubated for 2 h at 37°C on 0.45 μm-pore-size filters (Millipore) placed on CYE plus thymidine agar plates. Cells were resuspended in sterile water and plated on LB plus ampicillin (100 μg/ml) to determine the number of E. coli transconjugants. The conjugation frequency was determined by dividing the number of E. coli plasmid recipients by the number of L. pneumophila donors in each reaction. Shown are the averages and standard deviations for triplicate conjugation reactions.
Cytotoxicity assay.
Contact-dependent cytotoxicity assays were performed as described previously (23, 46). L. pneumophila strains were added at the indicated multiplicities of infection (MOI) to mouse bone marrow macrophages plated on glass coverslips in 24-well tissue culture plates at a density of 1.5 × 105 bone marrow macrophages per coverslip. Bacteria were pelleted onto the macrophages at 168 × g for 5 min at room temperature and then incubated for 1 hour at 37°C in 5% CO2. The coverslips were then inverted onto a drop of phosphate-buffered saline (PBS) containing 25 μg/ml ethidium bromide and 5 μg/ml acridine orange (23) and were immediately observed. To calculate cytotoxicity, the number of ethidium bromide-positive macrophages was divided by the total number of macrophages observed in each field of view. Shown are the averages and standard deviations for four randomly selected fields of view scored for each strain at each MOI.
Intracellular targeting assay.
Targeting assays were performed essentially as described previously (48). L. pneumophila cells grown to stationary phase were added to the macrophages at an MOI of approximately 5, and infections were allowed to proceed for 1 h at 37°C in 5% CO2. Infected macrophages were fixed by the addition of paraformaldehyde-lysine-periodate containing 5% sucrose, followed by incubation at room temperature for 20 min. Extracellular bacteria were stained with polyclonal serum to L. pneumophila (3, 49) diluted 1:10,000 in PBS containing 2% goat serum (PBSG), followed by goat anti-rabbit secondary antibody conjugated to Cascade Blue (Molecular Probes) diluted 1:10,000 in PBSG. Macrophages were then permeabilized with methanol, and intracellular bacteria were decorated with polyclonal serum to L. pneumophila diluted 1:10,000 in PBSG and goat anti-rabbit secondary antibody conjugated to Alexa Fluor 594 (Molecular Probes) diluted 1:10,000 in PBSG. LAMP-1 was labeled with the antibody ID4B, which was developed by J. Thomas August and was obtained from the Developmental Studies Hybridoma Bank, University of Iowa, Department of Biological Sciences, Iowa City. It was diluted 1:2.5 in PBSG and goat anti-rat secondary antibody conjugated to Alexa Fluor 488 (Molecular Probes) diluted 1:100 in PBSG. Only intracellular bacteria were scored for colocalization with the endocytic marker LAMP-1. The averages and standard deviations of numbers obtained from three sets of 100 intracellular bacteria are reported for each strain.
Immunofluorescence assay of SdeC secretion.
Immunofluorescence staining of secreted SdeC was performed as described previously (4). Cells were stained with affinity-purified polyclonal serum raised to SdeC diluted 1:10, followed by incubation with goat anti-rabbit antibody conjugated to Oregon green (Molecular Probes) diluted 1:100. Bacterial and macrophage DNAs were stained with 4′,6-diamidino-2-phenylindole (DAPI). For each strain, three counts of 100 bacteria were averaged. Results were normalized to wild-type secretion of SdeC set at 100%.
RESULTS
ΔdotL lethality can be suppressed by dot/icm and non-dot/icm mutations.
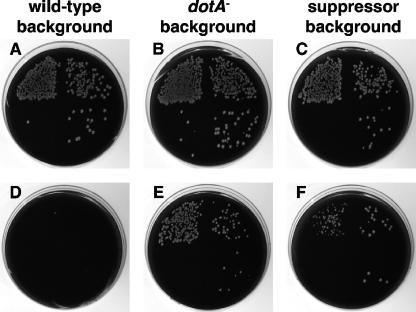
Although the dot/icm genes are required for the intracellular growth of L. pneumophila, most of these genes are dispensable for growth on bacteriological media (39, 40, 49). In contrast, dotL is essential for the viability of the L. pneumophila strain Lp02 under all growth conditions (6). We previously took advantage of ΔdotL lethality to perform a screen for mutations that allow growth in the absence of dotL (6). The assay was based on resolution of a dotL/ΔdotL::Cmr merodiploid strain, as observed in Fig. 1. Total resolution events can be detected by plating on CYE-sucrose plates, as the merodiploid cassette contains a counterselectable marker that confers sensitivity to sucrose. Specific resolution to the ΔdotL::Cmr locus can be identified by growth of the strain on CYE plates containing sucrose and chloramphenicol. Therefore, by comparing growth of the merodiploid on the two types of media, it can be determined if a gene is essential or dispensable. The essentiality of dotL can be observed by the lowered plating efficiency of the wild-type dotL/ΔdotL::Cmr merodiploid on CYE-sucrose plates containing chloramphenicol (Fig. 1D). Of the total resolution events (Fig. 1A), fewer than 1 in 1,000 resolved to the ΔdotL::Cmr locus.
FIG. 1.
dotL is an essential gene, and mutations that suppress ΔdotL lethality can be isolated. The essential nature of dotL can be demonstrated by the failure of a dotL+/ΔdotL::Cmr merodiploid JV1003 to resolve to the ΔdotL::Cmr locus when grown on CYE-sucrose plates containing chloramphenicol (D). Resolution of the dotL+/ΔdotL::Cmr merodiploid to either the wild-type dotL+ or the ΔdotL::Cmr locus can be detected by colony formation on CYE-sucrose plates lacking chloramphenicol (A, B, and C). Growth on CYE-sucrose plates containing chloramphenicol (E and F) is indicative of the presence of a mutation capable of suppressing the lethality caused by loss of dotL. Shown are the resolution abilities of three dotL+/ΔdotL::Cmr merodiploid strains (the wild-type strain Lp02 JV1003, the dotA mutant JV1005, and a strain containing a novel suppressor mutation, JV1372). In each case, resolution was assayed by plating 10-fold serial dilutions in a clockwise manner starting in the upper left quadrant on CYE-sucrose plates (A, B, and C) or CYE-sucrose plates containing chloramphenicol (D, E, and F).
Although dotL is an essential gene, plating the dotL/ΔdotL::Cmr merodiploid strain on sucrose-chloramphenicol plates occasionally did result in growth of a few colonies. These colonies were confirmed to contain dotL deletions and were deduced to be alive because they contained an additional mutation(s) capable of suppressing ΔdotL lethality (6). Based on the frequency at which these suppressors were observed, it seemed likely that there must be a large number of genes whose inactivation could alleviate the loss of dotL. This prediction proved true, as inactivation of almost any dot/icm gene, including dotA, is sufficient to suppress loss of dotL (Fig. 1B and E).
To identify additional suppressors, we previously reported a screen for mini-Tn10 insertions that allowed L. pneumophila to survive without the dotL gene (6). We identified 33 independent strains containing insertions that suppressed the loss of dotL. Nineteen of these strains possessed insertions in known dot/icm genes (6) or in dotV (Sexton et al., unpublished data). The remaining 14 suppressors contained insertions in open reading frames (ORFs) that did not resemble known dot/icm genes. These insertion mutations were able to suppress ΔdotL lethality at a frequency similar to that of a dotA mutation (Fig. 1C and 1F). In summary, dotL is an essential gene, but loss of dotL can be suppressed by insertions in dot/icm genes and non-dot/icm genes.
Identification of non-dot/icm suppressors of ΔdotL lethality.
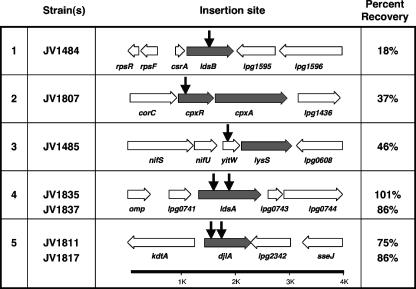
We report the sites of transposon insertion for 7 of the 15 non-dot/icm suppressors (Fig. 2 and Table 2). These seven insertions are in five genes: the L. pneumophila homologs of cpxR, djlA, and yitW, as well as two previously uncharacterized L. pneumophila open reading frames, lpg0742 and lpg1594 (9). Based on their ability to suppress loss of dotL, we have named these genes ldsA and ldsB for lethality of ΔdotL suppressor. We obtained a single insertion in ldsB, cpxR, and yitW and two independent insertions in ldsA and djlA (Fig. 2). However, it is worth noting that the original screen described by Buscher et al. was not performed to saturation (6).
FIG. 2.
Seven transposon insertions in non-dot/icm genes that suppress the lethality caused by loss of dotL were isolated. The insertions were found in five genes, i.e., ldsB, cpxR, yitW, ldsA, and djlA. A map of the region surrounding the site of the insertion(s) is shown, with the gene responsible for suppressing ΔdotL lethality indicated with gray arrows, the genes surrounding the site of insertion indicated with white arrows, and the sites of the transposon insertions shown with vertical black arrows. For each strain, the ability of the dotL+/ΔdotL::Cmr merodiploid to resolve to the ΔdotL::Cmr locus is indicated in the far right column, with the frequency of ΔdotL mutant recovery normalized to the ΔdotA strain JV1005 (100%).
TABLE 2.
Description of the genes surrounding the ΔdotL suppressors
| lpg no.a | Gene name | Protein product/homology |
|---|---|---|
| lpg1591 | rpsR | 30S ribosomal protein S18 |
| lpg1592 | rpsF | 30S ribosomal protein S6 |
| lpg1593 | csrA | Carbon storage regulator |
| lpg1594 | ldsB | Lethality ΔdotL suppressor B |
| lpg1595 | NDb | Hypothetical protein, ErfK homology |
| lpg1596 | yfcX | Enoyl coenzyme A hydratase |
| lpg1439 | corC | Mg2+ and Co2+ transporter |
| lpg1438 | cpxA | Cpx two-component sensor |
| lpg1437 | cpxR | Cpx two-component regulator |
| lpg1436 | ND | Hypothetical proteinc |
| lpg0604 | nifS | Aminotransferase |
| lpg0605 | nifU | Nitrogen fixation protein |
| lpg0606 | yitW | Metal-sulfur cluster biosynthetic enzyme |
| lpg0607 | lysS | Lysyl tRNA synthetase |
| lpg0608 | ND | S-Adenosylmethionine-dependent methyltransferase |
| lpg0740 | omp | Rickettsia 17-kDa surface-exposed antigen (lipoprotein) |
| lpg0741 | ND | Cystathionine-beta-synthase domain |
| lpg0742 | ldsA | Lethality ΔdotL suppressor A |
| lpg0743 | ND | Glutamate synthetase |
| lpg0744 | ND | Sensory transduction (GGDEF domain) |
| lpg2340 | kdtA | 3-Deoxy-d-manno-octulosonic acid transferase |
| lpg2341 | djlA | DnaJ-like protein |
| lpg2342 | ND | Hypothetical protein |
| lpg2343 | sseJ like | Lysophospholipase A |
Sites of insertion are indicated in boldface.
ND, no gene name designated.
Found only in Legionella species.
Of the known genes, cpxR encodes the regulatory portion of a two-component system that responds to the stress of misfolded proteins in the periplasm (34, 37). The djlA gene encodes a DnaJ homolog and is involved in sensing and repairing misfolded proteins in the bacterial inner membrane (11). A DjlA homolog has been shown to be required for the efficient intracellular growth of Legionella dumoffii, although its specific role was not determined (33). Finally, the yitW gene (lpg0606) encodes a protein with similarity to metal-sulfur cluster biosynthetic enzymes in the DUF59 Pfam family. However, we were able to determine that the suppression phenotype is not due to disruption of yitW but is instead solely due to a polar effect of the insertion on the expression of the downstream gene, lysS (see below). lysS encodes lysyl tRNA synthetase, which catalyzes the formation of Lys-tRNALys (31).
Of the novel suppressors, ldsA codes for a 417-amino-acid protein that is predicted to be localized to the inner membrane via eight transmembrane domains (9). Although homologs to LdsA are conserved in other L. pneumophila strains, including Paris and Lens (Lpp0807 and Lpl0778, respectively) (8), there do not appear to be highly homologous ldsA genes in any other bacterial species, including the closely related pathogen Coxiella burnetii (41). Four ORFs with very limited homology to ldsA can be detected in GenBank, including SO0975, GSU1615, MCA2787, and AdehDRAFT_0196 from Shewanella, Geobacter, Methylococcus, and Anaeromyxobacter species, respectively. Each of these ORFs is predicted to encode an inner membrane protein with a size similar to that of LdsA, although none has been assigned a function.
The ldsB gene is also predicted to encode a small inner membrane protein with eight transmembrane domains. Similar to the case for ldsA, the L. pneumophila strains Lens and Paris contain highly conserved homologs to ldsB (lpl1431 and lpp1552, respectively). Most gram-negative bacteria, including C. burnetii, do not possess an LdsB homolog. The exception may be Burkholderia pseudomallei, which is predicted to encode a protein with limited homology to LdsB (BLAST score of ∼1e−08 to LdsB). This B. pseudomallei hypothetical protein has been annotated as encoding a membrane-associated phospholipid phosphatase, although we could not detect this similarity and LdsB does not appear to resemble a phosphatase. In summary, LdsA and LdsB can be viewed as novel proteins that likely do not have true homologs outside of the L. pneumophila family.
Confirmation of suppressor phenotypes.
To confirm that the ΔdotL lethality suppressor phenotype selected for in our screen was specifically linked to the transposon insertions we identified, the transposon and surrounding DNA were transformed into an unresolved dotL/ΔdotL::Cmr merodiploid by using natural competence (44). The resulting strains were then assayed for their ability to suppress loss of dotL by resolving the strains on selective media as described above. In each case, resolution of merodiploids to the ΔdotL::Cmr locus was compared to that of a dotA mutant merodiploid. As shown in Fig. 2, each of the seven insertions was able to suppress ΔdotL lethality, with recovery rates ranging from 18% to 101% (normalized to a dotA mutant set at 100%). Although the percent recovery of ΔdotL mutants varied, we observed that the insertions with lower recovery rates of ΔdotL mutants also exhibited a corresponding diminished effect on Dot/Icm-related traits (see below). Significantly, no resolution events to the ΔdotL::Cmr locus were recovered from the wild-type merodiploid (0/300 colonies scored). Thus, each newly created insertion mutant appears able to suppress loss of dotL, demonstrating linkage of this phenotype to the identified insertion.
The ΔdotL suppressor mutants have varied effects on intracellular replication.
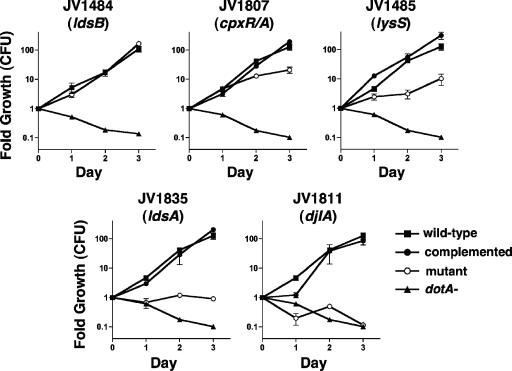
To determine the mechanism of ΔdotL lethality suppression, we assayed the effects of the insertion mutations on the virulence properties of L. pneumophila. Since the suppressor mutations were isolated in a strain that lacked dotL, they were first transformed into a wild-type strain of L. pneumophila by natural competence (44). These strains were then grown in vitro to confirm that each resembled the wild-type strain in growth rate, colony morphology, and motility (data not shown). The mutants were then assayed for their ability to replicate within macrophages derived from the bone marrow of A/J mice (47). In these cells, the wild-type strain Lp02 was able to multiply approximately 125-fold over the course of the assay, whereas a dotA mutant was unable to replicate (Fig. 3).
FIG. 3.
Intracellular growth of the suppressor mutants in bone marrow-derived macrophages from A/J mice. Each graph contains the same data for a control strain that can grow in macrophages (JV1139, squares) and a dot mutant that is unable to grow inside macrophages (JV1141, triangles). Also shown are growth data for each suppressor mutant containing the vector pJB908 (open circles) or the corresponding complementing clone (filled circles). JV1484 is a ldsB mutant, JV1807 is a cpxR cpxA mutant, JV1485 is a yitW lysS mutant, JV1835 is an ldsA mutant, and JV1811 is a djlA mutant. Complementation of the JV1484 mutant was not performed, since the strain grew similarly to a wild-type strain. Fold growth was calculated as the number of CFU recovered each day divided by the number of CFU recovered immediately after infection (day 0). Each time point represents the mean and standard deviation of CFU recovered from triplicate wells.
The seven insertion mutants exhibited a range of intracellular growth phenotypes, varying from wild-type growth to complete attenuation of virulence (Fig. 3). For example, the ldsB mutant (JV1484) grew robustly and was indistinguishable from the wild-type strain Lp02 (Fig. 3). In contrast, the cpxR mutant (JV1807) and the yitW mutant (JV1485) displayed partial defects in intracellular growth, with each having grown approximately 10- to 20-fold less than the wild-type strain at day 3. A strain containing an insertion in ldsA, JV1835, exhibited a strong growth defect, resulting in no observable increase in CFU over the course of the assay. Notably, this mutant did not display a decrease in intracellular CFU, whereas the CFU of the dotA mutant decreased approximately 10-fold by day 3. The other ldsA mutant, JV1837, showed a similar intracellular growth phenotype (data not shown). Finally, the djlA mutant (JV1811) was the most attenuated in that it was unable to replicate, lost viability, and had a phenotype resembling that of a dotA mutant (Fig. 3). The other djlA mutant, JV1817, exhibited the same level of attenuation (data not shown). Thus, the non-dot/icm ΔdotL lethality suppressors vary significantly in their intracellular replication defects, and we observed a positive correlation between the severity of their attenuation and the level of recovery of ΔdotL mutants.
The growth defects of the ldsA and djlA insertions could be fully restored by introduction of the corresponding gene on a plasmid (pJB3411 for ldsA+ or pJB3230 for djlA+) (Fig. 3). Since cpxR is in an operon with cpxA, and the genes each encode an element of a two-component regulator whose components function together, we assayed only complementation of the cpxR insertion by introduction of a plasmid encoding the cpxR-cpxA operon. The cpxR-cpxA operon plasmid pJB3243 was able to fully restore growth to the cpxR insertion strain JV1807 (Fig. 3).
Likewise, yitW is found in an operon with a second gene, lysS. Therefore, the growth defect of the yitW insertion could be due to inactivation of yitW, of the downstream gene lysS, or of both genes. Similar to the case for the cpxR insertion, addition of a plasmid (pJB3197) containing both genes to the yitW insertion strain (JV1485) was able to fully restore growth (data not shown), suggesting that both genes might be involved. However, JV1485 could also be fully complemented by introduction of just the lysS gene (pJB3198) (Fig. 3), leading to the conclusion that the phenotype of the yitW transposon insertion must be due to polar effects on expression of lysS. Complementation of the ldsB mutant (JV1484) was not performed, as this strain exhibited no intracellular growth defect. As a result, we can conclude that ldsB is dispensable for intracellular replication, the cpxR-cpxA operon and lysS are partially required, and ldsA and djlA are necessary. The requirement for djlA closely resembled that of most dot/icm genes.
The cpxR mutation, but not the other suppressor mutations, affects expression of the Dot/Icm apparatus proteins.
Based on the observation that inactivation of various dot/icm genes is able to suppress the lethality associated with loss of dotL (6), we hypothesized that these non-dot/icm suppressors might affect the expression and/or the stability of components of the Dot/Icm type IV secretion machinery. For example, it is well established for other bacterial species that cpxR/cpxA mutations can affect the expression of many proteins (34, 37). To determine if any of the ΔdotL suppressor mutations affected Dot/Icm protein levels, we performed Western blot analysis on whole-cell lysates by using antibodies to 20 Dot/Icm proteins and 2 secreted substrates. Six representative Dot/Icm proteins and the two secreted substrates, RalF and SdeC, are shown in Fig. 4A. An antibody that recognized the constitutively expressed housekeeping protein ICDH was included as a control. None of the suppressor mutations significantly affected expression of the majority of the Dot/Icm apparatus proteins or of the secreted substrates in either the wild-type or ΔdotL strain background in either the exponential or stationary phase of growth (Fig. 4A and data not shown).
FIG. 4.
Only the cpxR suppressor mutation affects the steady-state levels of the Dot/Icm secretion apparatus proteins. The following strains were assayed: Lp02 (wild-type [WT] L. pneumophila) in lane 1, JV4044 (a strain lacking all 26 dot/icm genes) in lane 2, JV1484 (an ldsB mutant) in lane 3, JV1807 (a cpxR cpxA mutant) in lane 4, JV1485 (a yitW lysS mutant) in lane 5, JV1835 (an ldsA mutant) in lane 6, and JV1811 (a djlA mutant) in lane 7. The seven strains were grown in liquid AYE, and lysates were analyzed by Western blotting using antibodies specific to the proteins listed to the right of each blot. (A) Representative proteins not affected by the suppressor mutations, using lysates generated from stationary-phase cultures (optical density at 600 nm of approximately 3.1). Shown are Western blots using antibodies recognizing six representative components of the Dot/Icm complex (DotL, DotB, DotG, DotO, IcmR, and IcmX), two secreted substrates (RalF and SdeC), and the constitutively expressed housekeeping protein ICDH. (B) Dot/Icm proteins affected by the cpxR::Kanr mutation, assaying lysates generated from early-stationary-phase cultures (optical density at 600 nm of approximately 3.1). (C) Dot/Icm proteins affected by the cpxR::Kanr mutation, assaying lysates generated from exponential-phase cultures (optical density at 600 nm of approximately 1.5). In both panels B and C, antibodies recognizing IcmV, IcmW, and ICDH were used.
However, reduced amounts of two Dot/Icm proteins, IcmV and IcmW, were observed in stationary-phase cultures of one suppressor, the cpxR mutant (Fig. 4B). This effect was more pronounced in exponentially growing cultures of the cpxR mutant (Fig. 4C). Diminished levels of DotA, encoded downstream of icmV, were also observed in the cpxR mutant (data not shown). Notably, IcmV levels appeared to be more affected than DotA or IcmW levels. Based on our previous observation that disruption of icmV or dotA, but not icmW, suppresses ΔdotL lethality (6), we hypothesized that decreased expression of IcmV and DotA was the mechanism of ΔdotL lethality suppression of the cpxR mutant. To test this, we transformed a plasmid containing the icmV dotA operon under a constitutive promoter or the corresponding vector into the cpxR::Kanr ΔdotL mutant. Although transformants were obtained with the vector, no transformants were recovered when the icmV dotA plasmid was transformed into this strain (data not shown). This result was dependent on inactivation of both dotL and cpxR, as transformants could be obtained in the cpxR::Kanr mutant background. Thus, the cpxR::Kanr mutant appears to suppress ΔdotL lethality through decreased levels of IcmV and DotA. However, the virulence defects of the remaining suppressor mutants do not appear to be due to altered expression or stability of the Dot/Icm T4SS but may instead be due to the improper assembly or activation of the secretion apparatus.
The ΔdotL suppressor mutants have varied effects on plasmid mobilization.
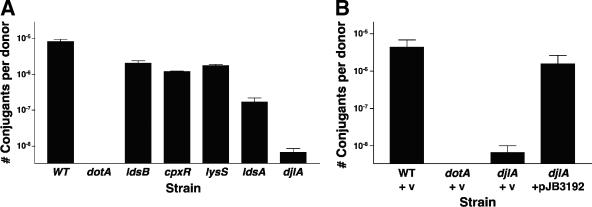
Having eliminated the most likely explanation for the ΔdotL suppressor phenotype, we employed a number of Dot/Icm-dependent assays to test the ΔdotL suppressor mutants for Dot/Icm T4SS functionality. The first assay we used is based on the ability of the L. pneumophila Dot/Icm T4SS to transfer a plasmid from one bacterium to another (39, 49). A wild-type L. pneumophila strain, JV4157, mobilized pKB5 with an efficiency of 8.1 × 10−6 transconjugants per donor, whereas a dotA mutant (JV4158) was completely deficient for plasmid transfer (limit of detection = 1 × 10−9) (Fig. 5A).
FIG. 5.
Ability of ΔdotL suppressors to mobilize a plasmid. Strains were assayed for the ability to mobilize an RSF1010 plasmid from one bacterium to another via the Dot/Icm secretion system. Strains containing the RSF1010 plasmid pKB5 were mated with E. coli strain ER1821, and transconjugants were selected on LB plates containing ampicillin. (A) Strains used were JV4157 (Lp02 plus pKB5), JV4158 (Lp03 plus pKB5), JV4163 (ldsB plus pKB5), JV4165 (cpxR cpxA plus pKB5), JV4164 (yitW lysS plus pKB5), JV4172 (ldsA plus pKB5), and JV4166 (djlA plus pKB5). (B) The conjugation defect of the djlA mutant can be complemented by the presence of the wild-type djlA gene. Strains JV4157 (wild type [WT] plus pKB5), JV4158 (dotA plus pKB5), JV4166 (djlA plus pKB5), and JV4313 (djlA plus djlA-complementing clone) were assayed for the ability to mobilize RSF1010 as described above. Data shown are the average number of transconjugants per L. pneumophila donor ± the standard deviation obtained from three reactions. v, vector.
The ldsB (JV4163), cpxR cpxA (JV4165), and lysS (JV4164) mutants were each able to conjugate pKB5 by the Dot/Icm system but with slightly lower efficiency than wild-type cells (2.0 × 10−6, 1.2 × 10−6, and 1.7 × 10−6 transconjugants per donor, respectively). Consistent with its more severe intracellular growth defect, the ldsA mutant (JV4172) was 48-fold less efficient than the wild type at plasmid transfer (1.7 × 10−7 transconjugants per donor). The djlA mutant (JV4166), which was severely attenuated for intracellular growth, was almost completely defective for plasmid transfer (6.7 × 10−9 transconjugants per donor). Although we had shown complementation of the intracellular growth defect of our djlA mutant (Fig. 3), we tested whether the conjugation defect of the djlA mutant could also be complemented. As expected, the plasmid mobilization defect of the djlA mutant could be restored to wild-type levels when a djlA-expressing plasmid, pJB3192, was present, confirming that the phenotype of the djlA mutant is specifically due to the transposon insertion (Fig. 5B). In summary, three of the suppressors exhibited only subtle defects in plasmid transfer, the ldsA mutant exhibited an intermediate defect, and the djlA mutant was severely defective.
The ΔdotL suppressor mutants have varied effects on contact-dependent cytotoxicity.
As an additional measure of Dot/Icm T4SS functionality, we assayed the ability of the ΔdotL suppressor mutants to cause rapid cytotoxicity of host macrophages. This form of cytotoxicity, also termed “contact-dependent cytotoxicity,” is observed at high MOI and results in host cell death consistent with the insertion of pores in the host cell membrane (23). Rapid contact-dependent cytotoxicity requires the majority of the Dot/Icm proteins, excluding the type IV adaptors IcmS and IcmW, and therefore accurately reflects the presence of an assembled and functional T4SS (12, 23).
To assay cytotoxicity, L. pneumophila strains were pelleted onto mouse bone marrow-derived macrophages at a range of MOI. After incubation for 1 hour, the infected macrophages were stained with acridine orange and ethidium bromide in order to determine their intactness. Live, intact cells stain green, whereas permeabilized cells stain red due to the uptake of ethidium bromide (23). Representative images of the cytotoxic effects of a wild-type strain, a dotA mutant, and the ΔdotL suppressor mutant strains are shown in Fig. 6A to H. Uninfected cells are green, as they are impermeable to ethidium bromide (Fig. 6A). Infection using a wild-type strain at an MOI of 50 bacteria/macrophage resulted in the majority of the macrophages being permeabilized (Fig. 6B). Infection using a dotA mutant at an identical MOI caused no cytotoxicity (Fig. 6C). Macrophages infected with the ldsB mutant (Fig. 6D), the cpxR cpxA mutant (Fig. 6E), and the lysS mutant (Fig. 6F) closely resembled those infected with wild-type cells (Fig. 6B). In contrast, the ldsA mutant (Fig. 6G) and the djlA mutant (Fig. 6H) were significantly less cytotoxic but did not appear to be as defective as the dotA mutant (compare Fig. 6G and H with Fig. 6C).
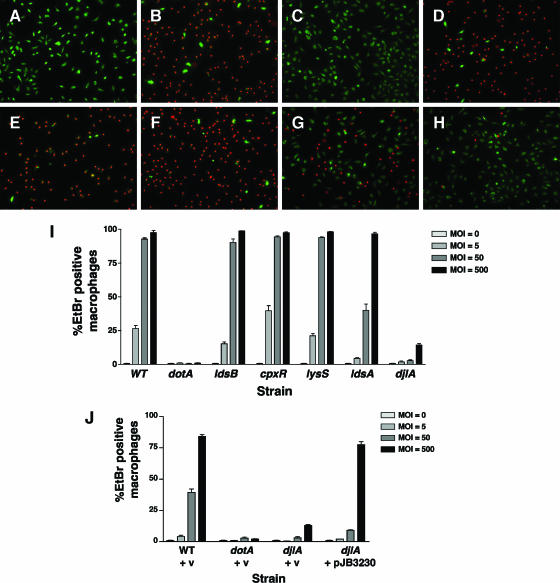
FIG. 6.
Contact-dependent cytotoxicity of the ΔdotL suppressors. Cytotoxic effects of L. pneumophila strains on mouse bone marrow-derived macrophages were examined using vital stains. Bacteria were incubated with mouse bone marrow-derived macrophages for 1 h. Cytotoxicity was assayed by staining with ethidium bromide and acridine orange. Live cells appear green, whereas permeabilized cells appear green with red nuclei. Representative images for infections done using an MOI of 50 are shown for the following strains: (A) uninfected macrophages, (B) Lp02 (wild type), (C) Lp03 (dotA), (D) JV1484 (ldsB), (E) JV1807 (cpxR cpxA), (F) JV1485 (yitW lysS)” (G) JV1835 (ldsA), and (H) JV1811 (djlA). (I) Quantitation of cytotoxicity, using four different MOI (0, 5, 50, or 500). Results are indicated as the percentage of ethidium bromide (EtBr)-positive-staining macrophages divided by the total number of macrophages observed per field. The averages and standard deviations for four randomly selected fields are shown for each strain at each MOI indicated. (J) Complementation of the cytotoxicity defect of the djlA mutant. Strains JV1139 (wild type [WT] plus pJB908), JV1141 (dotA plus pJB908), JV4077 (djlA plus pJB908), and JV4078 (djlA plus djlA-complementing clone) were assayed for contact-dependent cytotoxicity as described for panel I. v, vector.
To examine this phenotype in more detail, the infections were repeated using a range of MOI and cytotoxicity was quantitated (Fig. 6I). As before, the ldsB mutant (JV1484), the cpxR cpxA mutant (JV1807), and the lysS mutant (JV1485) closely resembled the wild-type strain (Lp02) at each MOI. The ldsA mutant appeared equally cytotoxic as the wild-type strain when assayed using the highest MOI (500), where both permeabilized nearly 100% of the macrophages. However, it was clearly attenuated when examined using an intermediate MOI (Fig. 6I). Quantitation of the djlA mutant cytotoxicity revealed that although it was severely attenuated at lower MOI, it retained some activity (14% cytotoxicity) when assayed using the highest MOI. This is strikingly in contrast to the dotA mutant, which was not cytotoxic at any MOI tested (Fig. 6I). As before, the defect in cytotoxicity of the djlA mutant could be complemented when djlA was supplied in trans on a plasmid (Fig. 6J). These results demonstrate that the Dot/Icm secretion complex functions normally in the ldsB, lysS, and cpxR cpxA mutants. However, the T4SS is not fully functional in the ldsA and djlA mutants, although both do retain some residual activity.
The ldsA and djlA mutants are defective in altering the endocytic pathway of host cells.
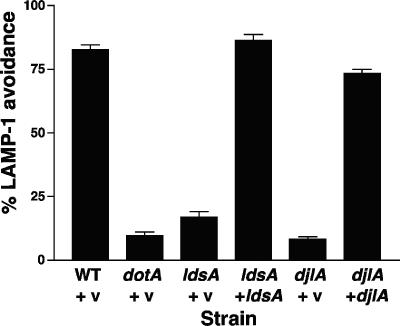
Since the ldsA and djlA mutants were the only suppressors that exhibited severe defects in dot/icm-related phenotypes, we examined the intracellular growth defect of these mutants further. It is possible that these mutants are impaired for growth within macrophages simply because they replicate at lower rates inside host cells. Alternatively, they could lack a function that is necessary for intracellular replication. For example, they could be unable to alter intracellular trafficking of the host cell, a process that absolutely requires a functional Dot/Icm type IV secretion system. To assess L. pneumophila avoidance of the endocytic pathway, we quantitated colocalization of Legionella-containing phagosomes with the endocytic marker LAMP-1 (48). As shown in Fig. 7, the majority of wild-type L. pneumophila bacteria did not colocalize with LAMP-1, whereas only 10% of dotA mutants were LAMP-1 negative. Both the ldsA mutant and the djlA mutant resembled the dotA mutant in that they were severely defective in altering the endocytic pathway. The ldsA mutant was slightly less attenuated than the dotA mutant for avoiding the endocytic marker LAMP-1 (17% LAMP-1 negative), while the djlA mutant was indistinguishable from the dotA mutant (8% LAMP-1 negative). As observed with intracellular growth, the targeting defect of each of the mutants was fully restored by introduction of complementing clones (86% and 73% LAMP-1 avoidance, respectively). Because the severity of the intracellular growth defect correlated with the defect in targeting for both mutants, it seems likely that the observed defects in intracellular growth are caused solely by the inability to alter the host endocytic pathway.
FIG. 7.
The ldsA mutant and the djlA mutant are defective in altering the host cell endocytic pathway. Colocalization of the endocytic marker LAMP-1 with intracellular bacteria was assayed by immunofluorescence microscopy. Strains JV1139 (wild type [WT] plus pJB908), JV1141 (dotA plus pJB908), JV4358 (ldsA plus pJB908), JV4359 (ldsA plus ldsA-complementing clone), JV4077 (djlA plus pJB908), and JV4078 (djlA plus djlA-complementing clone) were used to infect mouse bone marrow-derived macrophages for 1 hour prior to fixation and staining for intracellular versus extracellular bacteria and for LAMP-1. Avoidance of LAMP-1 by intracellular L. pneumophila is shown as the average and standard deviation of three counts of 100 bacteria. v, vector.
The ldsA and djlA mutants are defective in the secretion of the Dot/Icm substrate SdeC.
Alteration of the host endocytic pathway by the Dot/Icm T4SS is mediated by the export of protein substrates into the cytoplasm of the host cell (4, 13, 14, 26, 30, 32). Export of a representative substrate, SdeC, can be visualized by immunofluorescence staining of infected macrophages with an antibody raised against SdeC (4). Similarly to other L. pneumophila T4SS substrates, SdeC is secreted into the host cytoplasm, adjacent to the poles of the bacterium, where it remains associated with the phagosome (4).
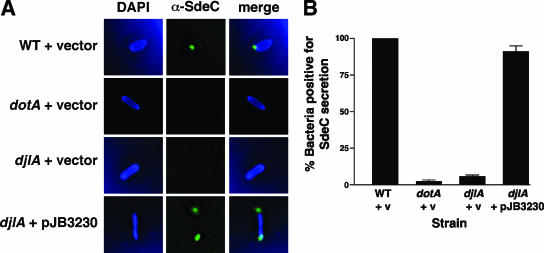
To assay SdeC export by the ldsA and djlA mutants, the strains were added to mouse bone marrow-derived macrophages, and infections were allowed to proceed for 30 min prior to fixation and staining. DAPI (blue) was used to stain macrophage and bacterial DNAs, whereas the SdeC antibody was decorated with a goat anti-rabbit antibody conjugated to Oregon green. Representative images of each strain are shown in Fig. 8A, with quantitation provided in Fig. 8B. As described previously (4), wild-type cells (JV1139) frequently exhibit foci of SdeC staining at one or both poles of the phagosome, whereas dotA mutants (JV1141) do not. Consistent with the defect in altering the endocytic pathway as observed by LAMP-1 colocalization, the djlA mutant (JV4077) was almost completely defective for SdeC secretion (Fig. 8A and B). The ldsA mutant was also defective for SdeC export, although not to the same extent (data not shown). Secretion of SdeC was fully restored in each mutant by the introduction of the corresponding complementing clone (Fig. 8 and data not shown). Thus, the intracellular growth defect of the ΔdotL suppressor mutants ldsA and djlA appears to be due to a failure to properly export T4SS substrates and therefore a failure to alter the endocytic pathway of the host cell.
FIG. 8.
The djlA mutant is defective in export of substrates of the Dot/Icm secretion system. Dot/Icm-mediated secretion of SdeC was assayed by immunofluorescence microscopy. Mouse bone marrow-derived macrophages were infected with various L. pneumophila strains for 30 minutes prior to fixation and staining with DAPI (to detect DNA) and a polyclonal serum that detects SdeC. (A) Representative immunofluorescence images of macrophages infected with strains JV1139 (wild type [WT] plus pJB908), JV1141 (dotA plus pJB908), JV4077 (djlA plus pJB908), and JV4078 (djlA plus djlA-complementing clone). DAPI staining for DNA is shown in the left panels, SdeC staining is shown in the center panels, and merged images are shown in the right panels. (B) The results in panel A were quantitated by scoring intracellular L. pneumophila cells for the presence of SdeC on the surface of the phagosome. The results were normalized to the amount of SdeC secretion observed with wild-type L. pneumophila set at 100%. Shown are the averages and standard deviations of three counts of 100 intracellular bacteria. v, vector.
DISCUSSION
We report here the characterization of five genes that were isolated in a screen for mini-Tn10 insertions able to suppress the lethality caused by loss of dotL. The insertions were in cpxR, djlA, yitW, and two novel genes, which we have named ldsA and ldsB (lethality of ΔdotL suppressor). Each of these suppressor mutations conferred the ability of L. pneumophila to survive in the absence of dotL. When assayed in a dotL+ background, these mutants exhibited differential effects on Dot/Icm-dependent assays. These results support the hypothesis that there are multiple means to suppress ΔdotL lethality, including by decreasing expression of dot/icm genes and by diminishing the assembly and/or activity of the L. pneumophila Dot/Icm secretion system.
The CpxRA (conjugative plasmid expression) stress response system is a two-component regulatory system induced in response to extracytoplasmic signals generated in the cell envelope, principally misfolded proteins in the periplasm (34, 37). Once these signals are detected, the system is responsible for activating the expression of a number of factors required for responding to cell envelope stress (35). In addition to a role in F plasmid conjugation (28), the Cpx system has been implicated in several virulence mechanisms, including invasion (Salmonella enterica), transcriptional regulation (Shigella sonnei), P-pilus biogenesis (uropathogenic E. coli), and assembly of type IV bundle-forming pili (enteropathogenic E. coli) (reviewed in reference 34).
Based on our model of ΔdotL toxicity and the important role of the Cpx system in responding to periplasmic stress in other organisms, it is not surprising that this system is involved in the response to loss of dotL. We hypothesized that the cpx mutation could affect expression of dot/icm genes, thereby suppressing ΔdotL lethality due to lowered levels of Dot/Icm proteins. This hypothesis was supported by a previous study, using β-galactosidase reporters, that showed decreased transcription of icmR, icmV, and icmW in a cpxR mutant (16). Although we did not observe that IcmR protein levels were significantly affected by a cpxR insertion (Fig. 4A), we did observe an effect on DotA, IcmV, and IcmW. Interestingly, the cpxR::Kanr mutant had only a minor effect on the levels of IcmX (Fig. 4A), a protein encoded downstream of icmW. By assaying the effects of overexpression of IcmV and DotA, we were able to demonstrate that decreased levels of IcmV and DotA were solely responsible for suppression of ΔdotL lethality in the cpxR::Kanr mutant. This is consistent with our previous observation that disruption of icmV or dotA, but not icmW, suppresses ΔdotL lethality (6). Surprisingly, although decreased IcmV and DotA levels were sufficient to suppress loss of dotL in the cpxR::Kanr mutant, it was still able to conjugate and remained cytotoxic. Thus, the ΔdotL lethality phenotype is more sensitive to decreased levels of Dot/Icm proteins than other Dot/Icm-associated traits.
The remaining non-dot/icm suppressors described here likely represent mutations that indirectly affect the activity of the L. pneumophila T4SS or mutations that do not affect the Dot/Icm complex itself but allow the strain to survive in the absence of dotL. These mutations can be subdivided into three phenotypic classes: (i) mutations that affect virulence by disrupting the assembly and/or the activation of the Dot/Icm system (e.g., ldsA and djlA), (ii) mutations that negatively affect the ability of the strain to grow in macrophages but do not appear to grossly affect the assembly and function of the Dot/Icm complex (e.g., lysS), and (iii) mutations that do not affect any of the virulence-related assays (e.g., ldsB). Before examining the overall implications of these suppressors, we will discuss each class separately.
The first class of mutants reported here includes the ldsA and djlA mutants. Each of these mutants was much more attenuated in intracellular growth than the other suppressor mutants, and the djlA mutant was completely defective for growth in macrophages. Further analysis revealed that the reason for the attenuation of these mutants is that they likely affect the assembly and/or activity of the Dot/Icm apparatus. Although these mutants still make wild-type levels of Dot/Icm proteins and substrates, they are less efficient than the wild type at avoiding phagosome-lysosome fusion and exhibit decreased Dot/Icm-mediated plasmid transfer and contact-dependent cytotoxicity. Finally, both mutants are severely attenuated for the secretion of a T4SS substrate, SdeC, into the cytoplasm of macrophages. Thus, as predicted, our ΔdotL lethality suppressor screen did identify factors involved in the assembly or activity of the Dot/Icm apparatus.
The ldsA gene encodes a polytopic inner membrane protein that does not contain any distinguishing motifs, making prediction of its molecular function difficult. Based on the protein's putative membrane localization and the Dot/Icm-related defects associated with loss of LdsA, it is formally possible that LdsA is an additional Dot/Icm protein. However, we do not favor this possibility since LdsA lacks homology to a component of any known type IV secretion system and is encoded distantly from the two Dot/Icm pathogenicity islands (49).
In contrast to the ldsA mutant, it is easier to propose an explanation for the djlA mutant. DjlA (for DnaJ-like protein) is an inner membrane-anchored homolog of DnaJ with its J domain located in the cytoplasm (11). Similarly to DnaJ, E. coli DjlA is known to interact with the DnaK (Hsp70) chaperone and stimulate its ATPase activity (50). DjlA has been proposed to function as a chaperone for the assembly and/or activity of membrane proteins and may play a role as a sensor of envelope stress (21). The involvement of a stress response system in the suppression of ΔdotL lethality is consistent with our model that the absence of DotL leads to the accumulation of a toxic subcomplex in the envelope of cells. Based on the proposed activities of E. coli DjlA, the highly conserved L. pneumophila homolog is likely to be involved in sensing misfolded proteins in the membrane and assisting in their proper folding. Therefore, L. pneumophila may require DjlA to properly fold and assemble components of the Dot/Icm complex. Loss of djlA would therefore phenocopy dot/icm mutations and suppress ΔdotL lethality by a similar mechanism. Additionally, djlA has also been implicated as being required for the intracellular growth of another Legionella species, Legionella dumoffii (33). Because our djlA mutant still appears to synthesize components of the Dot/Icm apparatus and yet is severely defective for all Dot/Icm-dependent traits, it likely serves as a key factor in the assembly/quality control of the L. pneumophila T4SS. To our knowledge, DjlA is the first such factor identified.
The second class of mutations reported here includes an insertion in yitW that affects the expression of the lysyl-tRNA synthetase gene lysS. This mutation caused subtle defects in intracellular growth in mouse bone marrow-derived macrophages. However, the mutant did not display a significant defect in plasmid transfer or contact-dependent cytotoxicity, traits requiring a functional Dot/Icm secretion apparatus. LysS is an aminoacyl-tRNA synthetase that catalyzes the formation of Lys-tRNALys, which is then used to insert lysine into proteins (31). It was surprising to obtain an insertion that appeared to down-regulate the expression of lysS, since most tRNA synthetases are essential. However, it is possible that the yitW::Kanr allele exhibited only an incomplete polar effect on lysS, thereby partially down-regulating its expression. Alternatively, lysS may not be essential because Legionella possesses a homolog of lysU, which encodes a secondary, inducible form of lysyl-tRNA synthetase in other bacterial species (31). Although the molecular mechanism of suppression by inactivation of lysS remains cryptic, the Lys-tRNALys molecule has been implicated in additional roles, including functioning as a signaling molecule (31). It is thus possible that the mechanism of lysS suppression of ΔdotL lethality is indirect and may be unlinked to its role in protein synthesis.
The insertion in ldsB was the only mutant in the third class identified in this screen whose inactivation suppressed ΔdotL lethality but did not affect the virulence of a dotL+ strain of L. pneumophila. LdsB is predicted to be a novel polytopic, inner membrane protein that does not possess any apparent protein motifs, thus precluding an obvious prediction of function. Although we do not understand the molecular mechanism of ldsB suppression of ΔdotL lethality, the ability of this mutant to replicate normally in macrophages suggests that the suppression may be mediated by an indirect mechanism of action (see below). Nevertheless, the identification of ldsB is important for two reasons. First, since a ΔdotL strain is not viable in the Lp02 background, it was previously only possible to examine this strain lacking dotL in the presence of another dot/icm mutation, e.g., ΔdotL ΔdotA, thus severely complicating the analysis of the ΔdotL phenotype. As a result of this discovery, it is now possible to examine the dot/icm phenotypes of Lp02 lacking dotL in the ΔdotL ΔldsB strain. Second, the existence of a ΔdotL lethality suppressor that does not affect the intracellular replication of L. pneumophila provides credence to our proposal that the JR32 ΔdotL strain is viable because the JR32 strain background contains a suppressor mutation (6).
Based on our initial observation that dot/icm mutations could suppress loss of dotL, we proposed that an L. pneumophila ΔdotL strain is not viable because it accumulates a toxic substrate in the cell envelope. This poisonous structure could be a misfolded Dot/Icm subcomplex, similar to pilin subunits that accumulate in the absence of the P-pilus chaperone PilD (25). Alternatively, the toxic substrate might be a normally assembled Dot/Icm subcomplex that functions improperly in the absence of DotL, perhaps as an unregulated secretion pore that leads to a loss of homeostasis. The former model implies that accumulation of a subcomplex, likely to consist of a few Dot/Icm proteins, is responsible for the toxicity. If this were the case, inactivation of only a few dot/icm genes should suppress ΔdotL lethality. Instead, inactivation of almost any dot/icm gene suppresses loss of dotL, which is more consistent with the toxicity being due to an improperly functioning Dot/Icm complex.
Taken in this context, inactivation of factors that are required for the proper assembly or activity of the Dot/Icm complex, such as DjlA, should also suppress ΔdotL lethality. However, the existence of suppressors that replicate in macrophages and have only subtle or no effects on the Dot/Icm complex in a dotL+ background is perplexing. We propose two possible explanations for this conundrum. First, the number of functional Dot/Icm complexes required for intracellular growth may be significantly less than the number of toxic ΔdotL complexes required for cell death. In this case, a mutation that decreases the number of functional complexes may be able to suppress ΔdotL lethality but have no effect on intracellular growth in a wild-type background. Alternatively, one could imagine a type of mutation that induces a response capable of allowing a ΔdotL strain to live that is not needed for the normal assembly/activity of the complex. For example, inactivation of a repressor of a periplasmic protease might allow the elimination of a toxic Dot/Icm subcomplex but yet not be required in a wild-type cell. In either case, further examination of the ΔdotL lethality suppressors should reveal insights into both the normal assembly/activity of the T4SS and envelope stress response systems in L. pneumophila.
Acknowledgments
We thank James Kirby for the generous gift of pJK211-2 and Petra Levin and Jessica Sexton for critical reading of the manuscript.
This work was funded by NIH grant AI48052 to J.P.V.
Footnotes
Published ahead of print on 22 September 2006.
REFERENCES
- 1.Abu-Zant, A., M. Santic, M. Molmeret, S. Jones, J. Helbig, and Y. Abu Kwaik. 2005. Incomplete activation of macrophage apoptosis during intracellular replication of Legionella pneumophila. Infect. Immun. 73:5339-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amer, A. O., and M. S. Swanson. 2005. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell. Microbiol. 7:765-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, H. L., J. P. Vogel, and R. R. Isberg. 1998. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect. Immun. 66:950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardill, J. P., J. L. Miller, and J. P. Vogel. 2005. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol. Microbiol. 56:90-103. [DOI] [PubMed] [Google Scholar]
- 5.Berger, K. H., and R. R. Isberg. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7-19. [DOI] [PubMed] [Google Scholar]
- 6.Buscher, B. A., G. M. Conover, J. L. Miller, S. A. Vogel, S. N. Meyers, R. R. Isberg, and J. P. Vogel. 2005. The DotL protein, a member of the TraG-coupling protein family, is essential for viability of Legionella pneumophila strain Lp02. J. Bacteriol. 187:2927-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazalet, C., C. Rusniok, H. Bruggemann, N. Zidane, A. Magnier, L. Ma, M. Tichit, S. Jarraud, C. Bouchier, F. Vandenesch, F. Kunst, J. Etienne, P. Glaser, and C. Buchrieser. 2004. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36:1165-1173. [DOI] [PubMed] [Google Scholar]
- 9.Chien, M., I. Morozova, S. Shi, H. Sheng, J. Chen, S. M. Gomez, G. Asamani, K. Hill, J. Nuara, M. Feder, J. Rineer, J. J. Greenberg, V. Steshenko, S. H. Park, B. Zhao, E. Teplitskaya, J. R. Edwards, S. Pampou, A. Georghiou, I. C. Chou, W. Iannuccilli, M. E. Ulz, D. H. Kim, A. Geringer-Sameth, C. Goldsberry, P. Morozov, S. G. Fischer, G. Segal, X. Qu, A. Rzhetsky, P. Zhang, E. Cayanis, P. J. De Jong, J. Ju, S. Kalachikov, H. A. Shuman, and J. J. Russo. 2004. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305:1966-1968. [DOI] [PubMed] [Google Scholar]
- 10.Christie, P. J., and J. P. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke, D. J., A. Jacq, and I. B. Holland. 1996. A novel DnaJ-like protein in Escherichia coli inserts into the cytoplasmic membrane with a type III topology. Mol. Microbiol. 20:1273-1286. [DOI] [PubMed] [Google Scholar]
- 12.Coers, J., J. C. Kagan, M. Matthews, H. Nagai, D. M. Zuckman, and C. R. Roy. 2000. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol. Microbiol. 38:719-736. [DOI] [PubMed] [Google Scholar]
- 13.Conover, G. M., I. Derre, J. P. Vogel, and R. R. Isberg. 2003. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol. Microbiol. 48:305-321. [DOI] [PubMed] [Google Scholar]
- 14.de Felipe, K. S., S. Pampou, O. S. Jovanovic, C. D. Pericone, S. F. Ye, S. Kalachikov, and H. A. Shuman. 2005. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J. Bacteriol. 187:7716-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gal-Mor, O., and G. Segal. 2003. Identification of CpxR as a positive regulator of icm and dot virulence genes of Legionella pneumophila. J. Bacteriol. 185:4908-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz, M. A., and F. R. Maxfield. 1984. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell Biol. 99:1936-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagan, J. C., and C. R. Roy. 2002. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4:945-954. [DOI] [PubMed] [Google Scholar]
- 21.Kelley, W. L., and C. Georgopoulos. 1997. Positive control of the two-component RcsC/B signal transduction network by DjlA: a member of the DnaJ family of molecular chaperones in Escherichia coli. Mol. Microbiol. 25:913-931. [DOI] [PubMed] [Google Scholar]
- 22.Kessler, B., V. de Lorenzo, and K. N. Timmis. 1992. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol. Gen. Genet. 233:293-301. [DOI] [PubMed] [Google Scholar]
- 23.Kirby, J. E., J. P. Vogel, H. L. Andrews, and R. R. Isberg. 1998. Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol. 27:323-336. [DOI] [PubMed] [Google Scholar]
- 24.Kolter, R., M. Inuzuka, and D. R. Helinski. 1978. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell 15:1199-1208. [DOI] [PubMed] [Google Scholar]
- 25.Lee, Y. M., P. A. DiGiuseppe, T. J. Silhavy, and S. J. Hultgren. 2004. P pilus assembly motif necessary for activation of the CpxRA pathway by PapE in Escherichia coli. J. Bacteriol. 186:4326-4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo, Z. Q., and R. R. Isberg. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 101:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marra, A., and H. A. Shuman. 1989. Isolation of a Legionella pneumophila restriction mutant with increased ability to act as a recipient in heterospecific matings. J. Bacteriol. 171:2238-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen, J., and P. Silverman. 1980. Chromosomal mutations of Escherichia coli that alter expression of conjugative plasmid functions. Proc. Natl. Acad. Sci. USA 77:513-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merriam, J. J., R. Mathur, R. Maxfield-Boumil, and R. R. Isberg. 1997. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect. Immun. 65:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679-682. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura, Y., and K. Ito. 1993. Control and function of lysyl-tRNA synthetases: diversity and co-ordination. Mol. Microbiol. 10:225-231. [DOI] [PubMed] [Google Scholar]
- 32.Ninio, S., D. M. Zuckman-Cholon, E. D. Cambronne, and C. R. Roy. 2005. The Legionella IcmS-IcmW protein complex is important for Dot/Icm-mediated protein translocation. Mol. Microbiol. 55:912-926. [DOI] [PubMed] [Google Scholar]
- 33.Ohnishi, H., Y. Mizunoe, A. Takade, Y. Tanaka, H. Miyamoto, M. Harada, and S. Yoshida. 2004. Legionella dumoffii DjlA, a member of the DnaJ family, is required for intracellular growth. Infect. Immun. 72:3592-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raivio, T. L. 2005. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol. Microbiol. 56:1119-1128. [DOI] [PubMed] [Google Scholar]
- 35.Raivio, T. L., and T. J. Silhavy. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179:7724-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson, C. G., and C. R. Roy. 2006. Attachment and fusion of endoplasmic reticulum with vacuoles containing Legionella pneumophila. Cell Microbiol. 8:793-805. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz, N., and T. J. Silhavy. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122-126. [DOI] [PubMed] [Google Scholar]
- 38.Sadosky, A. B., L. A. Wiater, and H. A. Shuman. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61:5361-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA 95:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segal, G., and H. A. Shuman. 1999. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 67:2117-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seshadri, R., I. T. Paulsen, J. A. Eisen, T. D. Read, K. E. Nelson, W. C. Nelson, N. L. Ward, H. Tettelin, T. M. Davidsen, M. J. Beanan, R. T. Deboy, S. C. Daugherty, L. M. Brinkac, R. Madupu, R. J. Dodson, H. M. Khouri, K. H. Lee, H. A. Carty, D. Scanlan, R. A. Heinzen, H. A. Thompson, J. E. Samuel, C. M. Fraser, and J. F. Heidelberg. 2003. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. USA 100:5455-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sexton, J. A., J. L. Miller, A. Yoneda, T. E. Kehl-Fie, and J. P. Vogel. 2004. Legionella pneumophila DotU and IcmF are required for stability of the Dot/Icm complex. Infect. Immun. 72:5983-5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sexton, J. A., J. S. Pinkner, R. Roth, J. E. Heuser, S. J. Hultgren, and J. P. Vogel. 2004. The Legionella pneumophila PilT homologue DotB exhibits ATPase activity that is critical for intracellular growth. J. Bacteriol. 186:1658-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sexton, J. A., and J. P. Vogel. 2004. Regulation of hypercompetence in Legionella pneumophila. J. Bacteriol. 186:3814-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sexton, J. A., and J. P. Vogel. 2002. Type IVB secretion by intracellular pathogens. Traffic 3:178-185. [DOI] [PubMed] [Google Scholar]
- 46.Sexton, J. A., H. J. Yeo, and J. P. Vogel. 2005. Genetic analysis of the Legionella pneumophila DotB ATPase reveals a role in type IV secretion system protein export. Mol. Microbiol. 57:70-84. [DOI] [PubMed] [Google Scholar]
- 47.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanson, M. S., and R. R. Isberg. 1996. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect. Immun. 64:2585-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel, J. P., H. L. Andrews, S. K. Wong, and R. R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873-876. [DOI] [PubMed] [Google Scholar]
- 50.Wall, D., M. Zylicz, and C. Georgopoulos. 1994. The NH2-terminal 108 amino acids of the Escherichia coli DnaJ protein stimulate the ATPase activity of DnaK and are sufficient for lambda replication. J. Biol. Chem. 269:5446-5451. [PubMed] [Google Scholar]