Abstract
An extensive literature has established that the synthesis of wall teichoic acid in Bacillus subtilis is essential for cell viability. Paradoxically, we have recently shown that wall teichoic acid biogenesis is dispensable in Staphylococcus aureus (M. A. D'Elia, M. P. Pereira, Y. S. Chung, W. Zhao, A. Chau, T. J. Kenney, M. C. Sulavik, T. A. Black, and E. D. Brown, J. Bacteriol. 188:4183-4189, 2006). A complex pattern of teichoic acid gene dispensability was seen in S. aureus where the first gene (tarO) was dispensable and later acting genes showed an indispensable phenotype. Here we show, for the first time, that wall teichoic acid synthesis is also dispensable in B. subtilis and that a similar gene dispensability pattern is seen where later acting enzymes display an essential phenotype, while the gene tagO, whose product catalyzes the first step in the pathway, could be deleted to yield viable mutants devoid of teichoic acid in the cell wall.
The bacterial cell wall is a complex meshwork of carbohydrates and amino acids linked as a rigid structure termed peptidoglycan, which is responsible for a variety of cellular functions, including growth, division, maintenance of shape, and protection from osmotic stress (10). In gram-positive organisms, in addition to this dense layer of peptidoglycan, the cell wall contains an equal amount of a highly charged anionic polymer of polyol phosphate, called wall teichoic acid. Although variability exists among the polymers from various organisms, these polymers have been found in all gram-positive bacteria studied. Remarkably, despite its discovery nearly 50 years ago, the cellular function of wall teichoic acid remains speculative. Nevertheless, a significant body of literature using the model organism Bacillus subtilis has identified a requirement for teichoic acid polymers in cell viability (3).
Beginning with temperature-sensitive mutants and more recently with the creation of deletion strains that were conditionally complemented using a tightly regulated promoter, nearly every gene responsible for wall teichoic acid biosynthesis has been shown to be required for viability in B. subtilis (2, 4, 6, 7, 15). In contrast, we recently demonstrated that wall teichoic acid was dispensable in Staphylococcus aureus (8). Paradoxically, that work indicated that the first step in polymer synthesis was dispensable, while the later steps were not (8). This apparent contradiction was resolved with the finding that a lesion in the first step of the biochemical pathway (TarO) suppressed the lethal phenotype associated with mutations in the later steps. Here, we have reevaluated the dispensability of teichoic acid biosynthesis genes in B. subtilis, with particular attention to the dispensability of the first biosynthetic step encoded in tagO (orthologue of tarO).
The tagO gene was the subject of a relatively recent dispensability study of B. subtilis where the failure to create insertional mutants led to the conclusion that disruption of tagO was lethal to the cell (16). In the work reported here, we employed a precise deletion strategy using double recombination of a PCR product targeting tagO. The PCR product contained a central erythromycin cassette flanked by 1,000-bp regions 5′ and 3′ of tagO. To our surprise, we were able to successfully create a strain with a deletion in tagO (EB1451) that was viable but slow growing (Table 1 shows the strains and plasmids used in this study). The failure in the previous study (16) to isolate mutants in tagO by insertional inactivation may stem from the slow growth and altered colony morphology of this mutant. These colonies were significantly smaller and smoother than colonies of wild-type B. subtilis but could be repeatedly subcultured onto fresh medium (data not shown). Additionally, transformation (11) of chromosomal DNA from the deletion strain back into the wild-type background (EB6) occurred at a frequency within twofold that obtained by an unlinked, dispensable marker (data not shown) and gave rise to colonies with growth rates and morphology identical to those of the donor strain, arguing against the existence of a secondary site mutation leading to viability.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| B. subtilis | ||
| EB6 | hisA1 argC4 metC3 | L5087 (2) |
| EB240 | hisA1 argC4 metC3 amyE::xylR PxylAtagD cat86 tagD::Specr | 2 |
| EB633 | hisA1 argC4 metC3 amyE::xylR PxylAtagB cat86 tagB::Specr | 4 |
| EB669 | hisA1 argC4 metC3 amyE::xylR PxylAtagF cat86 tagF::Specr | 4 |
| EB892 | EB6 transformed with pRBtagBgfp | 5 |
| EB1451 | hisA1 argC4 metC3 tagO::Ermr | This study |
| EB1453 | hisA1 argC4 metC3 amyE::xylR PxylAtagB cat86 tagB::SpecrtagO::Ermr | This study |
| EB1559 | hisA1 argC4 metC3 amyE::xylR PxylAtagD cat86 tagD::SpecrtagO::Ermr | This study |
| EB1560 | hisA1 argC4 metC3 amyE::xylR PxylAtagF cat86 tagF::SpecRtagO::Ermr | This study |
| Plasmids | ||
| pMUTIN4 | IPTGa-inducible integration vector, source of Ermr cassette | 17 |
| pUS19 | pUC19 derivative containing Specr cassette | 1 |
IPTG, isopropyl-β-d-thiogalactopyranoside.
Because a deletion in tagO is expected to disrupt the first step of wall teichoic acid biosynthesis, we reasoned that the deletion strain should be devoid of any wall teichoic acid. Using previously established protocols, the cell walls from both the wild type and the deletion strain were isolated, and the phosphate content was analyzed (4). Compared to the wild type, the cell wall phosphate content was decreased by nearly 95% in the tagO null mutant (EB1451) (2.01 ± 0.04 μg phosphate/mg cell wall versus 0.14 ± 0.02 μg phosphate/mg cell wall). These data support the absence of teichoic acid in the cell wall and indicate that the activity of TagO was not bypassed by an alternative biosynthetic mechanism.
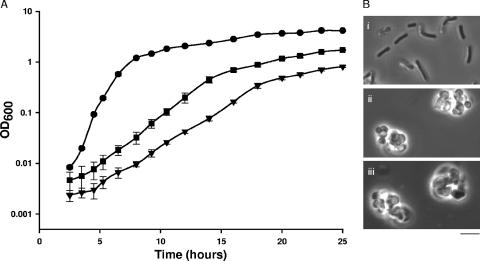
Further characterization of the tagO deletion strain was performed through the investigation of the growth kinetics by comparison to the wild type in Luria-Bertani broth (Fig. 1A). It is clear that the failure to synthesize teichoic acid had a drastic effect on the growth of B. subtilis. The lag phase of the mutant strain was considerably longer than that of the wild type and was coupled with a decreased growth rate. The growth kinetics were also examined with the addition of 20 mM MgCl2 in the medium. Previous reports have demonstrated that Mg2+ supplement in the medium has a positive effect on the growth of certain morphology mutants (9, 13). The most dramatic effect was observed with an mreB mutant whose viability was dependent on the addition of Mg2+. Although the addition of MgCl2 does not restore growth of the tagO deletion mutant to wild-type levels, supplementation resulted in a shorter lag phase and increased growth rate (doubling time of 1.4 ± 0.1 h for the supplemented cultures versus 2.1 ± 0.1 h for the nonsupplemented cultures). Although the effect of Mg2+ on the enhancement of growth is not well understood, several explanations have been suggested. Most proposals have implied some impact on peptidoglycan structure or the stabilization of cell wall-enzyme complexes that are relevant to cell wall remodelling or synthesis (9). Furthermore, given the potential role for teichoic acid polymers in binding Mg2+ ions (12), supplementation of this ion might compensate for the loss of teichoic acid polymers in the cell wall.
FIG. 1.
Growth of tagO deletion mutant. (A) Growth analysis was performed in LB for EB6, i.e., the wild-type B. subtilis (•), and the tagO deletion strain (EB1451) grown in the presence (▪) and absence (▾) of MgCl2. Cultures were inoculated at a starting optical density at 600 nm (OD600) of 0.001, and absorbance measurements were taken every 1 or 2 h. (B) Phase-contrast microscopy was performed on stationary-phase cultures of the (i) wild-type strain and the tagO deletion strain grown in the (ii) presence and (iii) absence of MgCl2. Bar, 5 μm.
Light microscopy and transmission electron microscopy in the presence and absence of MgCl2 are shown in Fig. 1B and 2, respectively. Light microscopy of the tagO mutant revealed a loss of the rod shape and swelling of the cell volume in addition to cell aggregation. These phenotypes were not alleviated by the addition of MgCl2. Interestingly, these characteristics were previously evident in micrographs of a TagO-depleted strain that were published by Soldo et al. (16). Transmission electron microscopy in the work reported here revealed aberrant septation and nonuniform thickening of the peptidoglycan layer, hallmarks associated with a loss of teichoic acid in B. subtilis (2). From these findings, it is clear that the loss of teichoic acid polymers has a dramatic effect on the cellular morphology of B. subtilis.
FIG. 2.
Ultrastructure of B. subtilis lacking wall teichoic acid. Strains of B. subtilis 168 were harvested at late log phase of growth and conventionally embedded in thin sections for examination with transmission electron microscopy as described previously (14). The (A) wild-type strain (EB6) along with the tagO deletion mutant (EB1451) in the (B) absence and (C) presence of MgCl2 are depicted. Arrows highlight areas of thickened cell wall. Bar, 500 nm.
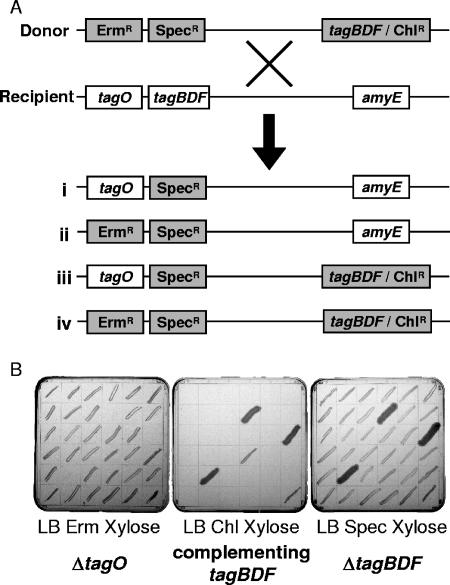
Given the surprising dispensability pattern associated with teichoic acid biosynthesis genes in S. aureus, where the first step was dispensable and remaining steps had an essential phenotype (8), we were interested, in this work, to reevaluate the dispensability of several late-acting teichoic acid genes (tagB, tagD, and tagF) in B. subtilis. The low transformability of B. subtilis makes it difficult to differentiate between a failed transformation and a lethal event; therefore, we endeavored to examine the dispensability of these late-acting genes by congression analysis (transformation of chromosomal DNA into the recipient strain and analysis of resistance markers transferred). Strains were generated that contained a deletion of tagO (marked with Ermr, ermAB); a deletion of the late-acting gene tagB, tagD, or tagF [marked with Specr, AAD(9)]; and a complementing copy of the late-acting gene at amyE (marked with Chlr, cat86). Each strain was produced by transforming chromosomal DNA from the tagO deletion strain (EB1451) into the complemented deletion strains of tagB (EB633), tagD (EB240), and tagF (EB669), giving rise to strains EB1453, EB1559, and EB1560.
Chromosomal DNA from each of the strains constructed (EB1453, EB1559, and EB1560) was transformed into a wild-type background (EB6) and growth selected on LB medium containing spectinomycin (150 μg/ml) and xylose (2%). After 2 days, 100 colonies from each transformation were examined for erythromycin and/or chloramphenicol resistance. Figure 3A provides a schematic of the experimental methodology and possible outcomes expected. Figure 3B shows the outcome of a typical experiment where 36 clones were chosen from the transformation of chromosomal DNA of strain EB1559 into strain EB6. Here, 31 clones were Specr Ermr, 3 clones were Specr Chlr, and 2 clones were Specr Ermr Chlr. Notably, we were unable to generate any clones that were solely Specr, suggesting that tagD is indeed essential and that it is only possible to obtain a deletion of tagD if it is accompanied by a complementing copy or by a deletion of tagO. These results were echoed in larger scale screens performed for tagB, tagD, and tagF outlined in Table 2. In each case, the majority of clones (80 to 90%) were Specr Ermr. Under no circumstances were clones generated that were exclusively Specr. To confirm that Specr could be unlinked from Ermr and/or Chlr, a similar congression sought to transform chromosomal DNA from strain EB1453 into EB892 (a strain containing a plasmid-borne copy of tagB). Here 24 of the 25 clones selected were Specr Erms Chls, demonstrating that the Specr marker could be unlinked from the other two, indicating that the tagO locus can be unlinked from the tagB locus and therefore the entire tag operon. Taken together, these data support the conclusion that the first enzyme of the teichoic acid biosynthesis pathway is dispensable, yet the remaining enzymes, at least tagB and beyond, are indispensable for viability. Furthermore, the ability to isolate clones that were Specr and Ermr yet Chls indicates that the essential nature of tagB, tagD, and tagF can be suppressed by a deletion in tagO. These data parallel those obtained using S. aureus as a model, and thus, the peculiar dispensability pattern seen in these organisms may be a mechanistic feature associated with teichoic acid biosynthesis genes in gram-positive bacteria.
FIG. 3.
Testing tag gene dispensability in B. subtilis. (A) To address the dispensability of tagB, tagD and tagF donor strains were created containing deletions of tagO (marked with Ermr) and one copy of tagB, tagD, or tagF (marked with Specr) (tagBDF) that contained a complementing copy of tagBDF at amyE (marked with Chlr). Transformation into a recipient (wild-type) strain and selection on spectinomycin (Spec) (150 μg/ml) and xylose (2%) could allow for four possible outcomes (i to iv). (B) The outcome of this selection procedure performed to test the dispensability of tagD is depicted. In addition to showing Specr, all of the clones selected were also Ermr and/or Chlr. Erm, erythromycin; Chl, chloramphenicol.
TABLE 2.
Testing the dispensability of the late-acting teichoic acid gene product
| Gene tested | Donor strain | Recipient strain | No. of clones that were phenotype:
|
|||
|---|---|---|---|---|---|---|
| Specr Ermr Chlsc | Specr Erms Chlrd | Specr Ermr Chlre | Specr Erms Chlsf | |||
| tagBa | EB1453 | EB6 | 95 | 4 | 1 | 0 |
| tagDa | EB1559 | EB6 | 92 | 6 | 2 | 0 |
| tagFa | EB1560 | EB6 | 83 | 17 | 0 | 0 |
| tagBb | EB1453 | EB892 | 1 | 0 | 0 | 24 |
A total of 100 colonies was examined.
A total of 25 colonies was examined.
Resistance profile of a double mutant (e.g., ΔtagO ΔtagB).
Resistance profile of a complemented deletion strain (e.g., EB633).
Resistance profile of the donor strain (e.g., EB1453).
Resistance profile of a strain with a chromosomal deletion for the tested gene (e.g., ΔtagB).
Here we show that despite a significant literature to the contrary, teichoic acid polymers are not essential to the viability of B. subtilis but nevertheless appear to play a crucial role in maintaining the shape of this organism. Through the replacement of tagO with an erythromycin resistance cassette in the absence of complementation, we have circumvented the ability of the organism to produce cell wall containing teichoic acid polymers, as shown by the drastic reduction in phosphate content. The creation of this mutant is in contradiction to the work by Soldo et al., who reported the inability to generate viable mutants in tagO through insertional inactivation (16). We attribute this discrepancy to the slow growth and altered morphology of this mutant that may have mistakenly led these authors to conclude that these mutants were not viable. Perhaps most remarkable is that, despite the dispensability of tagO, late-acting gene products are required for viability. This is in agreement with the peculiar dispensability pattern seen in S. aureus teichoic acid genes. Indeed it may reflect a mechanistic feature that is paradigmatic of the dispensability patterns of these genes in all gram-positive bacteria. As speculated in our previous work, we believe that the essentiality of the late-acting gene products may arise from the build up of toxic intermediates or from the sequestration of a crucial metabolite, such as undecaprenol phosphate, which is also required for the production of peptidoglycan.
Acknowledgments
We thank Bob Harris of the University of Guelph for his technical assistance in preparing samples for electron microscopy. Microscopy was performed in the NSERC Guelph Regional Integrated Imaging Facility (GRIIF).
This work was supported, in part, by a Canadian Institutes of Health Research (CIHR) operating grant (MOP-15496). M.A.D. holds a CIHR Canadian Graduate Scholarship. Both E.D.B. and T.J.B. hold Canada Research Chairs. Microscopy performed at the NSERC Guelph Regional Integrated Imaging Facility (GRIIF) is partially funded by an NSERC Major Facility Access grant to T.J.B.
Footnotes
Published ahead of print on 29 September 2006.
REFERENCES
- 1.Benson, A. K., and W. G. Haldenwang. 1993. Regulation of σB levels and activity in Bacillus subtilis. J. Bacteriol. 175:2347-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhavsar, A. P., T. J. Beveridge, and E. D. Brown. 2001. Precise deletion of tagD and controlled depletion of its product, glycerol-3-phosphate cytidylyltransferase, leads to irregular morphology and lysis of Bacillus subtilis grown at physiological temperature. J. Bacteriol. 183:6688-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhavsar, A. P., and E. D. Brown. 2006. Cell wall assembly in Bacillus subtilis: how spirals and spaces challenge paradigms. Mol. Microbiol. 60:1077-1090. [DOI] [PubMed] [Google Scholar]
- 4.Bhavsar, A. P., L. K. Erdman, J. W. Schertzer, and E. D. Brown. 2004. Teichoic acid is an essential polymer in Bacillus subtilis that is functionally distinct from teichuronic acid. J. Bacteriol. 186:7865-7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhavsar, A. P., R. Truant, and E. D. Brown. 2005. The TagB protein in Bacillus subtilis 168 is an intracellular peripheral membrane protein that can incorporate glycerol phosphate onto a membrane-bound acceptor in vitro. J. Biol. Chem. 280:36691-36700. [DOI] [PubMed] [Google Scholar]
- 6.Brandt, C., and D. Karamata. 1987. Thermosensitive Bacillus subtilis mutants which lyse at the non-permissive temperature. J. Gen. Microbiol. 133:1159-1170. [DOI] [PubMed] [Google Scholar]
- 7.Briehl, M., H. M. Pooley, and D. Karamata. 1989. Mutants of Bacillus subtilis 168 thermosensitive for growth and wall teichoic acid synthesis. J. Gen. Microbiol. 135:1325-1334. [DOI] [PubMed] [Google Scholar]
- 8.D'Elia, M. A., M. P. Pereira, Y. S. Chung, W. Zhao, A. Chau, T. J. Kenney, M. C. Sulavik, T. A. Black, and E. D. Brown. 2006. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J. Bacteriol. 188:4183-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Formstone, A., and J. Errington. 2005. A magnesium-dependent mreB null mutant: implications for the role of mreB in Bacillus subtilis. Mol. Microbiol. 55:1646-1657. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, I. C. 1997. Bacterial cell surface carbohydrates: structure and assembly. Biochem. Soc. Trans. 25:183-187. [DOI] [PubMed] [Google Scholar]
- 11.Harwood, C. R., and S. M. Cutting. 1990. Molecular biology methods for Bacillus. John Wiley and Sons, Toronto, Ontario, Canada.
- 12.Lambert, P. A., I. C. Hancock, and J. Baddiley. 1975. The interaction of magnesium ions with teichoic acid. Biochem. J. 149:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leaver, M., and J. Errington. 2005. Roles for MreC and MreD proteins in helical growth of the cylindrical cell wall in Bacillus subtilis. Mol. Microbiol. 57:1196-1209. [DOI] [PubMed] [Google Scholar]
- 14.Matias, V. R., and T. J. Beveridge. 2006. Native cell wall organization shown by cryo-electron microscopy confirms the existence of a periplasmic space in Staphylococcus aureus. J. Bacteriol. 188:1011-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pooley, H. M., F. X. Abellan, and D. Karamata. 1991. A conditional-lethal mutant of Bacillus subtilis 168 with a thermosensitive glycerol-3-phosphate cytidylyltransferase, an enzyme specific for the synthesis of the major wall teichoic acid. J. Gen. Microbiol. 137:921-928. [DOI] [PubMed] [Google Scholar]
- 16.Soldo, B., V. Lazarevic, and D. Karamata. 2002. tagO is involved in the synthesis of all anionic cell-wall polymers in Bacillus subtilis 168. Microbiology 148:2079-2087. [DOI] [PubMed] [Google Scholar]
- 17.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]