Abstract
Bacteria utilize quorum-sensing systems to modulate environmental stress responses. The quorum-sensing system of Streptococcus mutans is mediated by the competence-stimulating peptide (CSP), whose precursor is encoded by the comC gene. A comC mutant of strain GS5 exhibited enhanced antimicrobial sensitivity to a wide variety of different agents. Since the addition of exogenous CSP did not complement this phenotype, it was determined that the increased tetracycline, penicillin, and triclosan sensitivities resulted from repression of the putative bacteriocin immunity protein gene, bip, which is located immediately upstream from comC. We further demonstrated that the inactivation of bip or smbG, another bacteriocin immunity protein gene present within the smb operon in S. mutans GS5, affected sensitivity to a variety of antimicrobial agents. Furthermore, both the bip and smbG genes were upregulated in the presence of low concentrations of antibiotics and were induced during biofilm formation relative to in planktonic cells. These results suggest, for the first time, that the antimicrobial sensitivity of a bacterium can be modulated by some of the putative bacteriocin immunity proteins expressed by the organism. The implications of these observations for the evolution of bacteriocin immunity protein genes as well as for potential new chemotherapeutic strategies are discussed.
Streptococcus mutans has been implicated as a primary causative agent of dental caries in humans (6). One of the important virulence properties of these organisms is their ability to form biofilms known as dental plaque on tooth surfaces.
Biofilm formation is initiated by interaction between planktonic bacteria and a surface in response to appropriate environmental signals (2, 5, 11, 12, 16-18). In addition to responses to physical and chemical signals, bacteria regulate diverse physiological processes in a cell density-dependent manner; this is commonly called quorum sensing (1, 10). Bacteria utilize quorum-sensing systems to modulate environmental stress responses. Recently, some studies demonstrated that biofilm formation, acid tolerance, and the transformation of S. mutans are mediated by quorum sensing (3). The quorum-sensing system in S. mutans consists of at least five gene products, including a 21-amino-acid competence-stimulating peptide (CSP) whose precursor is encoded by comC, a histidine kinase sensor protein encoded by comD, and the cognate response regulator expressed from the comE gene (13). The comCDE genes are located in the same locus and together constitute a quorum-sensing system for generating and responding to CSP (13). By interfering with this cell-cell signaling mechanism, caries-causing S. mutans bacteria which use quorum sensing to control virulence could potentially be attenuated.
Many gram-positive bacteria produce antimicrobial peptides called bacteriocins (4, 8, 20, 24). Although these peptide molecules are not required for growth, they may help the microorganisms that produce them to compete for the limited nutrients in their environment (22). It has been shown that production of some of these antimicrobial peptides is regulated by the comC quorum-sensing system in S. mutans (9, 21). Bacteria in biofilms frequently express much greater resistance to antimicrobial agents than they do in the corresponding planktonic cultures (5). Since biofilm formation in some organisms is modulated by quorum sensing, we investigated the possibility that the comC quorum-sensing system in S. mutans modulates its sensitivity to antimicrobial compounds. Although some of the CSP-induced genes might be important for efficient genetic exchange under natural conditions, many of these gene products likely aid in other cell density-adaptive functions; i.e., several CSP-induced genes have stress-related roles or participate in the synthesis and transport of bacteriocin-like peptides (21, 24). The present results suggest for the first time that the antimicrobial sensitivity of S. mutans GS5 is modulated by some of the putative bacteriocin immunity proteins expressed by the organism. Since the bacteriocin operons are regulated, in turn, by quorum sensing, this suggests how quorum sensing can regulate the antimicrobial sensitivity of an organism.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All bacterial strains and plasmids used in this study are listed in Table 1. Strains of S. mutans were cultured and maintained in Todd-Hewitt broth (THB) (Difco Laboratories, Grand Island, N.Y.). Transformants of S. mutans were selected following growth on mitis salivarius agar (Difco Laboratories) plates supplemented with erythromycin (10 μg/ml).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| S. mutans strains | ||
| GS5 | wta; Ems Kms | SUNYaBb |
| UA159 | wt; Ems Kms | J. Ferretti, OU-ACGTc |
| Δbip | GS5::pMM503; Bip− Emr | This study |
| ΔsmbG | GS5::pMM504; SmbG− Emr | This study |
| H1 | GS5 with transposon Tn916 inserted at the upstream of Smb operon; Tetr | 24 |
| GS5 ΔcomC | GS5::pAYCA1301; ComC− Emr | 25 |
| UA159 ΔcomC | UA159::pAYCA1301; ComC− Emr | This study |
| E. coli DH5a | Cloning host | GIBCO BRL |
| Plasmids | ||
| pCR2.1 | Cloning vector; Ampr | Invitrogen |
| pUC19 | Cloning vector | Promega |
| pMM501 | pUC19 harboring a bip gene; Ampr | This study |
| pMM502 | pUC19 harboring an smbG gene; Ampr | This study |
| pMM503 | pUC19 harboring an inactivated bip gene with an Emr cassette; Emr Ampr | This study |
| pMM504 | pUC19 harboring an inactivated smbG gene with an Emr cassette; Emr Ampr | This study |
wt, wild type.
SUNYaB, culture collection at the Department of Oral Biology, State University of New York, Buffalo, N.Y.
OU-ACGT, University of Oklahoma Advanced Center for Genome Technology.
DNA manipulations.
DNA isolation, endonuclease restriction, ligation, and transformation of competent Escherichia coli were carried out as previously described (19). Transformation of S. mutans was accomplished by procedures routinely carried out in this laboratory (19).
Construction of the bip and smbG mutants.
The bip and smbG mutants (Fig. 1) were constructed following double-crossover homologous recombination via insertion of an erythromycin resistance determinant into each gene. The plasmids used for disruption of the bip and smbG genes were prepared as follows. A 1.6-kb DNA fragment containing the entire bip or smbG gene together with the respective flanking regions were PCR amplified from GS5 genomic DNA with the following primers: for bip, forward primer 1736A (5′-TGCGGTCTATTGACCTCCTC-3′) and reverse primer ComCsmmC (5′-CGGGGTACCTTGATTATTTAACCC-3′); for smbG, forward primer SMBT2/PCRFO (5′-CGTATGGAGCTGTGGGAATAC-3′) and reverse primer SMBT2/PCRRE (5′-GGTTACTGTGAGCCAATCCAC-3′). Initially, the DNA fragments including the bip and smbG genes were cloned into the pCR2.1-TOPO vector (Invitrogen), and the resulting plasmids were digested with EcoRI. The fragments were then cloned into pUC19 to generate resulting plasmids pMM501 and pMM502, respectively. Both plasmids were digested with SpeI and BamHI at their respective unique sites, which were located within the target genes, followed by blunt ending and ligation with an erm resistance cassette from pUC119Em (19). The resulting plasmids, pMM503 and pMM504, were linearized with KpnI and HindIII, respectively, downstream from the inserted fragments, and the linearized plasmids were used to transform S. mutans GS5 (19). Confirmation of plasmid insertions causing gene disruption was performed by PCR (data not shown).
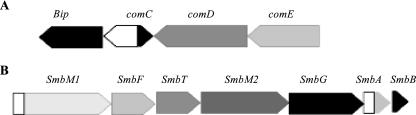
FIG. 1.
Relative orientations of the bip and smbG genes in S. mutans GS5. (A) The bip gene is located upstream of the comCDE locus. (B) The smbG gene is located upstream of the bacteriocin structural genes, smbA and smbB, within the smb operon. Open boxes, putative promoter regions of the operons. The transcription of the genes is depicted by the orientation of the boxed arrows.
The bip H1 double mutant was constructed as follows. The plasmid pMM503, which included the bip gene and an erm resistance cassette, was linearized with KpnI and transformed into strain H1 (24). The bip H1 double mutant construction was confirmed by PCR.
Growth inhibition by antibiotics.
Each S. mutans strain grown overnight at 37°C was inoculated into THB containing several concentrations of each antimicrobial in duplicate. After the strains were grown overnight at 37°C, the cell density was measured by determining the optical density at 600 nm.
Agar plate bacteriocin assays.
Loopfuls of stationary-phase cultures of the S. mutans strains were stabbed into a THB agar plate. After 24 h of incubation at 37°C under anaerobic conditions, the plates were overlaid with the bip H1 double mutant in 3 ml of THB with 1% low-melting-point agarose (Bioproducts, Rockland, Maine). After 24 h of additional incubation at 37°C under anaerobic conditions, the diameters of the clearance zones surrounding the inoculated bacteria were measured.
Biofilm formation.
S. mutans GS5 was inoculated into 5-ml portions of half-strength THB in six-well polystyrene microtiter plates to form biofilms. After 48 h of incubation, the planktonic cells and biofilm cells were separated for quantitation as previously described (25). Briefly, biofilm formation was quantitated following crystal violet staining of biofilms, while growth was determined by measuring the turbidities (optical density at 570 nm) of parallel wells following resuspension of the sessile organisms with planktonic cells.
Real-time quantitative RT-PCR.
Total RNA was isolated from 15 ml of log-phase cell cultures. After centrifugation, the cells were suspended in 0.3 ml of diethylpyrocarbonate-treated water. The samples were transferred to FastRNA tubes with blue caps (Qbiogene, Inc., Carlsbad, Calif.), and 0.9 ml of TRIzol reagent (Invitrogen) was then added. Cells were broken with a FastPREP FP120 homogenizer (Qbiogene) at a speed setting of 0.6 for 30 s. After samples were placed on ice for 2 min, 0.2 ml of chloroform was added and the tubes were vortexed and centrifuged again as described above. The RNA was finally precipitated from the aqueous phase with isopropanol, and the resulting pellets were dried and resuspended in 20 μl of diethylpyrocarbonate-treated water. For reverse transcription-PCR (RT-PCR) analysis, RNA samples were treated for 15 min at 37°C with 1.0 U of RNase-free DNase (Amersham Biosciences Corp., Piscataway, N.J.) per ml to remove contaminating DNA. Reverse transcription was carried out with SuperScript III (Invitrogen) according to the directions of the supplier. The real-time RT-PCR was performed on cDNA samples with either the 16S rRNA primers (as internal controls) or bip-specific primers (1737/F, 5′-CAGCGCAGCTGATAGCTGTTTGTCT-3′; 1737/R, 5′-CTGCTGGCAAATTCGCTTACTTG-3′), using IQ-Supermix PCR reagent (Bio-Rad) in the iCycler thermal cycler according to the manufacturer's recommendations (Bio-Rad Laboratories). Relative expression levels of bip transcripts were then calculated by normalizing the levels of bip-specific RNA to the levels of 16S rRNA. By normalizing the cycle threshold (CT) values for bip to the total amount of 16S rRNA, all samples were compared, and the relative fold changes in the samples were calculated using the −ΔΔCT method described for the MyIQ real-time PCR detection system (Bio-Rad Laboratories). A similar approach was used to quantitate smbG mRNA
Fluorescence efflux measurement.
The tested strains were grown to mid-log phase, and then cells were then harvested at 7,000 × g for 10 min, washed once with 100 mM NaCl-50 mM sodium phosphate buffer (pH 7.0), and suspended again in the same buffer at an optical density at 600 nm of 0.1 in the presence of glycerol. Experimental measurements were generally performed within 2 h after cell preparation. Fluorescence measurements were performed by a modification of methods described previously (15). The fluorescent probe, N-phenyl-1-naphtylamine (NPN; Sigma), was initially dissolved in absolute methanol. Fluorescence emission intensity was measured with a Twinkle LB970 fluorometer (Berthold Technologies GmbH & Co. KG, Bad Wildbad, Germany). Excitation and emission wavelengths for NPN were 355 nm and 460 nm, respectively.
Statistical analysis.
Intergroup differences between various factors were estimated using a statistical analysis of variance for factorical models. Fisher's protected least-significant differences (PLSD) test was used to compare individual groups.
RESULTS
Antimicrobial sensitivity and quorum sensing.
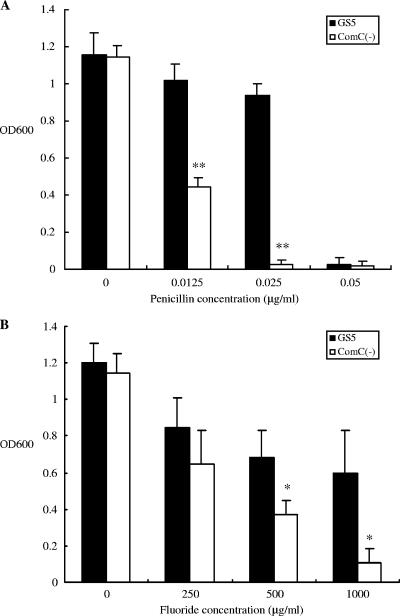
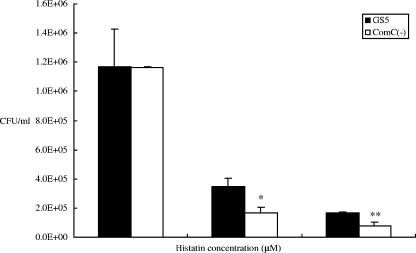
Since relatively dense biofilms have been shown to be more resistant to antimicrobial agents than planktonic cells (5), it was of interest to determine the role of quorum sensing in this property. Therefore, the effects of a comC mutation in S. mutans GS5 on antimicrobial sensitivity were examined. These results clearly demonstrated that the mutation increased the sensitivity of the mutant to two distinct antimicrobials, fluoride and penicillin (Fig. 2). In addition, the comC mutant was much more sensitive to a variety of other distinct antimicrobial agents, including triclosan (an antimicrobial agent used in commercial toothpastes), tetracycline, and the endogenous antimicrobial compound histatin-5 (Fig. 2 and 3). These effects were not strain specific, as the comC mutants of strains UA159 (Fig. 4) and BM71 and NG8 (data not shown) also displayed increased sensitivity to the antimicrobial agents. However, surprisingly, the addition of synthetic CSP did not complement these effects in the comC mutant. Therefore, this prompted a closer examination of the method utilized to construct the comC mutant. Such an analysis revealed that the replacement of the comC gene with an Ermr gene also removed a portion of the nucleotide sequence immediately upstream of the gene. The erm cassette replaced part of the 3′ end of the comD gene, all of comC and some bases upstream of comC. This later region corresponded to a potential promoter region for a bacteriocin-like cassette containing open reading frames Smu1737 and Smu1738 (Los Alamos National Laboratory database [www.oralgen.lanl.gov]) (Fig. 1). The initial gene in this region, Smu1738, displayed weak similarity to a bacteriocin-like protein, BlpO. The adjacent open reading frame, Smu1737, corresponded to a putative immunity-like protein with a molecular size of approximately 15 kDa. This gene, recently referred to as immA (21), was named bip, for bacteriocin-like immunity protein, in the present study based upon its properties described below and to differentiate it from the other potential immunity protein genes present in the S. mutans genome. In order to examine the possibility that construction of the comC mutant had also affected expression of the bacteriocin-like operon, expression of this operon was examined using RT-PCR, which showed no detectable expression of this operon in the mutant relative to that in wild-type GS5 (data not shown). This result suggested that the effects of the comC mutation on antimicrobial sensitivity could actually be due, in part, to attenuation of the expression of the bacteriocin immunity protein-like gene.
FIG. 2.
Antimicrobial sensitivities of GS5 and the comC mutant. Penicillin (A) and fluoride (B) at the indicated concentrations were added to cultures grown as described in the text. There are statistically significant differences between the growth of GS5 and that of the comC mutant (*, P < 0.05; **, P < 0.001 [Fisher's PLSD]). Error bars indicate standard deviations.
FIG. 3.
Histatin sensitivities of GS5 and the comC mutant. The GS5 and comC mutant strains were grown overnight, and cells were washed twice with sodium phosphate buffer. Cells were resuspended at 2.5 × 105 CFU/ml and incubated with the indicated concentration of histatin. There are statistically significant differences between the growth of GS5 and that of the comC mutant (*, P < 0.05; **, P < 0.01 [Fisher's PLSD]). Error bars indicate standard deviations.
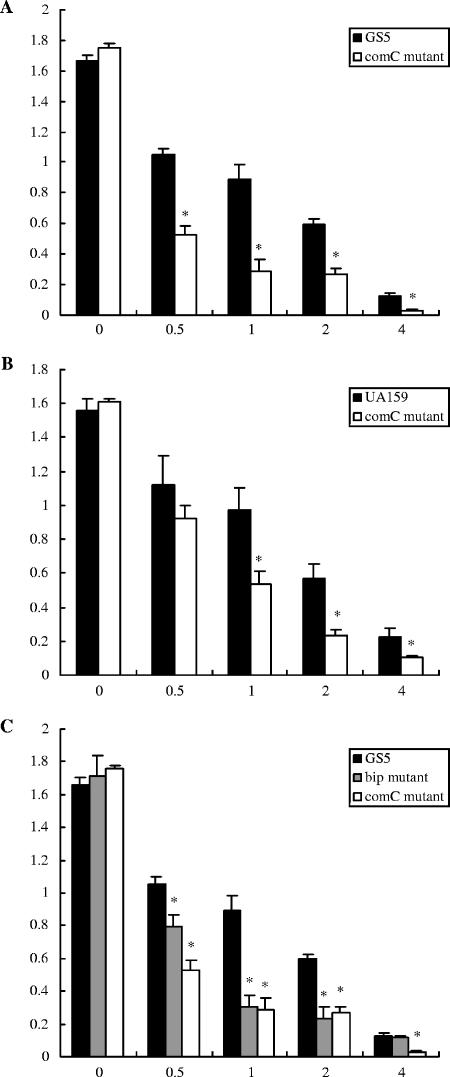
FIG. 4.
Tetracycline sensitivities of the bip and comC mutants. The mutants were incubated in THB containing tetracycline and grown as described in the text. (A) GS5 and GS5 comC mutant. (B) UA159 and UA159 comC mutant. (C) GS5, GS5 comC mutant, and GS5 bip mutant. There are statistically significant differences between the growth of the wild-type strains and that of the mutants (*, P < 0.05 [Fisher's PLSD]). Each experiment was repeated three times with triplicate samples. Error bars indicate standard deviations.
In order to directly test the hypothesis that alterations in the expression of the bacteriocin immunity protein upstream of the comC gene affected sensitivity of strain GS5 to a variety of antimicrobial agents, a monospecific bip mutant was constructed and was shown to be also altered in antimicrobial sensitivity (Fig. 4).
Therefore, these results suggested that the increased sensitivity of strain GS5 to a wide variety of antimicrobial agents was primarily due to the inactivation of the bip gene, which codes for a putative immunity protein.
The SmbG immunity protein also affects antimicrobial sensitivity in S. mutans GS5.
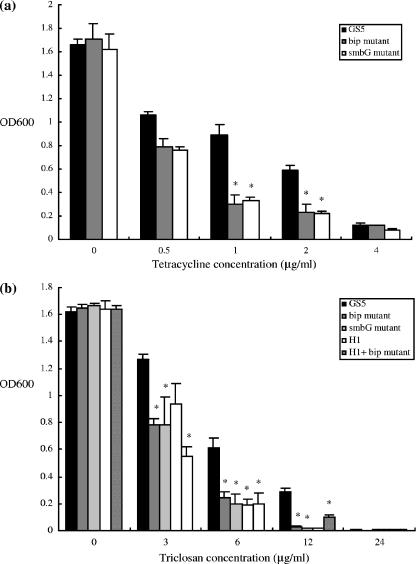
Since an operon coding for the bacteriocin Smb in strain GS5 has recently been described (24) and was shown to contain a putative immunity protein gene, smbG, it was of interest to determine if this gene also influences the sensitivity of strain GS5 to antimicrobial agents. Therefore, an smbG mutant was constructed and its antimicrobial sensitivities determined. Like the bip mutation, the smbG mutation also resulted in increased sensitivity to a variety of antimicrobial agents, including tetracycline and triclosan, relative to wild-type GS5 (Fig. 5). This suggested that the alteration in antimicrobial sensitivity resulting from the bip mutation was not limited to this putative immunity protein gene alone.
FIG. 5.
Antimicrobial sensitivities of GS5 and the smbG mutant. Tetracycline (a) and triclosan (b) at the indicated concentrations were added to cultures grown as described in the text. There are statistically significant differences between the growth of GS5 and that of the smbG mutant (*, P < 0.001 [Fisher's PLSD]). Each experiment was repeated three times with triplicate samples. Error bars indicate standard deviations.
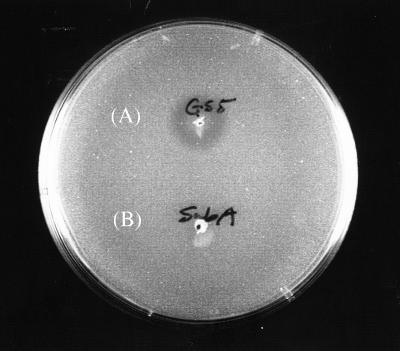
It was also of interest to examine the antimicrobial sensitivity of a double bip smbG mutant. However, repeated attempts to construct such a mutant were unsuccessful. Nevertheless, it was possible to isolate a mutant containing both the bip mutation and an smb promoter mutation (H1) (24) which prevented expression of all of the genes of this operon, including the Smb structural genes smbA and smbB (24). These results suggested that the bip gene could also play a role in the sensitivity of strain GS5 to the Smb bacteriocin. This further suggested that either the bip or smbG gene is required to protect strain GS5 against the inhibitory effect of the Smb bacteriocin. To test this hypothesis, a comparison of the sensitivities of the wild-type GS5 and the bip H1 double mutant to Smb was carried out (Fig. 6). These results indicated that the double mutant, but not the wild-type strain, was very sensitive to the bacteriocin. Moreover, the bip and smbG mutants each grew well in the presence of Smb. These results are consistent with a redundant role for both proteins in immunity to Smb. Therefore, attenuation of either gene significantly increased the sensitivity of strain GS5 to a variety of antimicrobial agents but not that to the endogenous bacteriocin Smb.
FIG. 6.
Sensitivity of the bip H1 double mutant to the Smb bacteriocin. Wild-type strain GS5 (A) and the smbA mutant (defective in Smb production) (B) were spotted onto a THB agar plate from overnight cultures. After incubation for 1 day, the plate was overlaid with the indicator strain, the bip H1 double mutant, and incubated for an additional 24 h.
bip gene expression in biofilm and planktonic cells.
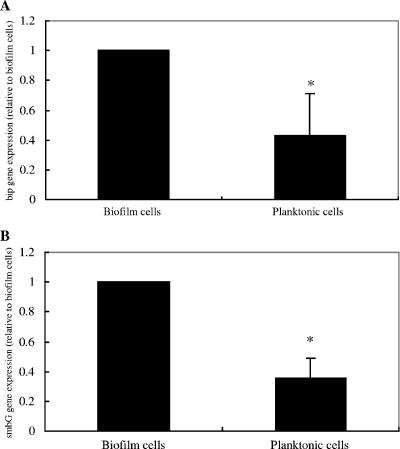
Since the present results suggested a role for bacteriocin immunity proteins in the relatively sensitivity of strain GS5 to a variety of antimicrobial agents and since biofilm cells are more resistant to such agents than planktonic cells, it was of interest to examine the regulation of immunity protein expression during biofilm formation. Therefore, a comparison of bip expression in planktonic versus biofilm cells was carried out (Fig. 7). bip gene expression appeared to be induced more than twofold during the development of biofilms. Similarly, expression of the smbG gene was also upregulated under these biofilm-forming conditions (Fig. 7). Therefore, these results are compatible with a role for the putative bacteriocin immunity proteins in the increased resistance of biofilm cells to antimicrobial agents, although the magnitude of the changes detected suggests that bip gene expression is not the sole determinant of this phenotype.
FIG. 7.
Expression of the bip (A) and smbG (B) genes in biofilms and planktonic cells. The quantity of bip gene cDNA measured by real-time RT-PCR was normalized to the 16S rRNA cDNA abundance within each unique reaction. Each experiment was repeated three times with triplicate samples. Error bars indicate the variance between triplicate samples within the real-time RT-PCR. There are statistically significant differences between biofilm cells and planktonic cells (*, P < 0.05 [Fisher's PLSD]).
bip and smbG gene expression in the presence of antimicrobial agents.
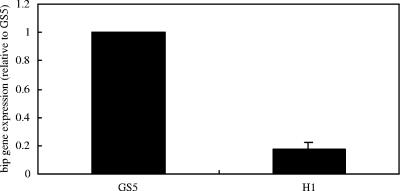
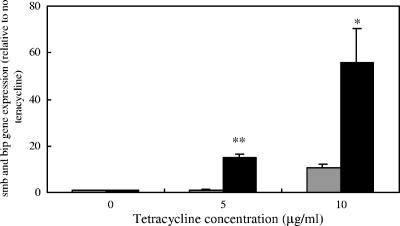
A role for bacteriocin immunity proteins in resistance to some noncognate bacteriocins was suggested by the demonstration that the H1 mutant defective in Smb production expressed lower levels of bip transcripts than wild-type GS5 (Fig. 8). This suggested that the expression of the immunity proteins could be regulated by heterologous antimicrobial agents. To determine whether the presence of antimicrobial agents could affect bip and smbG expression at the transcriptional level, we used real-time RT-PCR to compare the transcriptional levels of bip and smbG in the presence of low concentrations of tetracycline (Fig. 9). The expression of both the bip and smbG genes was significantly increased in the presence of low concentrations of tetracycline, but the expression of the bip gene was induced much more than that of the smbG gene.
FIG. 8.
Effects of smb mutation on bip gene transcription. The RNA was extracted and quantified by real-time RT-PCR for bip gene expression. The quantity of bip cDNA measured by real-time RT-PCR was normalized to the 16S cDNA abundance within each unique reaction.
FIG. 9.
Immunity protein gene expression in the presence of tetracycline. Shaded bars, smbG gene; solid bars, bip gene. There are statistically significant differences between strain GS5 in the presence and absence of different concentrations of tetracycline (*, P < 0.01; **, P < 0.001 [Fisher's PLSD]). Each experiment was repeated three times with triplicate samples. Error bars indicate standard deviations.
Extrusion of intracellular compounds in the bip and smbG mutants.
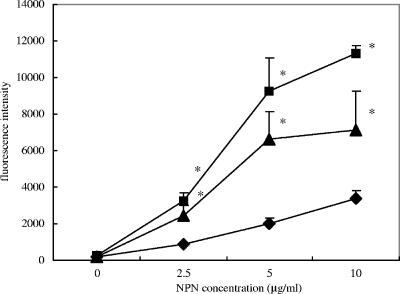
Immunity proteins commonly are export proteins which secrete intracellularly accumulated antimicrobial agents. Therefore, it was of interest to determine if both Bip and SmbG might be involved in the export of antimicrobial compounds. For this purpose, the fluorescent model export agent NPN was utilized to compare the export properties of the mutants with those of the parental GS5 strain. This agent has been utilized previously to quantitate the export of drugs from bacteria (15). Both the bip and smbG mutants were impeded in exporting NPN relative to the wild-type strain (Fig. 10). Another Ermr mutant, altered in the gtfB gene (23), was demonstrated to export NPN similarly to the wild-type strain, indicating that the Ermr marker was not responsible for these differences. Therefore, these results are consisted with a role for both the Bip and SmbG proteins in the export of a variety of distinct antimicrobial agents.
FIG. 10.
Roles of Bip and SmbG in export as determined using a model export molecule, fluorescent NPN. Fluorescence of the cells was determined in the presence of different concentrations of NPN as described in the text. ⧫, GS5; ▪, bip mutant; ▴, smbG mutant. There are statistically significant differences between GS5 and the two mutants (*, P < 0.05 [Fisher's PLSD]). Each experiment was repeated three times with triplicate samples. Error bars indicate standard deviations.
DISCUSSION
Although some of the CSP-induced genes are obviously important for transformation, it is becoming increasingly clear that CSP mediates a variety of different physiological responses (3, 19, 25). Since relatively dense biofilms express increased resistance to antimicrobial agents, we hypothesized that the antimicrobial sensitivity of S. mutans might be affected by CSP-dependent quorum-sensing systems. Indeed, the comC mutant of strain GS5 exhibited enhanced antimicrobial sensitivity to a variety of different agents. However, CSP did not complement the phenotype of increased antimicrobial sensitivity. These results suggested that attenuation of comC expression alone was not responsible for this alteration. The present results suggested that the attenuation of bip gene expression was primarily responsible for the increased antimicrobial sensitivity displayed by the comC mutant used in this study. Therefore, the gene replacement strategy for generating the comC mutant used in this investigation apparently altered the promoter activity corresponding to the bip-containing operon. Despite the fact that it has been recently demonstrated that CSP is involved in the regulation of expression of this operon (21), the inability to complement these effects in the comC mutant by addition of exogenous CSP suggests that it is the promoter alteration which is primarily responsible for the phenotype displayed by the comC mutant. Nevertheless, it is predicted that a true comC mutant of S. mutans without an alteration in the bip operon should also be more sensitive to a variety of antimicrobial agents due to repression of bip and smbG expression. Thus, in this manner, quorum sensing mediated by CSP can indirectly affect the relative sensitivity of S. mutans to antimicrobial agents. In addition, to our knowledge, this is the first demonstration that putative bacteriocin immunity proteins display broad activities with a variety of heterologous antimicrobial agents.
The present results further suggest that other bacteriocin immunity proteins in addition to Bip in strain GS5 could also affect antimicrobial sensitivity. For example, the smbG gene, coding for another putative immunity protein, also appears to play a role in this sensitivity. Recent results have also suggested that CSP is also involved in the regulation of expression of the smb operon (24). However, the inability to complement the comC mutation and alter antimicrobial sensitivity with exogenous CSP under conditions where bacteriocin production is complemented (24) suggests that smbG is not primarily responsible for the increased comC mutant antimicrobial sensitivities. Nevertheless, the smbG mutation also increased the sensitivity of strain GS5 to antimicrobial agents, indicating that this gene product does have an effect on this property. Thus, it is suggested that the multiple bacteriocin-like immunity proteins expressed by organisms such as S. mutans could play roles in the resistance of these organisms to a variety of antimicrobial agents.
It has been widely reported that bacteria growing as a surface biofilm are more resistant to various stress challenges and antimicrobial agents than planktonic cells (2, 7, 14). Of medical importance is the observation that biofilm cells can be 1,000-fold more resistant to antibiotics than planktonic cells. The present results suggesting increased expression of the putative immunity protein genes during in vitro biofilm formation are compatible with a general role for the bacteriocin immunity proteins in such resistance. However, the magnitude of the induction demonstrated suggests that this may not be the major factor associated with biofilm resistance.
The present study also demonstrated that the expression of the bip and smbG genes was markedly increased in the presence of tetracycline. The induced expression of the bip gene in the presence of low concentrations of the antibiotic was more pronounced, approximately 1,000-fold higher than that of the smbG gene. Such a large increase might reflect the weak expression of the bip gene in the absence of exogenous antimicrobial agents. In addition, the present results suggesting a redundant role for SmbG and Bip relative to Smb sensitivity further suggest that these proteins are not absolutely specific for resistance to their cognate bacteriocins. However, the putative bacteriocin associated with Bip has not yet been characterized.
The present results are also consistent with a role for both the Bip and SmbG proteins in the export of antimicrobial agents, as suggested for many bacteriocin immunity proteins (12). Both the bip and smbG mutants were attenuated in exporting NPN relative to the wild-type strain. An interesting question raised by these results involves the molecular basis for the broad specificity displayed by the S. mutans putative immunity proteins. Although it would appear that these proteins evolved to ensure the relative resistance of the producer strain to their respective bacteriocins, it appears that these proteins have much broader specificity. Further investigation into the structures and mechanisms of action of these proteins will be required to determine the molecular basis for these properties.
Several general implications of the present results are suggested both for the evolution of immunity proteins and for antimicrobial therapy. It is reasonable to propose that bacteriocin-producing bacteria may have evolved as a result of the environmental advantage of producing bacteriocins. These antimicrobial products generally affect organisms closely related to the producers. The present results suggest that the selection for bacteriocin immunity proteins may also have been an important factor in the evolution and transmission of these operons. The broad specificity of these proteins as demonstrated in the present study may provide protection against a variety of inhibitory substances (i.e., metals, antibiotics, bacteriocins, etc.) in different environments. The demonstration of the presence of genes such as those in the bip-containing operon of S. mutans without demonstrated antimicrobial activity is also compatible with such a hypothesis. Thus, the activity of the immunity proteins, rather than their cognate bacteriocins, may have become important for the survival of some bacteria in specific environments.
The present results further suggest a novel approach for chemotherapy against certain bacteria. It may be possible to increase the effectiveness of commonly used antimicrobial agents by specifically inhibiting the bacteriocin immunity protein activities of these organisms. For example, the effectiveness of the commonly utilized antiplaque agent triclosan against cariogenic S. mutans could be enhanced by developing specific inhibitors of Bip activity. Moreover, since CSP is involved in regulating the expression of multiple bacteriocin operons in these organisms, specific inhibitors of CSP activity may also produce comparable effects. Such approaches would also have the advantage of targeting specific pathogenic organisms without comparable effects on endogenous bacteria. Further investigation will be required to determine if such approaches can prove to be useful in chemotherapy against specific diseases.
Acknowledgments
This investigation was supported in part by NIH grant DE03258.
Footnotes
Published ahead of print on 22 September 2006.
REFERENCES
- 1.Bassler, B. L. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582-587. [DOI] [PubMed] [Google Scholar]
- 2.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 3.Cvitkovitch, D. G., Y. H. Li, and R. P. Ellen. 2003. Quorum sensing and biofilm formation in streptococcal infections. J. Clin. Investig. 112:1626-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diep, D. B., L. S. Havarstein, and I. F. Nes. 1996. Characterization of the locus responsible for the bacteriocin production in Lactobacillus plantarum C11. J. Bacteriol. 178:4472-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donlan, R. M., and J. W. Costerton. 2002. Biofilm: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoyle, B. D., and J. W. Costerton. 1991. Bacterial resistance to antibiotics: the role of biofilms. Prog. Drug Res. 37:91-105. [DOI] [PubMed] [Google Scholar]
- 8.Jack, R. W., J. R. Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleerebezem, M., and L. E. Quadri. 2001. Peptide pheromone-dependent regulation of antimicrobial peptide production in Gram-positive bacteria: a case of multicellular behavior. Peptides 22:1579-1596. [DOI] [PubMed] [Google Scholar]
- 10.Kleerebezem, M., L. E. Quadri, O. P. Kuipers, and W. M. de Vos. 1997. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol. Microbiol. 24:895-904. [DOI] [PubMed] [Google Scholar]
- 11.Kolenbrander, P. E. 2000. Oral microbial communities: biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 54:413-437. [DOI] [PubMed] [Google Scholar]
- 12.Kuramitsu, H. K. 1993. Virulence factors of mutans streptococci role of molecular genetics. Crit. Rev. Oral Biol. Med. 4:159-176. [DOI] [PubMed] [Google Scholar]
- 13.Li, Y. H., P. C. Y. Lau, J. H. Lee, R. P. Ellen, and D. G. Cvitkovitch. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilm. J. Bacteriol. 183:897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nes, I. F., D. B. Diep, L. S. Havarstein, M. B. Brurberg, V. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek 70:113-128. [DOI] [PubMed] [Google Scholar]
- 15.Ocaktan, A., H. Yoneyama, and T. Nakae. 1997. Use of fluorescence probes to monitor function of the subunit proteins of the MexA-MexB-OprM drug extrusion machinery in Pseudomonas aeruginosa. J. Biol. Chem. 272:21964-21969. [DOI] [PubMed] [Google Scholar]
- 16.O'Toole, G. A., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 17.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 18.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescence WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 19.Shiroza, T., and H. K. Kuramitsu. 1993. Construction of a model secretion system for oral streptococci. Infect. Immun. 61:3745-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagg, J. R., A. S. Dajani, and L. W. Wannamaker. 1976. Bacteriocins of gram-positive bacteria. Bacteriol. Rev. 40:722-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Ploeg, J. R. 2005. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J. Bacteriol. 187:3980-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vining, L. C. 1990. Functions of secondary metabolites. Annu. Rev. Microbiol. 44:395-427. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita, Y., W. H. Bowen, and H. K. Kuramitsu. 1992. Molecular analysis of a Streptococcus mutans strain exhibiting polymorphism in the tandem gtfB and gtfC genes. Infect. Immun. 60:1618-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yonezawa, H., and H. K. Kuramitsu. 2005. Genetic analysis of a unique bacteriocin, Smb, produced by Streptococcus mutans GS5. Antimicrob. Agents Chemother. 49:541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida, A., and H. K. Kuramitsu. 2002. Multiple Streptococcus mutans genes are involved in biofilm formation. Appl. Environ. Microbiol. 68:6283-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]