Abstract
Archaeoglobus fulgidus accumulates di-myo-inositol phosphate (DIP) and diglycerol phosphate (DGP) in response to heat and osmotic stresses, respectively, and the level of glycero-phospho-myo-inositol (GPI) increases primarily when the two stresses are combined. In this work, the pathways for the biosynthesis of these three compatible solutes were established based on the detection of the relevant enzymatic activities and characterization of the intermediate metabolites by nuclear magnetic resonance analysis. The synthesis of DIP proceeds from glucose-6-phosphate via four steps: (i) glucose-6-phosphate was converted into l-myo-inositol 1-phosphate by l-myo-inositol 1-phosphate synthase; (ii) l-myo-inositol 1-phosphate was activated to CDP-inositol at the expense of CTP; this is the first demonstration of CDP-inositol synthesis in a biological system; (iii) CDP-inositol was coupled with l-myo-inositol 1-phosphate to yield a phosphorylated intermediate, 1,1′-di-myo-inosityl phosphate 3-phosphate (DIPP); (iv) finally, DIPP was dephosphorylated into DIP by the action of a phosphatase. The synthesis of the two other polyol-phosphodiesters, DGP and GPI, proceeds via the condensation of CDP-glycerol with the respective phosphorylated polyol, glycerol 3-phosphate for DGP and l-myo-inositol 1-phosphate for GPI, yielding the respective phosphorylated intermediates, 1X,1′X-diglyceryl phosphate 3-phosphate (DGPP) and 1-(1X-glyceryl) myo-inosityl phosphate 3-phosphate (GPIP), which are subsequently dephosphorylated to form the final products. The results disclosed here represent an important step toward the elucidation of the regulatory mechanisms underlying the differential accumulation of these compounds in response to heat and osmotic stresses.
Archaeoglobus fulgidus is a hyperthermophilic archaeon first isolated from marine hydrothermal vents (1). In addition to this type strain, designated VC-16, a few others belonging to the same species were isolated from hot marine sediments (strain Z) (34) and oil field water (strain 7324) (2). The type strain is able to grow between 60°C and 90°C, with an optimum around 83°C. Like other marine hyperthermophiles, A. fulgidus is slightly halophilic, displaying optimal growth in medium containing 1.9% NaCl (wt/vol) and unable to grow in medium containing more than 5.5% NaCl. Osmoregulation involves accumulation of organic solutes, some of them very unusual (8, 14). The solute pool comprises DGP, DIP, minor amounts of glutamate, and the newly discovered GPI (14, 16). The most striking feature is the occurrence of polyol-phosphodiesters, a class of compatible solutes encountered in organisms thriving in hot environments but totally absent in mesophiles (25, 26). Accordingly, a putative thermo-protective function of cell components in vivo was ascribed to them, and their action as protein stabilizers in vitro was confirmed at least for DIP and DGP (13, 22, 28).
DIP was the first of these compounds to be discovered in members of the genus Pyrococcus (28). Since then, DIP accumulation has been reported in a large proportion of hyperthermophiles from the domains Archaea and Bacteria, and DIP is presently regarded as the canonical solute at extremely high temperatures (23, 26). The biosynthesis of DIP has been studied in Methanococcus igneus and Pyrococcus woesei (3, 29), and two distinct synthetic routes were proposed. For M. igneus, Chen and coworkers (3) proposed that myo-inositol-1-P is synthesized from glucose-6-P by myo-inositol-1-P synthase. Part of the myo-inositol-1-P is dephosphorylated into myo-inositol while another part is presumably activated to CDP-inositol, and then both molecules are condensed to yield DIP; biosynthesis of CDP-inositol was not observed. For P. woesei, it was proposed that two molecules of myo-inositol-1-P are condensed to yield DIP with the consumption of NTP (29).
In contrast to that of DIP, nothing is known about the biosynthesis of DGP or GPI. DGP has been found only in Archaeoglobus species, where it accumulates primarily in response to the supraoptimal salinity of the growth medium (8, 16); GPI, a structural chimera of DIP and DGP, was recently identified in bacteria of the genus Aquifex and in the archaeon A. fulgidus (14).
The curious structural relationships between the three polyol-phosphodiesters accumulated by A. fulgidus are also reflected in their pattern of accumulation. While DIP increases consistently in response to elevated temperature and DGP to osmotic stress, the level of GPI seems to respond to a combination of both stresses (8, 14). Thus, the synthesis and accumulation of these three solutes seem intertwined by a regulatory mechanism linked to osmotic pressure and heat, which can be unraveled only once the biosynthetic routes of these solutes are known. With this goal in mind, we decided to study the pathways for the synthesis of DIP, DGP, and GPI. For this purpose, CDP-inositol and the stereospecific forms of myo-inositol-1-P were obtained by chemical synthesis. Herein, we report the elucidation of the three new pathways based on the activities of relevant enzymes detected in cell extracts of A. fulgidus and the NMR structural characterization of intermediate metabolites.
MATERIALS AND METHODS
Abbreviations.
DIP, di-myo-inositol phosphate; DIPP, 1,1′-di-myo-inosityl phosphate 3-phosphate; DGP, diglycerol phosphate; DGPP, 1X,1′X-diglyceryl phosphate 3-phosphate; GPI, glycero-phospho-myo-inositol; GPIP, 1-(1X-glyceryl) myo-inosityl phosphate 3-phosphate; TSPSA, 3-(trimethylsisyl) propane sulfonic acid (sodium salt); GCT, CTP:l-glycerol-3-P cytidylyltransferase; HMQC, heteronuclear multiple quantum coherence; COSY, homonuclear correlation spectroscopy; DMF, dimethylformamide; p-TsOH, p-toluenesulfonic acid; BnBr, benzyl bromide; TFA, trifluoroacetic acid; EtOH, ethanol; MeOH, methanol; DCM, dichloromethane; TBDMSOTf, tert-butyldimethylsilyl trifluoromethanesulfonate; TBAF, tetrabutylammonium fluoride; MCPBA, m-chloroperbenzoic acid; NMR, nuclear magnetic resonance; PIPES, piperazine-N,N′-bis(2-ethanesulfonic acid); MOPS, morpholinepropanesulfonic acid; NTP, nucleoside triphosphate.
Materials.
l-Glycerol 3-phosphate, dl-glycerol 3-phosphate, glucose 6-phosphate, NAD+, CTP, ATP, UTP, GTP, CDP-glycerol, and myo-inositol were purchased from Sigma-Aldrich (St. Louis, MO). NaF was obtained from Pfizer (New York, NY). dl-CDP-inositol, l-myo-inositol 1-phosphate, and d-myo-inositol 1-phosphate were obtained by chemical synthesis (this work). DGP and DIP were provided by bitop AG (Witten, Germany): DIP was extracted from Pyrococcus woesei, and DGP was obtained by chemical synthesis. GPI was isolated from A. fulgidus biomass (14).
Organism and growth conditions.
Archaeoglobus fulgidus strain 7324 (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) was used in this study. Compared with the type strain (VC-16) that we used in previous studies (13, 16), strain 7324 displayed greater biomass yield, and different growths produced very reproducible results insofar as enzymatic activities were concerned. The organism was grown anaerobically in 2-liter static vessels with a gas phase composed of N2 and CO2 (80:20, vol/vol) in the medium described by Labes and Schönheit (12) with modifications. The basal medium contained (per liter) 0.5 g yeast extract, 10 mmol of l-lactate, 3 g PIPES, 30 g NaCl, 2 mg (NH4)2Fe(SO4)2 · 7H2O, 100 ml of salt solution (without NaCl), and 10 ml of a modified trace element solution. The salt solution contained (per liter) 74 g MgSO4 · 7H2O, 3.4 g KCl, 27.5 g MgCl2 · 6H2O, 2.5 g NH4Cl, 1.4 g CaCl2 · 2H2O, and 1.4 g K2HPO4. The modified trace element solution contained (per liter) 1.5 g nitriloacetic acid, 0.05 g CoSO4 · 6H2O, 0.1 g CaCl2 · 2H2O, 0.1 g ZnSO4 · 7H2O, 0.01 g KAl(SO4)2 · 12H2O, 0.1 g H3BO3, 0.01 g Na2WO4 · 2H2O, 0.01 g Na2MoO4 · 2H2O, 0.3 g Na2SeO3, and 0.1 g NiCl2 · 6H2O.
For the investigation of DGP synthesis, cells were grown under osmotic stress conditions (83°C, 3% NaCl), while the biosynthesis of GPI and DIP was studied in cells cultured under a combination of heat and osmotic stress (87°C, 3% NaCl). Cell growth was assessed by measuring optical density at 600 nm.
Preparation of A. fulgidus cell extracts.
Cells were harvested by centrifugation (1,000 × g, 10 min) during late exponential growth phase (optical density at 600 nm = 0.35) and washed twice with MOPS buffer (50 mM, pH 7.6) containing 3% NaCl under anaerobic conditions. The cell pellet was suspended in MOPS buffer (50 mM, pH 7.6) containing 3% NaCl. Cells were disrupted in a French press, and the cell debris was removed by centrifugation (30,000 × g, 4°C, 30 min). Low-molecular-mass compounds were removed in a PD-10 column (Amersham Biosciences) equilibrated with MOPS buffer (50 mM, pH 8.1) containing 5 mM MgCl2. Protein content was estimated by a bicinchoninic acid protein assay kit (Pierce).
Enzyme assays.
Enzymatic assays were performed at 80°C in a total volume of 0.5 ml, and no effort was made to avoid oxygen. All the reaction mixtures contained 50 mM MOPS buffer, pH 8.1, 5 mM MgCl2, and 5 mM of putative substrates for each investigated pathway. The reactions were initiated by the addition of 50 mg (total protein) of A. fulgidus cell extract. After 1 hour of incubation, the reaction mixtures were centrifuged; 5 mM EDTA (pH 8.0) and 50 μl of 2H2O were added to the supernatant, and the pH was adjusted to 7.6. The reaction products were analyzed by 31P NMR spectroscopy.
DGP synthesis was investigated in cell extracts by using the following substrates: CDP-glycerol, dl-glycerol-3-P, l-glycerol-3-P, and glycerol. The biosynthetic pathway of GPI was examined using the following substrates: CDP-glycerol and l-myo-inositol-1-P (or d-myo-inositol-1-P or myo-inositol) or CDP-inositol and dl-glycerol-3-P (or glycerol). To detect the activities leading to DIP synthesis, the following substrates were used: CDP-inositol and l-myo-inositol-1-P (or d-myo-inositol-1-P or myo-inositol) or glucose-6-P, NAD+, and CTP. To test the specificity of the enzymes regarding nucleoside triphosphate usage, all the reactions requiring CTP were also carried out in the presence of ATP, UTP, or GTP. To examine the presence of phosphorylated intermediate compounds, a phosphatase inhibitor (NaF) was added to the reaction mixtures to a final concentration of 5 to 50 mM.
Purification of the phosphorylated intermediates.
The reaction mixtures containing each of the phosphorylated intermediates were purified by anionic exchange chromatography as follows. The sample was applied onto a QAE-Sephadex A-25 column previously equilibrated with 5.0 mM sodium bicarbonate (pH 9.8) and eluted with one bed volume of the same buffer, followed by a linear gradient of 5.0 mM to 1 M NaHCO3. The eluted fractions were analyzed by 31P NMR spectroscopy and subsequently desalted using an activated Dowex 50W-X8 resin and distilled water for elution. The active fractions were pooled, and the pH was raised to 6 with KOH. The samples were lyophilized and dissolved in 2H2O prior to NMR analysis.
NMR spectroscopy.
The identification and characterization of the phosphorylated intermediates in the synthesis of DIP, DGP, and GPI were accomplished using 1H, 13C, and 31P NMR spectroscopy. One-dimensional and two-dimensional spectra were recorded on a Bruker DRX500 spectrometer by using standard Bruker pulse programs (Bruker, Rheinstetten, Germany), following a strategy previously described (15, 30). 13C-1H correlation spectra were recorded using a delay of 3.44 ms for the evolution of the scalar couplings, while a delay of 62.5 ms was used for the 31P-1H correlation spectra. 1H and 13C NMR spectra were referenced to the resonance of 3-(trimethylsilyl)propanesulfonic acid (sodium salt), and 31P NMR spectra were referenced to the resonance of external 85% H3PO4, both designated at 0 ppm. Assignment of resonances to DIP, GPI, DGP, CDP-glycerol, and CDP-inositol were confirmed by the addition of small amounts of the authentic compounds.
Chemical synthesis.
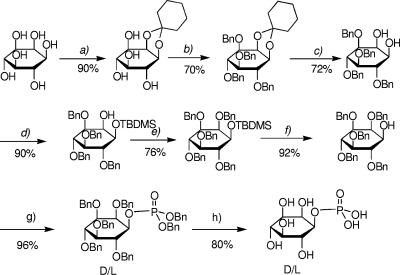
dl-myo-Inositol-1-P was obtained through a significant modification of the established procedures according to Fig. 1 (17, 32, 33). Enantiomerically pure d- and l-myo-inositol-1-P were prepared by optical resolution of dl-2,3,4,5,6-penta-O-benzyl-myo-inositol via separation of the diastereoisomeric camphanates (6). After hydrolysis, 1d- and 1l-2,3,4,5,6-penta-O-benzyl-myo-inositol were obtained separately and converted into optically pure d- and l-myo-inositol-1-P as with the racemic compound (Fig. 1). The synthesis of CDP-inositol was accomplished according to the established procedures (18, 24).
FIG. 1.
Scheme for the chemical synthesis of dl-myo-inositol 1-phosphate from myo-inositol. (a) (i) Cyclohexanone (16 eq), DMF-toluene, p-TsOH, 6 h, 150°C; (ii) EtOH, p-TsOH, 1 h, room temperature. (b) BnBr (12 eq), DMF, 16 h, room temperature. (c) TFA (7 eq), MeOH (1.2 eq), DCM. (d) TBDMSOTf (1.2 eq), (i-Pr)2Net (1.2 eq), DCM. (e) BnBr (4 eq), NaH (4 eq), DMF, 2 h, room temperature. (f) 1 M TBAF (1.5 eq), THF, 2 h, room temperature. (g) (i) (BnO)2PN(iPr)2 (1.5 eq), 1H-tetrazole (1.5 eq), DCM, 2 h 30 m, room temperature; (ii) MCPBA (2 eq), DCM, 1 h 30 m, −40°C to 0°C. (h) Pd-C, 20 lb/in2 H2, EtOH, 2 h, room temperature. The yields for the individual steps are indicated as percentages.
RESULTS
Biosynthesis of DIP.
To investigate DIP biosynthesis, glucose-6-P (the substrate of inositol-1-P synthase) plus NAD+ and CTP was incubated with a cell extract of A. fulgidus. DIP and CDP-inositol were identified by 31P NMR spectroscopy as final products in the reaction mixture, and the assignments were confirmed by spiking with the pure compounds. DIP and CDP-inositol were also formed when CTP and l-myo-inositol-1-P were provided as substrates (Fig. 2A). We verified that ATP, UTP, or GTP did not replace CTP in the synthesis of DIP or CDP-inositol. d-myo-Inositol-1-P and myo-inositol were also examined as possible substrates of CTP:l-inositol-1-P cytidylyltransferase, but none of them was used. Therefore, this new enzyme was specific for l-myo-inositol-1-P and CTP. By analogy with other cytidylyltransferases, pyrophosphate was expected as a product of the reaction but was not detected in the 31P NMR spectra of the reaction mixtures; this is explained by a very active pyrophosphatase present in A. fulgidus extracts (not shown).
FIG. 2.
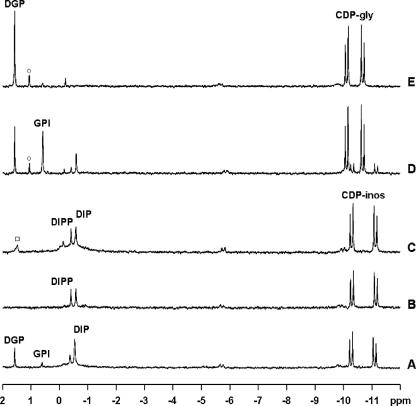
31P NMR spectra of the final products from incubation at 80°C of reaction mixtures containing A. fulgidus cell extract (50 mg total protein) in 50 mM MOPS buffer (pH 8.1), 5 mM MgCl2, and 5 mM of the following substrates: CTP plus dl-glycerol-3-P plus l-myo-inositol-1-P (A), CTP plus l-myo-inositol-1-P (B), CDP-inositol plus l-myo-inositol-1-P (C), CDP-glycerol plus l-myo-inositol-1-P (D), and CDP-glycerol plus dl-glycerol-3-P (E). Resonances due to CDP-inositol, CDP-glycerol, DIP, DIPP, DGP, and GPI are labeled. The spectra shown are the differences between the final spectra of the reaction mixtures with the substrates and the spectrum of the control (cell extract with no additions after 1 hour of incubation). The resonances labeled with ○ and □ are due to contaminants in the CDP-glycerol and CDP-inositol preparations, respectively.
These results definitely show that the synthesis of DIP proceeds from l-myo-inositol-1-P and CTP via the formation of the intermediate CDP-inositol. To our knowledge, this is the first demonstration of the biosynthesis of CDP-inositol. The subsequent reactions in the synthesis of DIP were further studied using CDP-inositol obtained by chemical synthesis and each of the following putative substrates: l-myo-inositol-1-P, d-myo-inositol-1-P, and myo-inositol. DIP synthesis was not detected from CDP-inositol and d-myo-inositol-1-P or myo-inositol but was clearly apparent when CDP-inositol and l-myo-inositol-1-P were used as substrates (Fig. 2C). Curiously, two resonances (−0.57 and −0.38 ppm) were detected in the phosphodiester region of the 31P NMR spectrum of the reaction mixture (Fig. 2B). The resonance at −0.57 ppm was due to DIP, but the other resonance could not be immediately assigned. The hypothesis that it was due to a phosphorylated form of DIP seemed a reasonable one. To confirm this hypothesis, the reaction was repeated with the addition of 7 mM NaF, a general phosphatase inhibitor. As a result, the intensity of the resonance at −0.38 ppm increased relative to the spectrum of the reaction mixture without fluoride. Moreover, after treatment with alkaline phosphatase, the new phosphodiester resonance disappeared, with the concomitant increase in the intensity of the DIP resonance (Fig. 3). The putative phosphorylated intermediate was purified by anion exchange chromatography and its structure established by NMR spectroscopy as DIPP (see below).
FIG. 3.
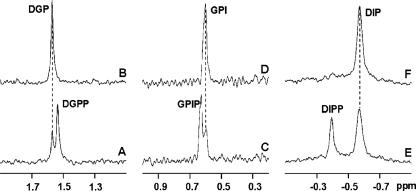
31P NMR spectra showing the conversion of the phosphorylated intermediates into the final products (DGP, GPI, and DIP) by the action of alkaline phosphatase. Panels A, C, and E show the spectra of the reaction mixtures containing cell extract, CTP, NaF, and the following: dl-glycerol-3-P (A), l-myo-inositol-1-P plus dl-glycerol-3-P (C), and l-myo-inositol-1-P (E). After spectral acquisition, samples A, C, and E were treated with alkaline phosphatase, originating from spectra B, D, and F, respectively. The phosphodiester resonances due to DIP (−0.57 ppm), DIPP (−0.38 ppm), GPI (0.60 ppm), GPIP (0.63 ppm), DGP (1.58 ppm), and DGPP (1.51 ppm) are shown.
Biosynthesis of DGP.
DGP synthesis was studied using CDP-glycerol, dl-glycerol-3-P, and glycerol as possible substrates. Maximal production of DGP was observed with CDP-glycerol and dl-glycerol-3-P (Fig. 2E). Production of DGP (around 55% of the maximum) was also detected when CDP-glycerol alone or in combination with glycerol was provided. This production was attributed to the presence of glycerol-3-P resulting from the thermal degradation of CDP-glycerol. No activity was observed when dl-glycerol-3-P and/or glycerol was used as a sole substrate. Additionally, it was checked that the pure enantiomeric form, l-glycerol-3-P, was a substrate for the enzyme; d-glycerol-3-P was not examined as it is not commercially available.
The synthesis of DGP from dl-glycerol-3-P and each of the nucleoside triphosphates CTP, ATP, UTP, and GTP was also examined. DGP was produced only when CTP was present, and in this case, the formation of CDP-glycerol was detected (not shown). To ascertain the presence of a possible phosphorylated intermediate, an assay using CDP-glycerol and dl-glycerol-3-P was performed in the presence of NaF (7.5 mM). A new resonance at 1.51 ppm was detected in the 31P NMR spectra, indicating the production of a new phosphodiester compound. After treatment with alkaline phosphatase, the new resonance disappeared, and the final intensity of the DGP resonance (1.58 ppm) increased by the corresponding amount (Fig. 3). This result provided strong evidence for the presence of a phosphorylated intermediate in the biosynthesis of DGP. The putative phosphorylated compound was partially purified and its structure determined by NMR spectroscopy (see below).
Biosynthesis of GPI.
An array of experiments was designed to investigate the synthesis of GPI. Given the chimeric structure of GPI containing glycerol and inositol moieties, CDP-glycerol and CDP-inositol were examined as putative polyol donors. CDP-glycerol was combined with myo-inositol, l-myo-inositol-1-P, or d-myo-inositol-1-P as a potential polyol acceptor; CDP-inositol was examined in combination with glycerol or dl-glycerol-3-P. Maximal formation of GPI (resonance at 0.60 ppm) was observed from CDP-glycerol and l-myo-inositol-1-P (Fig. 1D). Vestigial amounts of GPI were also detected when CDP-inositol and dl-glycerol 3-P were used (less than 10% of that for CDP-glycerol and l-myo-inositol-1-P), showing that the synthase is not absolutely specific for CDP-glycerol and l-myo-inositol-1-P. All the other assays were negative for GPI synthesis. As for DIP and DGP, CTP was the only nucleoside triphosphate that supported GPI formation.
DGP was synthesized whenever CDP-glycerol was supplied, a result explained by the generation of the additional substrate required for DGP synthesis during the thermal degradation of CDP-glycerol (see above). The addition of CDP-glycerol and l-myo-inositol-1-P led to the synthesis of GPI and DGP as major products, but minor amounts of DIP and CDP-inositol were also detected (Fig. 2D). This could be explained by the exchange between the glycerol and inositol moieties arising from the reversibility of the reaction catalyzed by “GPI synthase” in conjunction with the observed minor affinity for the alternative substrates (CDP-inositol and glycerol-3-P).
The presence of an intermediate phosphorylated compound in the synthesis of GPI was also confirmed: incubation of cell extracts with CDP-glycerol, l-myo-inositol-1-P, and 40 mM NaF led to the appearance of a new phosphorus resonance at 0.63 ppm that disappeared upon treatment with alkaline phosphatase (Fig. 3). The nature of the phosphorylated intermediate was established by NMR analysis after partial purification (see below).
NMR characterization of the phosphorylated intermediates.
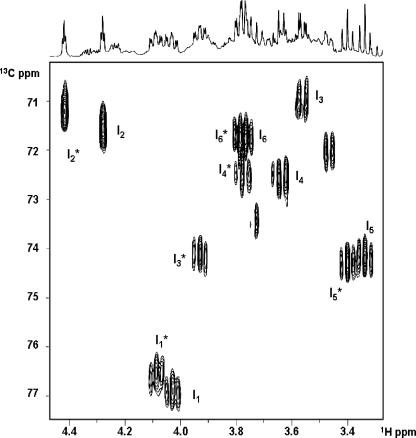
The proton correlation spectrum (COSY) of the purified DIPP allowed the sequential following of all resonances for the two distinct moieties in the compound. Proton-proton coupling patterns revealed these moieties as myo-inositol residues. The combination of the 13C-1H HMQC spectrum and the COSY spectrum permitted the assignment of all proton and carbon signals in the compound (Table 1). The signals belonging to one of the inositol moieties showed a complete overlap with those of DIP, while the other inositol moiety displayed shifts toward the lower field in the proton dimension (Fig. 4). In comparing the two moieties, it was interesting to note that the signal bearing the largest low-field shift was that of proton 3, while protons 2 and 4 showed moderate shifts, and protons 1 and 5 displayed even smaller ones. This made us suspect that the additional phosphate group was linked to position 3 of the inositol moiety. The proton-decoupled 31P NMR spectrum showed a resonance in the phosphomonoester and another in the phosphodiester region (4.78 and −0.38 ppm, respectively). Irradiation of the phosphodiester resonance resulted in the collapsing of the multiplets due to protons 1 in both inositol moieties, indicating that the two inositol moieties are linked via a phosphodiester bond at carbon 1. When the irradiation was performed at the monoester frequency, at 4.78 ppm, we observed changes only in the multiplet structure due to a proton at position 3 of the moiety originating shifted resonances, thus confirming the view that this moiety was phosphorylated and that the additional phosphate group was linked at position 3 of one of the inositol moieties. Therefore, the phosphorylated intermediate is 1,1′-di-myo-inosityl phosphate 3-phosphate.
TABLE 1.
NMR parameters of DIPP, GPIP, and DGPP
| Moiety | Chemical shifta (ppm)
|
|||||
|---|---|---|---|---|---|---|
| DIPP
|
GPIP
|
DGPP
|
||||
| 13C | 1H | 13C | 1H | 13C | 1H | |
| Inositol | ||||||
| C1 | 76.9 | 4.03 | ||||
| C2 | 71.6 | 4.28 | ||||
| C3 | 71.0 | 3.55 | ||||
| C4 | 72.6 | 3.64 | ||||
| C5 | 74.3 | 3.33 | ||||
| C6 | 71.9 | 3.76 | ||||
| Inositol-P | ||||||
| C1 | 76.6 | 4.08 | 76.5 | 4.03 | ||
| C2 | 71.2 | 4.41 | 71.3 | 4.39 | ||
| C3 | 74.2 | 3.92 | 74.7 | 3.92 | ||
| C4 | 72.5 | 3.77 | 72.3 | 3.78 | ||
| C5 | 74.3 | 3.39 | 74.5 | 3.39 | ||
| C6 | 71.8 | 3.78 | 71.3 | 3.78 | ||
| Glycerol | ||||||
| C1 | 67.3 | 4.00, 3.92 | 66.8 | 3.92, 3.85 | ||
| C2 | 71.2 | 3.90 | 71.0 | 3.90 | ||
| C3 | 62.4 | 3.73, 3.67 | 62.3 | 3.70, 3.60 | ||
| Glycerol-P | ||||||
| C1 | 67.0 | 3.94, 3.90 | ||||
| C2 | 70.7 | 3.98 | ||||
| C3 | 63.2 | 4.02, 3.94 | ||||
Referenced to the resonance of TSPSA, designated at 0.0 ppm. 31P NMR chemical shifts in the phosphodiester resonances of DIPP, GPIP, and DGPP were −0.38, 0.63, and 1.51 ppm, respectively; the phosphomonoester resonances were at 4.78, 4.81, and 4.88 ppm, respectively. The pH of the samples was 9.0.
FIG. 4.
13C-1H HMQC of DIPP partially purified from the reaction mixtures. Peaks due to the inositol and inositol-3-P moieties are labeled with I and I*, respectively. The subscript numerals represent proton numbering. Cross peaks correspond to connectivities between proton and carbon atoms covalently bonded.
The same overall strategy was used to discover the structure of the two other intermediates, DGPP and GPIP. In the case of the intermediate in the synthesis of GPI, partial purification of the compound led to a proton-decoupled 31P NMR spectrum bearing only two signals in the phosphomonoester and diester regions (4.81 and 0.63 ppm, respectively). When proton decoupling was removed, the phosphorus signal at 0.63 ppm revealed the complex structure expected for the phosphodiester signal of GPI, while the monoester signal at 4.81 ppm split into a doublet, meaning that it is coupled to a single proton. Therefore, the additional phosphate group could be linked only to the inositol moiety. Accordingly, the 13C-1H HMQC spectrum presented a group of six resonances that completely overlap with those of the phosphorylated inositol moiety of DIPP. The signals belonging to the glycerol moiety were assigned by comparison with those from GPI. To confirm the assignment, proton spectra were also acquired with selective irradiation of each of the phosphorous signals. Irradiation of the diester signal at 0.63 ppm caused the collapse of multiplicities in the signals at position 1 of the inositol and position 1 of the glycerol moiety, while irradiation at the frequency corresponding to 4.81 ppm affected only the proton signal assigned to position 3 of the inositol moiety. We conclude that the additional phosphate group in GPIP is located at position 3 of the inositol moiety and that the intermediate is 1-(1X-glyceryl) myo-inosityl phosphate 3-phosphate.
In the case of the intermediate in the synthesis of DGP, the proton-decoupled 31P NMR spectrum of the partially purified compound also showed a signal in the monoester and another signal in the diester region (4.88 and 1.51 ppm, respectively). Upon removal of proton decoupling, the diester signal at 1.51 ppm turned into a complex multiplet, while the signal at 4.88 ppm proved to be a triplet, meaning that it is coupled to a CH2 group. Therefore, the additional phosphate group is located at position 3 of one of the glycerol moieties. To strengthen the conclusion, two HMQC spectra were acquired. In the 1H-13C correlation spectrum, we found a set of signals that mimics those of DGP and another set shifted toward the lower field in the proton dimension. In the 1H-31P correlation spectrum, the phosphorous signal in the diester region at 1.51 ppm showed correlations with positions 1 of the glycerol moieties of both sets of signals, while the monoester signal at 4.88 ppm showed only one correlation to the signal assigned to position 3 of the low-field-shifted set of signals, thus establishing the phosphorylated intermediate as 1X,1′X-diglyceryl phosphate 3-phosphate. As indicated by the X,X′ notation, the stereochemistry of the reaction products was not established.
DISCUSSION
The biosynthetic pathways of the three major compatible solutes (DIP, DGP, and GPI) of Archaeoglobus fulgidus strain 7324 were elucidated based on the detection of the relevant enzymatic activities in cell extracts and the characterization of the intermediates and final products by NMR spectroscopy. The pattern of compatible solute accumulation in this strain was identical to that previously reported for the type strain A. fulgidus VC-16 (8, 16): the levels of DIP and DGP increased in response to heat and osmotic stress, respectively, while GPI increased primarily when the two stresses were combined (our unpublished data).
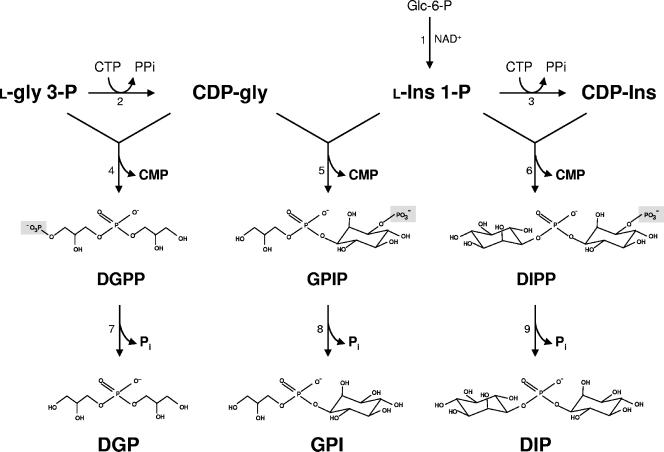
The routes for the synthesis of these three phosphodiester compounds have several features in common. (i) In all cases, CTP is the only nucleoside triphosphate used to activate the polyol, either glycerol or inositol; thus, CDP-glycerol or CDP-inositol is the polyol donor. (ii) The polyol acceptor is always in the phosphorylated form, i.e., l-glycerol 3-P or l-myo-inositol-1-P. (iii) The central biosynthetic step involves the condensation of CDP-polyol with the phosphorylated polyol to form a phosphorylated intermediate. (iv) Finally, the dephosphorylation of this intermediate leads to the final product, DIP, GPI, or DGP. This description establishes unequivocally the synthesis of DIP and DGP but not that of GPI, given the chimeric structure of this solute, involving the condensation of two different polyol moieties. Of the two possible substrate combinations, CDP-glycerol plus l-myo-inositol-1-P and CDP-inositol plus glycerol-3-P, the former is largely preferred in A. fulgidus. Therefore, the synthesis of GPI proceeds via the condensation of CDP-glycerol with l-inositol-1-P to yield GPIP, which is subsequently acted upon by a phosphatase (Fig. 5).
FIG. 5.
Proposed pathways for the synthesis of DIP, DGP, and GPI in A. fulgidus. Enzymes: 1, l-myo-inositol-1-P synthase; 2, CTP:glycerol-3-P cytidylyltransferase; 3, CTP:l-myo-inositol-1-P cytidylyltransferase; 4, DGPP synthase; 5, GPIP synthase; 6, DIPP synthase; 7, DGPP phosphatase; 8, GPIP phosphatase; and 9, DIPP phosphatase. Glc-6-P, glucose 6-phosphate; l-Ins-1-P, l-myo-inositol 1-phosphate; gly, glycerol; Ins, myo-inositol; and l-gly-3-P, l-glycerol 3-phosphate. The stereochemical configuration of the compounds has not been established.
This work provides definite evidence for the involvement of phosphorylated intermediates in the synthesis of either of these compatible solutes. There are no earlier reports on the synthesis of DGP or GPI, but the synthesis of DIP has been studied by other teams that suggested two different pathways (3, 29). None of the proposed pathways takes into account the occurrence of a phosphorylated intermediate. Chen et al. (3) propose that in M. igneus, DIP is formed in a single step from CDP-inositol and myo-inositol. On the other hand, the pathway proposed for P. woesei involves the condensation of two molecules of l-myo-inositol-1-P with the consumption of NTP, but no evidence for the formation of NDP-inositol was found (29).
The pathway for DIP synthesis proposed here for A. fulgidus is different from that found in M. igneus insofar as l-myo-inositol-1-P, instead of myo-inositol, is used by the synthase and the product of this reaction is a phosphorylated form of DIP instead of DIP itself. Our proposal for DIP synthesis in A. fulgidus is also different from that put forward a few years ago for P. woesei (29), but the coincidence of the input reagents in the two routes is a curious detail: two molecules of l-myo-inositol-1-P and one molecule of NTP. We demonstrated, however, that CDP-inositol and the phosphorylated form of DIP are intermediate metabolites in the pathway. To determine whether these discrepancies were associated with the distant phylogenetic relationship between the two organisms studied, the biosynthesis of DIP was also examined in Pyrococcus furiosus, an archaeon closely related to P. woesei. As with A. fulgidus, we observed the formation of CDP-inositol and the phosphorylated intermediate (DIPP) in cell extracts of P. furiosus when l-myo-inositol-1-P and CTP were provided as substrates (data not shown). Therefore, the biosynthetic routes proposed in Fig. 5 are supported by solid experimental evidence. It is intriguing, however, that this reaction scheme appears to point toward a d,l configuration of the inositol moieties in DIP rather than the l,l stereochemical configuration found in DIP isolated from Pyrococcus spp. (reference 31 and our unpublished data). This apparent paradox is expected to be solved after full characterization of the catalytic mechanism of DIPP synthase, an ongoing project in our laboratory.
To our knowledge, this is the first time that the synthesis of CDP-inositol has been demonstrated in a biological system. Although Chen et al. (3) have shown that CDP-inositol is used for DIP synthesis in cell extracts of M. igneus, those authors failed to detect the formation of this activated compound. Remarkably, thorough searches in the literature confirmed that CDP-inositol has not been found in any metabolic pathway other than DIP biosynthesis. This is somewhat surprising since the inositol moiety is found in a variety of biological molecules, e.g., as a constituent of phospholipid polar heads and glycolipid protein anchors (10, 11). On the other hand, this finding strengthens the view that hyperthermophiles have developed unique metabolic strategies for synthesizing unique molecules that are likely to play a role in the adaptation of these organisms to extremely hot environments.
In contrast with that of CDP-inositol, the synthesis of CDP-glycerol is widely distributed and has been studied in detail, particularly in Bacillus subtilis (19, 20). GCT catalyzes the reversible formation of CDP-glycerol and pyrophosphate from CTP and l-glycerol-3-P. A search of the A. fulgidus genome revealed an open reading frame (AF1418) with 36% identity with the GCT of B. subtilis that most likely encodes the activity responsible for CDP-glycerol synthesis in the archaeon. CTP:l-inositol-1-P cytidylyltransferase remains unknown, but it is obvious that it should be related to GCT, both enzymes belonging to the cytidylyltransferase family.
DGP has been found only in strains of the genus Archaeoglobus, but if the stereochemistry is ignored, the unit glycero-phospho-glycerol also appears in the lipid phosphatidylglycerol and in a linear 1,3-linked poly(glycerol phosphate) polymer which is a major component of teichoic acids in the walls of gram-positive cells. It is interesting to note the resemblance between the route disclosed here for DGP synthesis in A. fulgidus and the biosynthetic pathways reported for those biomolecules in different organisms (5, 27). For example, phosphatidylglycerol is also formed by dephosphorylation of a phosphorylated intermediate (phosphatidylglycerol phosphate) resulting from the condensation of CDP-phosphatidyl and glycerol-3-P (5).
Like DGP, GPI is a rare solute so far encountered only in hyperthermophiles of the genera Archaeoglobus and Aquifex. The occurrence of GPI as a cell metabolite has not been reported elsewhere; however, the unit glycero-phospho-inositol is found in phosphatidylinositol, an important constituent of lipid membranes. Interestingly, in this case, the synthesis proceeds in a single step involving the condensation of CDP-diacylglycerol with myo-inositol to yield the final product, phosphatidylinositol (11).
The preponderance of phosphodiester compounds in the solute pool of the hyperthermophile A. fulgidus is noteworthy, especially because this class of compounds was never reported to play a role in stress adaptation of mesophilic organisms. In contrast, sugars, polyols, amino acids, and derivatives are canonical compatible solutes of mesophiles. We have shown that the synthesis of DIP, DGP, and GPI proceeds in two steps, with the involvement of a phosphorylated intermediate that is subsequently dephosphorylated. Actually, this is the most common strategy for the synthesis of sugars or sugar derivatives, such as mannosylglycerate, glucosylglycerate, glucosylglycerol, trehalose, and sucrose, for the purpose of osmoprotection or thermoprotection (4, 7, 9, 16, 21). It seems as though the concerted action of a synthase and a phosphatase, resulting in the irreversible synthesis of the final product, was selected throughout evolution as the most efficient strategy for allowing accumulation of compatible solutes to high levels, which can easily reach the molar range in the cytoplasm.
In conclusion, the work presented here uncovered the routes for the synthesis of three major compatible solutes in A. fulgidus. These pathways must be differentially regulated by salt and temperature, as the pattern of accumulation of DIP, DGP, and GPI depends clearly on the type of stress imposed. This study represents an important step toward the elucidation of the role of these pathways in the strategies of osmo- and thermoadaptation in hyperthermophiles, a goal that can be achieved only with knowledge of the genes and enzymes implicated in these novel biosynthetic routes. This is the immediate purpose of current work in our laboratory.
Acknowledgments
This work was funded by the European Commission, contract COOP-CT-2003-508644, and Fundação para a Ciência e a Tecnologia/FEDER and POCTI Portugal, Project A004/2005 Action V.5.1., SFRH/BD/5076/2001 to L.G.G., SFRH/BD/25539/2005 to M.V.R., and SFRH/BPD/14841/2003 to N.B.
N.B. and L.G.G. provided equal contribution to this work. We thank Carla P. Almeida for technical support.
Footnotes
Published ahead of print on 6 October 2006.
We dedicate this work to the memory of the great scientist and founder of our institute, António V. Xavier, who died prematurely on 7 May 2006.
REFERENCES
- 1.Achenbach-Richter, L., K. O. Stetter, and C. R. Woese. 1987. A possible biochemical missing link among archaebacteria. Nature 327:348-349. [DOI] [PubMed] [Google Scholar]
- 2.Beeder, J., R. K. Nilsen, J. T. Rosnes, T. Torsvik, and T. Lien. 1994. Archaeoglobus fulgidus isolated from hot North Sea oil field waters. Appl. Environ. Microbiol. 60:1227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, L., E. T. Spiliotis, and M. F. Roberts. 1998. Biosynthesis of di-myo-inositol-1,1′-phosphate, a novel osmolyte in hyperthermophilic archaea. J. Bacteriol. 180:3785-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa, J., N. Empadinhas, L. Gonçalves, P. Lamosa, H. Santos, and M. S. da Costa. 2006. Characterization of the biosynthetic pathway of glucosylglycerate in the archaeon Methanococcoides burtonii. J. Bacteriol. 188:1022-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daiyasu, H., K. Kuma, T. Yokoi, H. Morii, Y. Koga, and H. Toh. 2005. A study of archaeal enzymes involved in polar lipid synthesis linking amino acid sequence information, genomic contexts and lipid composition. Archaea 1:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreef, C. E., M. Douwes, C. J. Elie, G. A. Marel, and J. H. Boom. 1991. Application of the bifunctional phosphonylating agent bis[6-(trifluoromethyl)benzotriazol-1-yl] methylphosphonate towards the preparation of isosteric d-myo-inositol phospholipid and phosphate analogues. Synthesis 1991:443-447. [Google Scholar]
- 7.Giaever, H. M., O. B. Styrvold, I. Kaasen, and A. R. Strom. 1988. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J. Bacteriol. 170:2841-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonçalves, L. G., R. Huber, M. S. da Costa, and H. Santos. 2003. A variant of the hyperthermophiles Archaeoglobus fulgidus adapted to grow at high salinity. FEMS Microbiol. Lett. 218:239-244. [DOI] [PubMed] [Google Scholar]
- 9.Hagemann, M., U. Effmert, T. Kerstan, A. Schoor, and N. Erdmann. 2001. Biochemical characterization of glucosylglycerol-phosphate synthase of Synechocystis sp. strain PCC 6803: comparison of crude, purified, and recombinant enzymes. Curr. Microbiol. 43:278-283. [DOI] [PubMed] [Google Scholar]
- 10.Ikezawa, H. 2002. Glycosylphosphatidylinositol (GPI)-anchored proteins. Biol. Pharm. Bull. 25:409-417. [DOI] [PubMed] [Google Scholar]
- 11.Kent, C., G. M. Carman, M. W. Spence, and W. Dowhan. 1991. Regulation of eukaryotic phospholipid metabolism. FASEB J. 5:2258-2266. [DOI] [PubMed] [Google Scholar]
- 12.Labes, A., and P. Schönheit. 2001. ADP-dependent glucokinase from the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus strain. Arch. Microbiol. 176:329-338. [DOI] [PubMed] [Google Scholar]
- 13.Lamosa, P., A. Burke, R. Peist, R. Huber, M. Y. Liu, G. Silva, C. Rodrigues-Pousada, J. LeGall, C. Maycock, and H. Santos. 2000. Thermostabilization of proteins by diglycerol phosphate, a new compatible solute from the hyperthermophile Archaeoglobus fulgidus. Appl. Environ. Microbiol. 66:1974-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamosa, P., L. G. Gonçalves, M. V. Rodrigues, L. O. Martins, N. D. H. Raven, and H. Santos. 2006. Occurrence of 1-glyceryl-1-myo-inosityl phosphate in hyperthermophiles. Appl. Environ. Microbiol. 72:6169-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamosa, P., L. O. Martins, M. S. da Costa, and H. Santos. 1998. Effects of temperature, salinity, and medium composition on compatible solute accumulation by Thermococcus spp. Appl. Environ. Microbiol. 64:3591-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martins, L. O., R. Huber, H. Huber, K. O. Stetter, M. S. da Costa, and H. Santos. 1997. Organic solutes in hyperthermophilic Archaea. Appl. Environ. Microbiol. 63:896-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massy, D. J., and P. Wyss. 1990. Chemical synthesis of GPIs and GPI-anchored glycopeptides. Helv. Chim. Acta 73:1037-1057. [Google Scholar]
- 18.Moffatt, J. G., and H. G. Khorana. 1961. Nucleoside polyphosphates. X. 1 The synthesis and some reactions of nucleoside-5′ phosphoromorpholidates and related compounds. Improved methods for the preparation of nucleoside-5′ polyphosphates J. Am. Chem. Soc. 83:649-658. [Google Scholar]
- 19.Park, Y. S., T. D. Sweitzer, J. E. Dixon, and C. Kent. 1993. Expression, purification, and characterization of CTP:glycerol-3-phosphate cytidylyltransferase from Bacillus subtilis. J. Biol. Chem. 268:16648-16654. [PubMed] [Google Scholar]
- 20.Pattridge, K. A., C. H. Weber, J. A. Friesen, S. Sanker, C. Kent, and M. L. Ludwig. 2003. Glycerol-3-phosphate cytidylyltransferase. Structural changes induced by binding of CDP-glycerol and the role of lysine residues in catalysis. J. Biol. Chem. 278:51863-51871. [DOI] [PubMed] [Google Scholar]
- 21.Porchia, A. C., and G. L. Salerno. 1996. Sucrose biosynthesis in a prokaryotic organism: presence of two sucrose-phosphate synthases in Anabaena with remarkable differences compared with the plant enzymes. Proc. Natl. Acad. Sci. USA 93:13600-13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramakrishnan, V., M. F. J. M. Verhagen, and M. W. W. Adams. 1997. Characterization of di-myo-inositol-1,1′-phosphate in the hyperthermophilic bacterium Thermotoga maritima. Appl. Environ. Microbiol. 63:347-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts, M. F. 2005. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Systems 1:5 [Online.] http://www.salinesystems.org/content/1/1/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roseman, S., J. J. Distler, J. G. Moffatt, and H. G. Khorana. 1961. Nucleoside polyphosphates. XI. 1 An improved general method for the synthesis of nucleotide coenzymes. Syntheses of uridine-5′, cytidine-5′ and guanosine-5′ diphosphate derivatives. J. Am. Chem. Soc. 83:659-663. [Google Scholar]
- 25.Santos, H., and M. S. da Costa. 2001. Organic solutes from thermophiles and hyperthermophiles. Methods Enzymol. 334:302-315. [DOI] [PubMed] [Google Scholar]
- 26.Santos, H., P. Lamosa, T. Q. Faria, N. Borges, and C. Neves. 2006. The physiological role, biosynthesis, and mode of action of compatible solutes from (hyper)thermophiles. In C. Gerday and N. Glandorf (ed.), Physiology and biochemistry of extremophiles. ASM Press, Washington, D.C.
- 27.Schertzer, J. W., and E. D. Brown. 2003. Purified, recombinant TagF protein from Bacillus subtilis 168 catalyzes the polymerization of glycerol phosphate onto a membrane acceptor in vitro. J. Biol. Chem. 278:18002-18007. [DOI] [PubMed] [Google Scholar]
- 28.Scholz, S., J. Sonnenbichler, W. Schäfer, and R. Hensel. 1992. Di-myo-inositol-1,1′-phosphate: a new inositol phosphate isolated from Pyrococcus woesei. FEBS Lett. 306:239-242. [DOI] [PubMed] [Google Scholar]
- 29.Scholz, S., S. Wolff, and R. Hensel. 1998. The biosynthesis pathway of di-myo-inositol-1,1′-phosphate in Pyrococcus woesei. FEMS Microbiol. Lett. 168:37-42. [Google Scholar]
- 30.Silva, Z., N. Borges, L. O. Martins, R. Wait, M. S. da Costa, and H. Santos. 1999. Combined effect of the growth temperature and salinity of the medium on the accumulation of compatible solutes by Rhodothermus marinus and Rhodothermus obamensis. Extremophiles 3:163-172. [DOI] [PubMed] [Google Scholar]
- 31.Van Leeuwen, S. H., G. A. van der Marel, R. Hensel, and J. H. van Boom. 1994. Synthesis of L,L di-myo-inositol-1,1′-phosphate: a novel inositol phosphate from Pyrococcus woesei. Recl. Trav. Chim. Pays-Bas 113:335-336. [Google Scholar]
- 32.Watanabe, Y., T. Yamamoto, and T. Okazaki. 1997. Synthesis of 2,6-di-O-α-D-mannopyranosylphosphatidyl-D-myo-inositol. Utilization of glycosylation and phosphorylation based on phosphite chemistry. Tetrahedron 53:903-918. [Google Scholar]
- 33.Yu, K.-L., and, B. Fraser-Reid. 1988. A novel reagent for the synthesis of myo-inositol phosphates: N,N-diisopropyl dibenzyl phosphoramidite. Tetrahedron Lett. 29:979-982. [Google Scholar]
- 34.Zellner, G., E. Stackebrandt, H. Kneifel, P. Messner, U. B. Sleytr, E. Conway de Macario, H.-P. Zabel, K. O. Stetter, and J. Winter. 1989. Isolation and characterization of a thermophilic, sulfate reducing archaebacterium, Archaeoglobus fulgidus strain Z. Syt. Appl. Microbiol. 11:151-160. [Google Scholar]