Abstract
In Escherichia coli the gad system protects the cell from the extreme acid stress encountered during transit through the host stomach. The structural genes gadA, gadB, and gadC encode two glutamate decarboxylase isoforms and a glutamate/γ-aminobutyrate (GABA) antiporter, respectively. Glutamate decarboxylation involves both proton consumption and production of GABA, a neutral compound which is finally exported via the GadC antiporter. Regulation of gadA and gadBC transcription is very complex, involving several circuits controlling expression under different growth phase, medium, and pH conditions. In this study we found that the AraC-like activators GadX and GadW share the same 44-bp binding sites in the gadA and gadBC regulatory regions. The common binding sites are centered at 110.5 bp and 220.5 bp upstream of the transcriptional start points of the gadA and gadBC genes, respectively. At the gadA promoter this regulatory element overlaps one of the binding sites of the repressor H-NS. The DNA of the gadBC promoter has an intrinsic bend which is centered at position −121. These findings, combined with transcriptional regulation studies, may account for the two different mechanisms of transcriptional activation by GadX and GadW at the two promoters studied. We speculate that while at the gadA promoter GadX and GadW activate transcription by displacing H-NS via an antirepressor mechanism, at the gadBC promoter the mechanism of activation involves looping of the DNA sequence between the promoter and the activator binding site.
Colonization of the intestine relies largely on the ability of enteric bacteria to survive in the extremely acidic environment of the stomach, the major bactericidal barrier of the gastrointestinal tract. In several gram-positive and gram-negative enteric bacteria, the glutamate-based acid resistance system (gad system) plays a major role in protection of the cell from the deleterious effects of a high-proton-concentration environment (6, 7, 10, 32). In Escherichia coli the gad system relies on the intracellular activity of two isoforms of glutamate decarboxylase, GadA and GadB, which catalyze the proton-consuming conversion of glutamate to γ-aminobutyrate (GABA), and on the activity of a membrane protein, GadC, which is responsible for glutamate/GABA antiport. The current model of amino acid-based acid resistance (30) postulates that under extremely acidic conditions (external pH, <2.5) protons leak into the cell, thereby causing both a decrease in the internal pH to about pH 4.5 and elimination of the electrical transmembrane potential (ΔΨ). At pH 4.5, glutamate decarboxylase, an enzyme with an acidic pH optimum, counteracts further decreases in the internal pH by producing intracellular positive charges (Glu−1 to GABA0) which lead to inversion of the transmembrane potential (positive inside) (30). However, excessive positive ΔΨ values do not occur because chloride ions enter the cell in exchange for H+ via chloride channels (1). As the external milieu becomes less acidic, the combined action of the decarboxylase (proton consumption) and the antiporter (GABA export) restores the internal pH to almost neutral and the internal ΔΨ to a negative value. Mild acidification of the intracellular environment and the presence of chloride are also responsible for glutamate decarboxylase activation, which is a highly cooperative process leading to active site opening and recruitment of the enzyme to the cytoplasmic side of the inner membrane, the cellular region thought to be most affected by protons leaking in (4, 16).
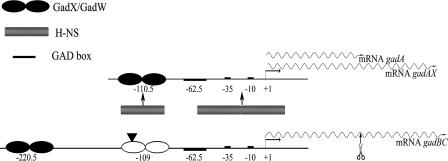
Two promoters are involved in transcription of the gad structural genes (Fig. 1). The gadA and gadB genes are located in two distinct loci of the chromosome, 2.1 Mb apart. The gadB gene is cotranscribed with gadC in a dicistronic gadBC mRNA, which is further processed to gadB- and gadC-specific transcripts in the gadBC intergenic region (10). The gadA gene is either independently transcribed (10) or cotranscribed with the downstream regulatory gene gadX, which is also independently transcribed from its indigenous promoter (37). Studies aimed at understanding the regulation of the gad system at the transcriptional level have demonstrated that the control is very complex and involves at least three circuits in which the contributions of the EvgAS two-component system and of specific transcriptional regulators (GadX, GadW, YdeO, and GadE) have been established (for a review, see reference 12). The central activator is the LuxR-like protein GadE (19), which binds at the so-called GAD box (Fig. 1). The role of GadX, GadW, and YdeO, all of which are members of the AraC-like family of transcriptional regulators, is restricted to specific circuits. The involvement of global regulators, such as the histone-like protein H-NS, the alternative RNA polymerase sigma factor of the stationary phase RpoS, and the cAMP receptor protein CRP, has also been demonstrated (for a review, see reference 12). The gad system is also subject to posttranscriptional control by the GTPase TrmE (15) and, at the level of the gadX mRNA, by the small GadY RNA (25).
FIG. 1.
Diagram of the gadAX and gadBC operons. The transcription start sites (+1) are indicated by bent arrows. The gadA gene is either cotranscribed with the downstream gadX gene (gadAX) (37) or transcribed as monocistronic gadA mRNA (10). The gadBC mRNA can be subjected to endonucleolytic processing within the gadBC intergenic region (10). The gadA (monocistronic) and gadB (processed) mRNAs are similar lengths (approximately 1.5 kb) and therefore have similar electrophoretic mobilities on an agarose gel. The H-NS binding sites responsible for gadA repression (14) are indicated. The GadX/GadW binding sites described in this paper are indicated by solid ellipses. The bending center in the gadBC regulatory region, identified in this work, is indicated by a solid triangle.
Most previous studies of regulation of the gad system were performed using immunoblot analyses with anti-GadA/B antibodies, which could not discriminate between the GadA and GadB isoforms. Therefore, the activation circuits described previously do not provide evidence for possible differences in the activation mechanisms for the structural genes gadA and gadBC. However, a recent report showed that H-NS directly binds the promoter region of gadX and gadA but not the promoter region of gadBC (14) (Fig. 1). In the present work, we obtained further evidence that distinct molecular mechanisms are responsible for the GadX- and GadW-dependent activation of the gadA and gadBC genes.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
The bacterial strains and plasmids used in this study are listed in Table 1. The growth of bacterial cultures was monitored by determining the optical density at 600 nm (OD600) with a diode array spectrophotometer (HP8452; Agilent Technologies). The media used were LB (2), LB-MES at pH 5.5 (LB buffered to pH 5.5 with 100 mM morpholineethanesulfonic acid [MES]), and LB-MOPS at pH 8.0 [LB buffered to pH 8.0 with 100 mM 3-(N-morpholino)propanesulfonic acid (MOPS)]. Antibiotics were added at the following concentrations: ampicillin, 100 μg/ml; streptomycin, 20 μg/ml; kanamycin, 25 μg/ml; and chloramphenicol, 35 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype | Reference or source |
|---|---|---|
| Strains | ||
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC ptsF25 rbsR | 5 |
| MG1655 | F− λ−rph−1 | GSC7740 |
| JM109 | F′ traD36 proA+ proB+ lacIqlacZΔM15 recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB) | 2 |
| FB8 | Wild-type E. coli K-12, UTH1038 | 17 |
| MC4100gadX | MC4100 gadX::Cmr | This study |
| MC4100gadW | MC4100 gadW::Cmr | This study |
| MC4100gadE | MC4100 gadE::Kanr | This study |
| MG1655gadW | MG1655 gadW::Cmr | This study |
| Plasmids | ||
| pBs | (pBluescriptSK) multicopy phagemid vector; ColE1 replicon, lacZα bla | Stratagene |
| pBsW | 1,080-bp EcoRI-BamHI fragment encompassing the gadW gene | This study |
| pBsX | 2.1-kb EcoRI-ClaI genomic fragment encompassing the gadX gene, the intergenic gadXW region, and first 162 nt of the gadW ORF in pBs | 37 |
| pBsX′ | 1.7-kb EcoRI-EcoRV fragment encompassing the gadX gene in pBs | This study |
| pTgadW | gadW ORF (735 bp) cloned in pBs-derived T/A vector | 22 |
| pBsPgadA | Promoter region of gadA (from position −176 to position 77) in pBs | 37 |
| pBsPgadBC | Promoter region of gadB (from position −173 to position 77) in pBs | 37 |
| pBsPgadB-307 | Promoter region of gadB (from position −307 to position 77) in pBs | This study |
| pMAL-c2 | Phagemid vector; ColE1 replicon, Ptac malE::lacZα lacIqbla | NEB |
| pmalE::gadW | gadW ORF and part of the multiple-cloning site of pBs (764 bp) ligated to the EcoRI-HindIII sites of pMAL-c2 | This study |
| pmalE::gadX | gadX ORF and part of the multiple-cloning site of pBs (832 bp) ligated to the EcoRI-HindIII sites of pMAL-c2 | 37 |
| pUC18 | Multicopy vector; ColE1 replicon, lacZα bla | NEB |
| pUCgadW | 761-bp gadW PCR fragment ligated to the EcoRI-BamHI sites of pUC18 | This study |
| pQE60 | Expression vector; ColE1 replicon, PT5-lacO RBSII | QIAGEN |
| pQEgadW | 785-bp EcoRI-HindIII gadW fragment from pUCgadW in pQE60 | This study |
| pRS415 | Operon fusion vector; ColE1 replicon lacZYA bla | 34 |
| pKgadB-244 | Promoter region of gadB (from position −244 to position 77) in pKK232.8 | 14 |
Construction of plasmids and mutant strains.
The gadX-overexpressing construct pBsX′ was generated from plasmid pBsX (37) by replacing a 700-bp EcoRV fragment (from nucleotide [nt] 652 in the gadX open reading frame [ORF] to nt 156 in the downstream gadW ORF) with a 271-bp PCR-generated fragment (from nt 652 in the gadX ORF to nt 98 downstream of the gadX stop codon).
Plasmid pBsW was constructed by cloning at the EcoRI and BamHI restriction sites of plasmid pBs a 1,080-bp PCR fragment obtained with oligonucleotides Wfor(−201) and Wrev(+879) (Table 2), using genomic DNA from E. coli ATCC 11246 as the template. The 1,080-bp PCR fragment spanned from position −201 upstream of the gadW ATG start codon to position 150 downstream of the gadW stop codon.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequencea | Restriction site |
|---|---|---|
| Wfor(−201) | 5′-GGGAATTCATAAACATAAGCTATACGCTG-3′ | EcoRI |
| Wfor(−14) | 5′-GGGAATTCTATAAGGGATAATCATGACT-3′ | EcoRI |
| Wfor(+1) | 5′-GGCATATGACTCATGTCTGCTC-3′ | NdeI |
| Wrev(+731) | 5′-CCGGATCCTGTCAGGAAAAGGTACC-3′ | BamHI |
| Wrev(+879) | 5′-GGGGATCCAAACATGCCGAGTCTTTTCC-3′ | BamHI |
| PgadB-307for | 5′-GGGGATCCGGGGATTTTATCAATAATCGC-3′ | BamHI |
| PgadBrev | 5′-GGAAGCTTCGTGAATCGAGTAGTTCCGAC-3′ | HindIII |
| PCgadArev | 5′-CCGGATCCATTTCGAACTCCTTAAA-3′ | BamHI |
| PCgadBrev | 5′-CCGGATCCATCCATTTTAAACTCCTTAA-3′ | BamHI |
| PCgadAforH | 5′-CCAAGCTTCCGTACTTCCATCGCG-3′ | HindIII |
| PCgadAforB | 5′-CCAGATCTCCGTACTTCCATCGCG-3′ | BglII |
| PCgadBforH | 5′-GGAAGCTTCGCTATTTTTATGTAATAATTT-3′ | HindIII |
| PCgadBforB | 5′-GGAGATCTCGCTATTTTTATGTAATAATTT-3′ | BglII |
| Xfor(−221) | 5′-GATATATGTTATATGAATGTTTA-3 ′ | |
| Xrev(+854) | 5′-CTATAATCTTATTCCTTCCG-3′ | |
| ΔgadEfor | 5′-AGCAAGTTATGATTTTTCTCATGACGAAAGATTCTTGTGTAGGCTGGAGCTGCTGCTTC-3 | |
| ΔgadErev | 5′-CTAAAAATAAGATGTGATACCCAGGGTGACGATGTCGCATTCCGGGGATCCGTCGACC-3′ | |
| rRNA16Sfor | 5′-CGTTACCCGCAGAAGAAGC-3′ | |
| rRNA16Srev | 5′-GTGGACTACCAGGGTATCTAATCC-3′ | |
| gadA/Brev | 5′-GGCGCATTCCACAGATCG-3′ | |
| gadBrev | 5′-TTGTCGATCCAGTTTTTGTTAATG-3′ | |
| gadBfor | 5′-GGAGTTTAAAATGGATAAGAAGCAAG-3′ | |
| gadXrev | 5′-CTTATTCTGCGATAGTTGCG-3′ | |
| gadXfor | 5′-AATATTAAGTCAACTATGCAATCACTAC-3′ |
Restriction sites are underlined.
To construct pQEgadW (containing the entire gadW ORF and its putative ribosome binding site under control of the PT5-lacO isopropyl-β-d-thiogalactopyranoside [IPTG]-inducible exogenous promoter of pQE60), the gadW gene was initially amplified by PCR from E. coli ATCC 11246 genomic DNA with oligonucleotides Wfor(−14) and Wrev(+731) (Table 2) and subsequently subcloned into pUC18 at the EcoRI and BamHI sites. The 785-bp gadW fragment, obtained by digestion of pUCgadW with EcoRI and HindIII, was finally ligated into the corresponding restriction sites of pQE60 (QIAGEN).
The 384-bp gadB fragment spanning from position −307 to position 77 (designated pBsPgadB-307) was generated by PCR using pBsB (11) as the template and oligonucleotides PgadB-307for and PgadBrev (Table 2). The amplicon was digested with the BamHI and HindIII restriction enzymes and cloned into the multiple-cloning site of plasmid pBs (Table 1), digested with the same restriction enzymes.
Following cloning, all PCR-amplified fragments were sequenced to check for the correct orientation within the plasmid and for the absence of unwanted mutations.
The gadW and gadX genes were disrupted by replacing with a cat cassette (derived from Campylobacter coli) (39) a 200-bp ClaI gadW internal fragment of pBsW and a 560-bp NcoI-EcoRV gadX internal fragment of pBsX′, respectively. PCR amplification of each cat-containing gene was carried out with the following pairs of oligonucleotides: primers Wfor(−14) and Wrev(+731) and primers Xfor(−221) and Xrev(+854) (Table 2). To generate the gadE mutant strain, template plasmid pKD13 (9) and primers ΔgadEfor and ΔgadErev (Table 2) were used for PCR amplification. The gadX, gadW, and gadE mutant strains were generated from wild-type strains E. coli MC4100 and MG1655 using the procedure described by Datsenko and Wanner (9).
RNA analyses.
RNA was isolated from cells grown under different conditions at specified optical densities, using a modified hot-phenol extraction method (10) or an RNeasy mini kit (QIAGEN). RNA concentration and quality were evaluated by determining (in 0.1 N NaOH) the OD260 and the 260 nm/280 nm ratio, respectively. Electrophoresis, Northern blotting, and membrane hybridization were carried out as previously described (10, 37).
Reverse transcription-PCR.
Quantitative real-time reverse transcription-PCRs were performed in two steps. Reverse transcription of DNase-treated RNAs (3 μg) was carried out using 10 U Transcriptor reverse transcriptase (Roche) and oligonucleotides rRNA16Srev, gadA/Brev, and gadXrev (Table 2). Real-time PCR was performed with a Chromo4 real-time PCR instrument (Bio-Rad Laboratories) using LightCycler FastStart DNA Master SYBR green I (Roche) and the following pairs of oligonucleotides (Table 2): gadBfor and gadBrev for amplification of gadB-specific mRNA, gadXfor and gadXrev for amplification gadX-specific mRNA, and rRNA16Sfor and rRNA16Srev for amplification of the internal control 16S rRNA.
Construction of single-copy gad::lacZ transcriptional fusions and β-galactosidase assay.
Construction of gadA(−176/77)::lacZ and gadB(−244/77)::lacZ chromosomal transcriptional fusions was described elsewhere (14). Lysogens were obtained by infecting E. coli strain MC4100 and its isogenic gadX, gadW, and gadE derivatives as described by Simons et al. (34). β-Galactosidase activity was determined as described by Miller (24), as follows: 1,000 × (OD420 − 1.75 × OD550)/(OD600 × reaction time × volume).
Expression and purification of MalE-GadW and MalE-GadX fusion proteins.
GadW was overexpressed as a MalE-GadW fusion protein. To generate the IPTG-inducible pmalE::gadW construct, the entire gadW gene was amplified by PCR from E. coli ATCC 11246 genomic DNA with oligonucleotides Wfor(+1) and Wrev(+731) (Table 2). The cloning strategy was essentially the strategy described elsewhere for pmalE::gadX construction (37). MalE-GadX and MalE-GadW were overexpressed under slightly different conditions. Overnight cultures of either E. coli JM109/pmalE::gadW or E. coli JM109/pmalE::gadX were diluted 1:100 in 125 ml of LB containing 0.2% glucose and 100 μg/ml ampicillin and incubated at 37°C with aeration. When the OD600 reached ∼0.5, expression of the relevant fusion protein was induced by addition of 0.3 mM IPTG, and the culture was incubated for 4 h at either 30°C (MalE-GadW) or 37°C (MalE-GadX). MalE-GadW was purified by using the same protocol that was used for MalE-GadX (36, 37).
Electrophoretic mobility shift assay.
DNA mobility shift assays were performed using the gadA fragment from positions −176 to 77, the gadBC fragment from positions −173 to 77, and the gadBC fragment from positions −244 to 77, derived from pBsPgadA, pBsPgadBC, and pKgadB-244, respectively (14, 37). The 5′ protruding ends of each fragment were filled in with Klenow DNA polymerase (Roche) in the presence of [α-32P]dATP, using standard protocols (2). Radiolabeled DNA fragments (10 fmol) were incubated at 22°C for 30 min in 20 μl binding buffer [10 mM Tris-HCl (pH 8.0) containing 50 mM KCl, 0.5 mM EDTA, 6% glycerol, 200 μg/ml of bovine serum albumin, and 100 ng/μl of poly(dI-dC)] with different concentrations of purified MalE-GadW or MalE-GadX. Samples were loaded onto 5% nondenaturing polyacrylamide gels in 0.5× TAE buffer (20 mM Tris, 0.5 mM EDTA, 2.5 mM sodium acetate; pH adjusted to 7.5 with concentrated acetic acid). The gels were run at room temperature in 0.5× TAE buffer for 4 h at 10 V/cm and immediately exposed for X-ray autoradiography.
DNase I footprinting.
The gadA fragment from position −176 to position 77 and the gadB fragment from position −307 to position 77, cloned in plasmids pBsPgadA (37) and pBsPgadB-307 (Table 1), were digested at one end and dephosphorylated using calf intestinal phosphatase (Roche). The linearized plasmids were then 5′ end labeled with T4 polynucleotide kinase (Roche) using [γ-32P]ATP (GE Healtcare) and were digested further to release the radioactive fragment. The probes were separated from the plasmid by electrophoresing the digestion mixture on a 6% polyacrylamide gel, extracted from the gel, and eluted in 3 ml of elution buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 300 mM sodium acetate, 0.2% sodium dodecyl sulfate [SDS]) overnight at 37°C. The radioactive probes were purified further and concentrated by phenol-chloroform extraction and ethanol precipitation. Radiolabeled DNA probes (20,000 cpm) were incubated at room temperature for 30 min with different amounts of MalE-GadX or MalE-GadW in 50 μl of 20 mM Tris-HCl (pH 8.0) containing 50 mM KCl, 0.5 mM CaCl2, 6% glycerol, and 20 ng/μl sonicated herring sperm DNA. DNase I digestion was carried out with 0.02 U of enzyme (Roche) at room temperature and after 1 min was stopped by adding 150 μl of stop buffer (192 mM sodium acetate, 32 mM Na2EDTA, 0.14% SDS, 64 μg/ml sonicated herring sperm DNA). Samples were extracted with phenol-chloroform, precipitated with ethanol, resuspended in loading buffer (formamide containing 2 mM EDTA, 0.1% xylene cyanol, and 0.1% bromophenol blue), and subjected to electrophoresis on a 6% sequencing gel. The radioactive probes were also subjected to sequencing, performed using the chemical method of Maxam and Gilbert (23), and loaded on the same gel.
Circular permutation assay.
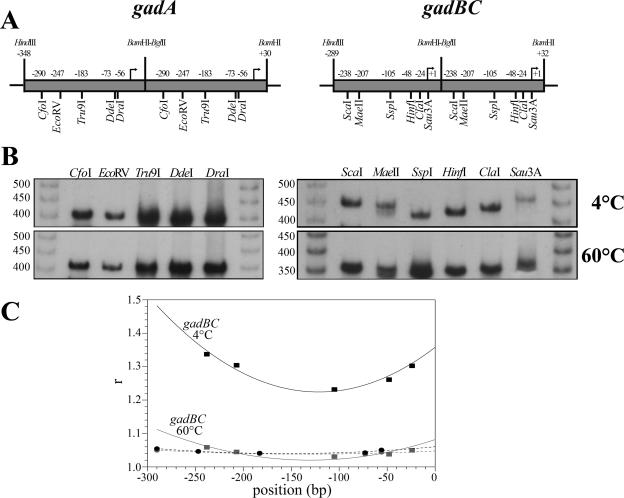
DNA fragments were obtained by PCR amplification using the following combination of primers (Table 2): a reverse primer containing a BamHI restriction site (PCgadArev for gadA and PCgadBrev for gadB) and one of the two forward primers containing either a HindIII restriction site or a BglII restriction site (PCgadAforH and PCgadAforB for gadA; PCgadBforH and PCgadBforB for gadB). The two pairs of gadA and gadBC amplicons were ligated using the compatibility of BamHI and BglII restriction sites and were cloned as tandem copies at the HindIII and BamHI restriction sites of pBs. The tandem copies, extracted using the XhoI and XbaI restriction sites of pBs, were digested with different restrictions enzymes (see Fig. 5). The digestion mixtures were radiolabeled using alkaline phosphatase and T4 polynucleotide kinase (Roche) in the presence of [γ-32P]ATP and were resolved on a 5% nondenaturing polyacrylamide gel. Electrophoresis was performed either at 60°C or at 4°C in buffer containing 90 mM Tris-HCl, 90 mM boric acid, and 2.5 mM EDTA (pH 8.6). A nonbent 50-bp ladder (GE Healthcare) was used as the mobility reference. The bending center was determined by fitting the values for the ratios of apparent size to real size to the parabolic function y = ax2 + bx + c and by solving for x with dy/dx = 0.
FIG. 5.
Gel electrophoretic mobility assay of the circularly permutated gadA and gadB fragments. (A) Maps of the gadA and gadB head-to tail dimers. The positions of single cutting restriction sites are indicated. The bent arrows indicate the transcriptional start sites. (B) Gel electrophoresis of the radiolabeled gadA and gadB permutated fragments, performed with a 5% polyacrylamide gel at 4°C (upper panel) and 60°C (lower panel). The sizes of members of a nonbent 50-bp ladder are indicated on the left. (C) Ratios apparent size to real size (r) of gadA (•) and gadB (▪) permutated fragments plotted against map positions (bp). The dashed (gadA) and solid (gadB) lines are the lines of fit to a parabolic function.
Immunoblot analysis and Gad activity.
Aliquots containing 2.5 μg of proteins from the soluble fraction of cell lysates were used for Western blot analysis. Samples were electrophoresed on a 10% SDS-polyacrylamide gel electrophoresis gel and directly electroblotted onto polyvinylidene difluoride membranes (Immobilon-P; Millipore). Both GadA and GadB were detected with affinity-purified anti-GadA rabbit polyclonal primary antibodies (10) and horseradish peroxidase-labeled secondary antibody provided with a BM chemiluminescence Western blotting kit (Roche).
Glutamate decarboxylase activity assays and GABA measurement were performed as previously described (11).
RESULTS
GadW positively affects expression of the gad structural genes.
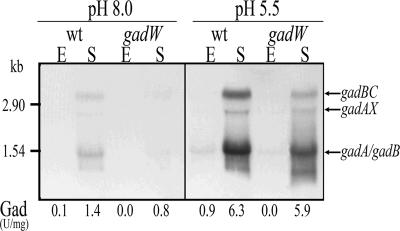
It has been reported that, independent of pH, GadW represses transcription of the gad structural genes indirectly by repressing gadX expression. However, in the absence of GadX and at pH 8.0, GadW directly activates the gadA and gadBC genes (21). Although the mechanism by which GadW can exert these dual effects is not known, it is possible that the relative intracellular levels of these two regulators could account for activation or repression of the gad system. This hypothesis is supported by the finding that in minimal medium under fermentative growth conditions and independent of the pH, GadW repression of gadA and gadBC is not due to inhibition of GadX and the latter protein is responsible for gadW repression (20, 38). Because the data currently available for the role of GadW were obtained using E. coli K-12 strain EK227 and mutant derivatives of this strain, we decided to further analyze the role of GadW in E. coli K-12 strains MC4100 and MG1655, the former because it is a very common laboratory strain and the latter because its genome has been completely sequenced and for this reason it is often used in microarray studies. To obtain insight into the role of GadW in controlling transcription of the gad genes in response to growth phase and pH, we constructed a gadW mutant and analyzed transcription of the gad genes in wild-type and mutant strains by performing Northern blot and Gad enzymatic assays; the results are shown in Fig. 2.
FIG. 2.
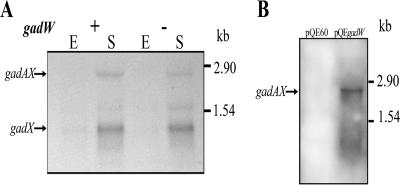
Effect of gadW mutation on expression of gad structural genes. Total RNA and total proteins were extracted from cultures of E. coli strains MC4100 and MC4100gadW grown in LB-MOPS at pH 8.0 or LB-MES at pH 5.5 to the exponential phase (E) (OD600, ∼0.5) and stationary phase (S) (OD600, ∼2). Northern blot analysis was performed with 10 μg of RNA subjected to electrophoresis, transferred onto nylon filters, and hybridized with a gadA/B probe (10). The sizes of rRNAs are indicated on the left for reference. The positions of the gadBC, gadAX, gadA, and gadB transcripts are indicated by arrows on the right. The corresponding decarboxylase activity (Gad), expressed for each sample in units per milligram of total protein, is shown below each lane, and the values are the means of four independent experiments with 10% standard deviations.
The effect of the gadW mutation in strains MC4100 (Fig. 2) and MG1655 (data not shown) consisted of decreases in the levels of gadA, gadAX, and gadBC transcripts, which were more pronounced at an alkaline pH. Gad enzymatic measurements (U/mg total protein) (Fig. 2) and immunoblots stained with GadA/B antibodies (data not shown) correlated with the results of Northern analyses of RNA. Indeed, in the stationary phase at pH 8 the Gad activity of mutant MC4100gadW was 57% of the Gad activity of the wild-type strain, and it was approximately the same (94%) at pH 5.5.
Subsequently, β-galactosidase assays were used to analyze the effect of gadW mutation on transcription of the gadA and gadBC promoters fused to the lacZ reporter gene. The results were then compared to those obtained with wild-type strain MC4100 and the isogenic gadX and gadE mutants (Table 3). Overall, the absence of GadW resulted in a decrease in gadA and gadBC induction that was more pronounced at an alkaline pH. In particular, transcription of gadA was affected only at pH 8.0, while transcription of gadBC was reduced at pH 8.0, as well as in the stationary phase at pH 5.5 (residual activity, 72%). Moreover, the stationary-phase gadB-specific transcript was quantified by real-time PCR for the wild-type and gadW mutant strains, and the results showed that there was reduction to 30% and 63% of the transcripts for the mutant at pH 8 and pH 5.5, respectively.
TABLE 3.
Expression of gadA and gadBC promoters in different genetic backgrounds
| Strain | β-Galactosidase activity (Miller units)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
gadA
|
gadBC
|
|||||||
| pH 8.0
|
pH 5.5
|
pH 8.0
|
pH 5.5
|
|||||
| Exponential phase | Stationary phase | Exponential phase | Stationary phase | Exponential phase | Stationary phase | Exponential phase | Stationary phase | |
| Wild type | 1,488 ± 290 | 5,994 ± 1,151 | 2,009 ± 440 | 11,928 ± 2,611 | 1,375 ± 320 | 3,950 ± 796 | 2,395 ± 438 | 12,833 ± 2,829 |
| gadW | 1,044 ± 203 (70) | 2,245 ± 489 (37) | 1,776 ± 373 (88) | 12,493 ± 1,989 (104) | 944 ± 166 (67) | 2,130 ± 457 (54) | 2,897 ± 577 (120) | 9,245 ± 1,545 (72) |
| gadX | 177 ± 35 (12) | 672 ± 262 (11) | 313 ± 64 (16) | 3,177 ± 660 (27) | 177 ± 35 (13) | 621 ± 150 (16) | 301 ± 64 (13) | 4,055 ± 865 (32) |
| gadE | 102 ± 15 (7) | 252 ± 38 (4) | 165 ± 25 (8) | 416 ± 87 (3) | 130 ± 29 (9) | 420 ± 91 (10) | 215 ± 39 (9) | 670 ± 11 (5) |
The values are means ± standard deviations for 10 to 16 independent experiments. Cultures of E. coli MC4100 (wild type), MC4100gadW (gadW), MC4100gadX (gadX), and MC4100gadE (gadE) containing the chromosomal fusions gadA(−176/77)::lacZ and gadBC(−244/77)::lacZ were grown in LB-MOPS at pH 8.0 and LB-MES at pH 5.5 to the exponential and stationary phases. The values in parentheses are percentages of the corresponding activities for the MC4100 wild-type strain.
As expected (19), the gadE mutation completely abolished transcription of the gadA and gadBC genes, independent of the conditions (Table 3). For the gadX mutant a similar trend was observed, but in the stationary phase at an acidic pH approximately 30% of the wild-type level of β-galactosidase activity was detected (Table 3), in agreement with a previous report (37). Therefore, GadW may play a more important role in induction of gad structural genes at alkaline pH values.
In order to demonstrate that GadW positively contributes to induction of gad genes and thus to acid resistance, we used FB8, an acid-sensitive E. coli strain, to perform acid resistance assays, using the procedure described by Hommais et al. (17, 18). Wild-type E. coli strain FB8 was reported previously to become acid resistant when GadX or GadE was overexpressed (17, 18). To examine this, the gadW gene and its flanking regions (the 201-bp region upstream of the ATG start codon and the 150-bp region downstream of the stop codon) were cloned into the multicopy plasmid pBs (pBsW), which allowed expression of gadW from its indigenous promoter. E. coli FB8 carrying pBsW was found to be more acid resistant than the wild-type strain carrying the empty vector pBs (the levels of survival were 0.02% for FB8/pBs and 1% for FB8/pBsW), although it was much less acid resistant than FB8 carrying pBsX′ encoding GadX (level of survival, 29%). Measurement of Gad activity and GABA export in E. coli FB8 carrying pBsW grown to the stationary phase at an acidic pH demonstrated that Gad activity increased fourfold compared to the activity of the same strain carrying the vector plasmid alone (0.90 U/mg total protein for FB8/pBsW versus 0.24 U/mg total protein for FB8/pBs), and the level of exported GABA was even higher than that in the same strain carrying pBsX′ (0.47 mM GABA for FB8/pBsW versus 0.05 mM GABA for FB8/pBsX′).
Taken together, these data suggest that GadW has a greater impact on the gadBC promoter.
GadW and GadX bind with different affinities to the same DNA regions of gadA and gadBC promoters.
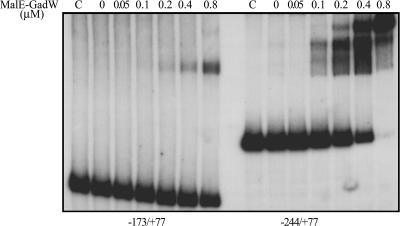
To investigate the binding properties and map the binding sites of the GadW protein in the gadA and gadBC promoter regions, we purified GadW as a fusion protein with MalE. We observed that the chimera MalE-GadW, overexpressed in E. coli JM109, was able to activate the gad structural genes in vivo like the native GadW overexpressed in the same strain under control of the PT5-lacO IPTG-inducible exogenous promoter, as in pQEgadW (data not shown). An electrophoretic mobility shift assay in the presence of MalE-GadW showed that the fusion protein was able to retard the promoter regions of both gadA (positions −176 to 77) (data not shown) and gadBC (positions −244 to 77) (Fig. 3), and this was in agreement with the results of previous studies (21). However, the longer of the two gadBC fragments tested (positions −173 to 77 and positions −244 to 77) (Fig. 3) exhibited higher affinity. This fragment was completely shifted with 0.8 μM MalE-GadW, giving rise to different DNA-protein complexes, while at the same protein concentration the shorter fragment was present mainly in the unbound form. The formation of multiple DNA-protein complexes in the longer gadBC fragment suggested that GadW bound at two sites with different affinities. One of these sites, the site with the higher affinity, bound at 0.1 μM MalE-GadW and only when the fragment from position −244 to position 77 was used (Fig. 3, right panel). At 0.2 μM MalE-GadW the other DNA-protein complex appeared, suggesting that there was a second binding site that was probably fully included in the region from position −173 to position 77, as deduced by the fact that when the gadBC shorter fragment was used, a DNA-protein complex was detected at the same protein concentration (Fig. 3, left panel).
FIG. 3.
Binding of MalE-GadW to the gadBC promoter region: gel retardation assays of in vitro binding of the purified MalE-GadW protein to the gadBC promoter-containing regions encompassing nt −173 to 77 (left) and nt −244 to 77 (right). The radiolabeled DNA fragments (10 fmol) were incubated in 20-μl mixtures with different amounts of the MalE-GadW protein under the conditions described in Materials and Methods. MalE-GadW/DNA complexes were separated from the unbound probe on a 5% polyacrylamide gel run in 0.5× TAE buffer. Lanes C show the results for incubation with 0.8 μM MalE-GadW in the presence of 100 fmol of the specific unlabeled DNA fragments.
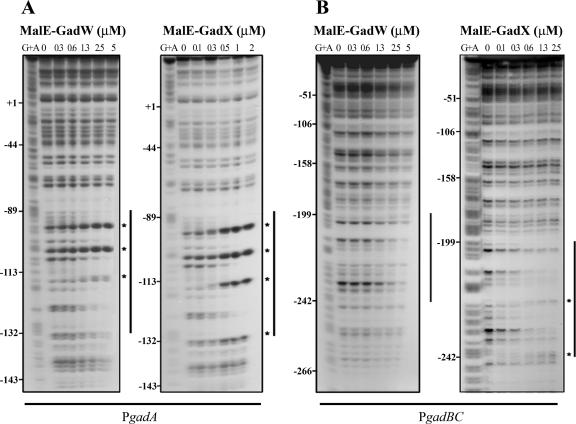
The GadW and GadX binding sites in both gadA and gadBC promoter regions were mapped by DNase I footprinting analyses using the MalE-GadW and MalE-GadX recombinant proteins, and the results are shown in Fig. 4. MalE-GadW protected the gadA promoter region from position −132 to position −89 with respect to the gadA transcriptional start site (Fig. 4A, left panel). The same region was bound by MalE-GadX (Fig. 4A, right panel). The gadBC promoter region in the presence of both regulators was protected from DNase I digestion from position −242 to position −199 with respect to the gadBC transcription start site (Fig. 4B). This represented a newly identified binding site that was not detected in a previous study (37) when a shorter gadBC promoter fragment (positions −173 to 77) was used. Overall, these results demonstrated that GadW and GadX directly bind the same DNA regions of both gad promoters, but apparently with different affinities. Moreover, the protection patterns mediated by GadX and GadW were not identical. The enhanced DNase I cleavage caused by GadX at positions −132 and −115 on the gadA promoter and at positions −242 and −225 on the gadBC promoter might indicate that greater distortion of the target promoters occurs upon GadX binding.
FIG.4.
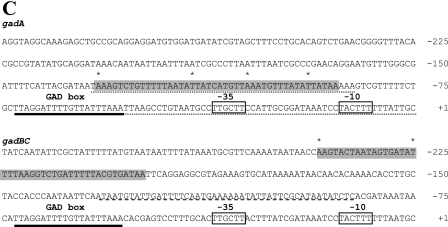
Identification of MalE-GadW and MalE-GadX binding sites in gadA and gadBC regulatory regions. DNase I footprinting assays were performed using PgadA (positions −176 to 77) (A) and PgadBC (positions −307 to 77) (B) with different amounts of MalE-GadW and MalE-GadX. Samples were processed as described in Materials and Methods. The protected regions are indicated by vertical lines, and the hypersensitive sites are indicated by asterisks. (C) Nucleotide sequences of the gadA and gadBC regulatory regions. The MalE-GadX and MalE-GadW binding sites, identified by DNase I footprinting assays (panels A and B), are indicated by shading, and the hypersensitive sites are indicated by asterisks. The low-affinity MalE-GadX site in the gadBC promoter (37) is underlined with a dashed line. The H-NS binding sites in the gadA promoter (14) are underlined with dotted lines. The −35 and −10 hexamers are enclosed in boxes. The GAD box is underlined with a boldface line in both promoter regions.
gadBC promoter region has intrinsic bending.
The newly identified GadX and GadW binding site, centered at 220.5 bp upstream of the transcription start site of gadBC, is consistent with the hypothesis that stimulation of transcription mediated by these two regulators may occur through a DNA looping mechanism (for a review, see reference 26). The DNA looping might arise from either intrinsic or protein-induced bending of the DNA region between the RNA polymerase and activator binding sites. In order to test for the presence of an intrinsic bend in the gadBC promoter region, we analyzed the electrophoretic mobility of this region at four temperatures (4°C, 20°C, 40°C, and 60°C) and compared it with that of the gadA promoter region. This experimental approach provided an indication of the presence of sequence-mediated DNA bending as the electrophoretic mobility of an intrinsically bent DNA fragment is reduced at low temperatures (4°C) and enhanced at high temperatures (60°C) (28). Interestingly, the two DNA fragments behaved differently; i.e., only the mobility of the gadBC fragment was significantly reduced at low temperatures (data not shown). A circular permutation assay was performed to identify the location of the bending center (Fig. 5). To do this, the DNA regions of gadA from position−348 to position 30 and gadBC from position −289 to position 32 were cloned as tandem copies and digested with the appropriate restriction enzymes that cut only once within each copy (Fig. 5A). The behavior of gadBC permutated fragments, analyzed after nondenaturing gels were electrophoresed at 4°C and at 60°C, indicated that a bending locus was present because each DNA fragment had a different mobility (Fig. 5B, right panels), and the extent of retardation was more evident at 4°C. By contrast, we observed no changes in the mobility properties of the gadA permutated fragments (Fig. 5B, left panels). By fitting the r values, corresponding to the ratios of apparent size to real size, to a parabolic function it was possible to extrapolate the location of the bending center in the gadBC promoter region (Fig. 5C), which mapped at −121 bp from the transcription start site both at 4°C and at 60°C.
GadW activates gadX expression via the gadAX promoter.
GadW was shown to repress gadX expression in rich LB medium both at an alkaline pH and at an acidic pH (21), and the repression was proposed to occur indirectly because GadW does not bind to the gadX promoter (20). By performing an electrophoretic mobility shift assay and β-galactosidase assays with the gadX promoter, we confirmed that GadW did not bind in vitro to the gadX promoter region (data not shown). However, the results of our analysis of the effect of GadW on gadX expression are in contrast with the results described in previous reports (21) because we found that in different gadW mutants (from E. coli MG1655 or MC4100) gadAX transcription decreased slightly (Fig. 6A). Again, the discrepancy might have been due to the strain used in previous studies (20). Quantitative real-time PCR confirmed that in the gadW mutant gadX-specific transcripts (gadX and gadAX mRNAs) from stationary-phase cultures exhibited 64% of the wild-type level at pH 8 and 78% of the wild-type level at pH 5.5. These data suggested that the activating effect of GadW on gadX was not a direct effect on the gadX promoter but occurred at the level of the gadAX promoter, where GadW bound (Fig. 4) (21). This hypothesis was corroborated by the observed increase in gadAX transcription in GadW-overexpressing strains, such as E. coli JM109/pQEgadW (Fig. 6B) and MC4100/pBsW (data not shown).
FIG. 6.
Effect of gadW mutation and GadW overexpression on gadAX expression. (A) Northern blot analysis of total RNA extracted from E. coli wild-type strain MC4100 (+) and mutant MC4100gadW (−) grown in LB-MES at pH 5.5 to the exponential phase (E) (OD600, ∼0.5) and stationary phase (S) (OD600, ∼2). Aliquots (10 μg of total RNA) were subjected to electrophoresis, transferred onto nylon filters, and hybridized with the gadX-specific probe (37). The sizes of reference rRNAs are indicated on the right. The positions of the gadX and gadAX transcripts are indicated by arrows on the left. (B) Northern hybridization analysis of total RNA extracted from IPTG-induced E. coli JM109 cultures carrying the pQE60 and pQEgadW plasmids. Cells were grown in LB at pH 7.4 until the mid-exponential phase (OD600, ∼1). Aliquots (10 μg of total RNA) were subjected to electrophoresis, transferred onto nylon filters, and hybridized with the gadX-specific probe (37). The sizes of reference rRNAs are indicated on the right. The position of the gadAX transcript is indicated by an arrow on the left.
DISCUSSION
In the present study we demonstrated that even in the presence of a functional gadX gene, GadW is an activator of gad structural genes in E. coli K-12 strains MC4100 and MG1655. This is in contrast to the results of previous studies in which the less-characterized E. coli K-12 strain EK227 was used (20-21). Our conclusions were corroborated by transcriptional analyses (Northern blotting, β-galactosidase assays, and real-time PCR) showing that in E. coli strains MC4100 and MG1655 the gadW mutation caused reductions in the gadA, gadAX, and gadBC transcript levels. These reductions were also clear at the protein level, as deduced by measurement of Gad activity and by immunoblot analyses. The activator role of GadW, although less crucial than the roles of GadX and GadE, was particularly evident in LB and at the level of the gadBC promoter. Our findings, corroborated by analyzing other E. coli strains, such as FB8 in acid resistance assays and JM109 to examine expression of gadW under control of a heterologous promoter, indicated that there may have been altered behavior of E. coli EK227 in previous studies (20, 21, 38).
The finding that GadX and GadW bind to the same 44-bp sites in the gadA and gadBC promoter regions highlights a common mechanism of recognition of both promoters, which may be controlled by the relative intracellular levels of these two activators. This observation is in line with a report showing that GadX and GadW, via their N-terminal domains, form not only homodimers but also heterodimers (21). GadW and GadX are members of the AraC/XylS protein family of transcriptional regulators, which are characterized by the presence in the C-terminal domain of two helix-turn-helix motifs (13). The association of GadX and GadW in heterodimers is likely to occur because these proteins exhibit certain levels of sequence identity and similarity (14% and 38%) in the N-terminal dimerization domain. The high levels of sequence identity and similarity (41% and 66%) at the level of the C-terminal DNA-binding domain may account for the binding to the same DNA site. Interestingly, the 44-bp gadA and gadBC binding sites identified in this work exhibit 57% sequence identity. Overall, the greater distortion of the target promoters observed as a consequence of GadX binding, as deduced by the enhanced DNase I cleavage at specific positions (positions −132 and −115 in the gadA promoter and positions −242 and −225 in the gadBC promoter), might reflect the different efficiencies of transcription activation observed for GadX and GadW (Table 3).
As far as the gadA regulatory region is concerned, the presence of common DNase I-hypersensitive sites within the 44-bp binding site suggests that there is similar geometry of the nucleoprotein complexes formed upon GadX or GadW binding. The 44-bp gadA binding site encompasses the two GadX higher-affinity sites (sites III and IV) previously identified (37). The GadX low-affinity sites (sites I and II) (37) were not detected in the present study, likely because of the different experimental conditions used (in this study the target DNA was incubated with MalE-GadX or MalE-GadW in the presence of the scavenger herring sperm DNA and was not subjected to PCR amplification, which is typically used to detect weakly protected DNase I sites). The length of the binding site suggests that GadW and GadX bind as dimers to the gadA promoter region, with the recognition helix of each of the four helix-turn-helix motifs occupying one of four consecutive DNA major grooves. The presence of strong DNase I-hypersensitive sites at positions −115, −105, and −94, regularly separated by 10 or 11 nucleotides, also suggests that both regulators induce a conformational change in the DNA when there is binding. This was observed in the crystal structure of the AraC-like activator MarA bound to the mar promoter (29). In a recent report it was shown that GadX is capable of directly stimulating gadA transcription both in vivo and in vitro (14). GadW, expressed from a multicopy plasmid, causes an increase in the β-galactosidase activity of the gadA::lacZ fusion (Tramonti, unpublished data) to the same extent as GadX expressed in the same plasmid (14). The position of the GadX (GadW) binding site, centered 110 bp from the gadA transcriptional start site, is compatible with a model in which GadX and GadW contact this promoter as class I activators, i.e., activators that bind to a site upstream of the promoter −35 element and contact the C-terminal domain of the α subunit of RNA polymerase, thereby recruiting the polymerase to the promoter (3, 35). Furthermore, given that the MalE-GadW (and MalE-GadX) binding site encompasses H-NS binding site II (14), the antirepressor mechanism proposed for GadX at the gadA promoter (14) is likely also valid for GadW. The positive effect of GadW on the gadA promoter was also observed through an increase in gadX expression which was cotranscribed with gadA.
The location of the GadW and GadX binding sites identified in this work (positions −242 to −199 relative to the gadBC transcription start site) implies that the mechanism of activation of gadBC is different from the mechanism of activation of gadA. The antirepressor mechanism proposed for activation of gadA is unlikely to be responsible for gadBC activation because H-NS does not bind to the gadBC promoter (14) (Fig. 1). Often contact of a transcriptional regulator, which binds further upstream of the canonical class I activators (i.e., upstream of position −91) (3), with the initiation complex requires looping out of the intervening DNA sequence (for a review, see reference 26). This is what occurs in the regulatory regions of the upper TOL operon (27), the narGHJI operon (33), and the malK gene (31). In all these cases the functional bending induced by CRP or IHF can be reproduced with a DNA fragment containing a sufficient degree of static curvature (intrinsically bent). In this work we obtained experimental evidence that the gadBC promoter region has an intrinsic bend and that the bending center, as deduced by a circular permutation assay, is located at position −121 with respect to the transcription start site. Such bending may bring the activator protein, GadX or GadW at around position −220.5, into contact either with the RNA polymerase or with an additional factor binding in the nucleotide region close to the −35 sequence. It should be recalled that the primary activator GadE binds as a classical class I activator at the so-called GAD box, centered at position −62.5 relative to the gadA and gadBC transcriptional start site (19) (Fig. 1). According to our model the binding of GadX to a previously identified low-affinity site, centered at position −109 (37) (Fig. 1), might help the formation of the activation loop by emphasizing the intrinsic curvature already present.
On the basis of a previous study (14) and the present study, we provide a more detailed picture of the different requirements for gadA and gadBC activation, which several previous reports have treated as the same thing. The gadA gene is part of a 17-kb genomic region encompassing many acid-inducible genes with promoters having a high DNA melting capacity (18). H-NS acts as a repressor of most of these genes, which include gadX, gadW, and gadE (17). It is thus likely that this genomic region is organized into a supercoiled loop in which DNA is bridged by H-NS molecules (8) which operate a very efficient locking mechanism preventing premature induction of all these genes. The genomic context in which gadBC is placed is different. In the 20-kb genomic region (from yddS to ydeN) encompassing the gadBC operon, none of the genes is known to be H-NS repressed or acid induced. The hypothesis that the molecular mechanism leading to activation of gadA or gadBC is different was validated in the present study, which also provided the basis for a more detailed understanding of the sequence of molecular events switching on acid resistance in E. coli.
Acknowledgments
This work was supported by grants from the Istituto Pasteur-Fondazione Cenci Bolognetti to D.D.B. and from the Italian Ministero dell'Istruzione dell'Università e della Ricerca (MIUR) to D.D.B and V.S.
Footnotes
Published ahead of print on 15 September 2006.
REFERENCES
- 1.Accardi, A., and C. Miller. 2004. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature 427:803-807. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley and Sons, New York, NY.
- 3.Browning, D. F., and J. W. Busby. 2004. The regulation of bacterial transcription initiation. Nat. Rev. 2:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Capitani, G., D. De Biase, C. Aurizi, H. Gut, F. Bossa, and M. G. Grütter. 2003. Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J. 22:4027-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadaban, M. J., J. Chou, and S. N. Cohen. 1980. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J. Bacteriol. 143:971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castanie-Cornet, M. P., T. A. Penfound, D. Smith, J. F. Elliot, and J. W. Foster. 1999. Control of acid resistance in Escherichia coli. J. Bacteriol. 181:3525-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter, P. D., C. G. M. Gahan, and C. Hill. 2001. A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol. Microbiol. 40:465-475. [DOI] [PubMed] [Google Scholar]
- 8.Dame R. T. 2005. The role of nucleoid-associated proteins in the organization and compaction of bacterial chromatin. Mol. Microbiol. 56:858-870. [DOI] [PubMed] [Google Scholar]
- 9.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Biase, D., A. Tramonti, F. Bossa, and P. Visca. 1999. The response to stationary-phase stress conditions in Escherichia coli: role and regulation of the glutamic acid decarboxylase system. Mol. Microbiol. 32:1198-1211. [DOI] [PubMed] [Google Scholar]
- 11.De Biase, D., A. Tramonti, R. A. John, and F. Bossa. 1996. Isolation, overexpression and biochemical characterization of the two isoforms of glutamic acid decarboxylase from Escherichia coli. Protein Expr. Purif. 8:430-438. [DOI] [PubMed] [Google Scholar]
- 12.Foster, J. W. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nat. Rev. Microbiol. 2:898-907. [DOI] [PubMed] [Google Scholar]
- 13.Gallegos, M. T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giangrossi, M., S. Zattoni, A. Tramonti, D. De Biase, and M. Falconi. 2005. Antagonistic role of H-NS and GadX in the regulation of the glutamate decarboxylase-dependent acid resistance system in Escherichia coli. J. Biol. Chem. 280:21498-21505. [DOI] [PubMed] [Google Scholar]
- 15.Gong, S., Z. Ma, and J. W. Foster. 2004. The Era-like GTase TrmE conditionally activates gadE and glutamate-dependent acid resistance in Escherichia coli. Mol. Microbiol. 54:948-961. [DOI] [PubMed] [Google Scholar]
- 16.Gut, H., E. Pennacchietti, R. A. John, F. Bossa, G. Capitani, D. De Biase, and M. G. Grütter. 2006. Escherichia coli acid resistance: pH-sensing, activation by chloride and autoinhibition in GadB. EMBO J. 25:2643-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hommais, F, E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertuy, J. P. Le Caer, A. Danchin, and P. Bertin. 2001. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol. Microbiol. 40:20-36. [DOI] [PubMed] [Google Scholar]
- 18.Hommais, F., E. Krin, J. Y. Coppee, C. Lacroix, E. Yeramian, A. Danchin, and P. Bertin. 2004. GadE (YhiE): a novel activator involved in the response to acid environment in Escherichia coli. Microbiology 150:61-72. [DOI] [PubMed] [Google Scholar]
- 19.Ma, Z., S. Gong, H. Richard, D. L. Tucker, T. Conway, and J. W. Foster. 2003. GadE (YhiE) activates glutamate decarboxylase-dependent acid resistance in Escherichia coli K-12. Mol. Microbiol. 49:1309-1320. [DOI] [PubMed] [Google Scholar]
- 20.Ma, Z., H. Richard, and J. W. Foster. 2003. pH-dependent modulation of cyclic AMP levels and GadW-dependent repression of RpoS affect synthesis of the GadX regulator and Escherichia coli acid resistance. J. Bacteriol. 185:6852-6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma, Z., H. Richard, D. L. Tucker, T. Conway, and J. W. Foster. 2002. Collaborative regulation of Escherichia coli glutamate-dependent acid resistance by two AraC-like regulators, GadX and GadW (YhiW). J. Bacteriol. 184:7001-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchuk, D., M. Drumm, A. Saulino, and F. S. Collins. 1991. Construction of T-vectors, a rapid and general system for direct cloning unmodified PCR products. Nucleic Acids Res. 19:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maxam, A. M., and W. Gilbert. 1980. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 65:499-560. [DOI] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 25.Opdyke, J. A., J.-G. Kang, and G. Storz. 2004. GadY, a small-RNA regulator of acid response genes in Escherichia coli. J. Bacteriol. 186:6698-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Martín, J., F. Rojo, and V. De Lorenzo. 1994. Promoters responsive to DNA bending: a common theme in prokaryotic gene expression. Microbiol. Rev. 58:268-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Martin, J., K. N. Timmis, and V. De Lorenzo. 1994. Co-regulation by bent DNA. Functional substitutions of the integration host factor site at sigma 54-dependent promoter Pu of the upper-TOL operon by intrinsically curved sequences. J. Biol. Chem. 269:22657-22662. [PubMed] [Google Scholar]
- 28.Prosseda, G., P. A. Fradiani, M. Di Lorenzo, M. Falconi, G. Micheli, M. Casalino, M. Nicoletti, and B. Colonna. 1998. A role of H-NS in the regulation of the virF gene of Shigella and enteroinvasive Escherichia coli. Res. Microbiol. 149:15-25. [DOI] [PubMed] [Google Scholar]
- 29.Rhee, S., R. G. Martin, J. L. Rosner, and D. R. Davies. 1998. A novel DNA-binding motif in MarA: the first structure for an AraC family transcriptional activator. Proc. Natl. Acad. Sci. USA 95:10413-10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard, H., and J. W. Foster. 2004. Escherichia coli glutamate- and arginine-dependent acid resistance system increases internal pH and reverse transmembrane potential. J. Bacteriol. 186:6032-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richet, E., and L. Søgaard-Andersen. 1994. CRP induces the repositioning of MalT at the Escherichia coli malKp promoter primarily through DNA bending. EMBO J. 13:4558-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders, J. W., K. Leenhouts, J. Burghoorn, J. R. Brands, G. Venema, and J. Kok. 1998. A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol. Microbiol. 27:299-310. [DOI] [PubMed] [Google Scholar]
- 33.Schröder, I., S. Darie, and R. P. Gunsalus. 1993. Activation of the Escherichia coli nitrate reductase (narGHJI) operon by NarL and Fnr requires integration host factor. J. Biol. Chem. 268:771-774. [PubMed] [Google Scholar]
- 34.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 35.Tebbutt, J., V. A. Rhodius, C. L. Webster, and S. J. W. Busby. 2002. Architectural requirements for optimal activation by tandem CRP molecules at a class I CRP-dependent promoter. FEMS Microbiol. Lett. 210:55-60. [DOI] [PubMed] [Google Scholar]
- 36.Tramonti, A., M. De Canio, F. Bossa, and D. De Biase. 2003. Stability and oligomerization of recombinant GadX, a transcriptional activator of the Escherichia coli glutamate decarboxylase system. Biochim. Biophys. Acta 1647:376-380. [DOI] [PubMed] [Google Scholar]
- 37.Tramonti, A., P. Visca, M. De Canio, M. Falconi, and D. De Biase. 2002. Functional characterization and regulation of gadX, a gene encoding an AraC/XylS-like transcriptional activator of the Escherichia coli glutamic acid decarboxylase system. J. Bacteriol. 184:2603-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tucker, D. L., N. Tucker, Z. Ma, J. W. Foster, R. L. Miranda, P. S. Cohen, and T. Conway. 2003. Genes of the GadX-GadW regulon in Escherichia coli. J. Bacteriol. 185:3190-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, Y., and D. E. Taylor. 1990. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene 94:23-28. [DOI] [PubMed] [Google Scholar]