Abstract
The fcb gene cluster involved in the hydrolytic dehalogenation of 4-chlorobenzoate is organized in the order fcbB-fcbA-fcbT1-fcbT2-fcbT3-fcbC in Comamonas sp. strain DJ-12. The genes are operonic and inducible with 4-chloro-, 4-iodo-, and 4-bromobenzoate. The fcbT1, fcbT2, and fcbT3 genes encode a transporter in the secondary TRAP (tripartite ATP-independent periplasmic) family. An fcbT1T2T3 knockout mutant shows a much slower growth rate on 4-chlorobenzoate compared to the wild type. 4-Chlorobenzoate is transported into the wild-type strain five times faster than into the fcbT1T2T3 knockout mutant. Transport of 4-chlorobenzoate shows significant inhibition by 4-bromo-, 4-iodo-, and 4-fluorobenzoate and mild inhibition by 3-chlorobenzoate, 2-chlorobenzoate, 4-hydroxybenzoate, 3-hydroxybenzoate, and benzoate. Uptake of 4-chlorobenzoate is significantly inhibited by ionophores which collapse the proton motive force.
Bacterial transport systems have traditionally been divided into four general classes based on energy coupling mechanisms, primary sequence, and mode of transport (7, 16, 25, 28). Primary and secondary transporters facilitate solute transport into the cell coupled with a source of energy (i.e., a chemical reaction, light absorption, or electron flow) or an ion electrochemical gradient, respectively (28). Third, group translocators modify their substrates during transport such as phosphorylation. The fourth group are channel-type proteins allowing energy-independent diffusion of the substrate. The first two families of solute transport systems occupy the majority among the known and predicted transporters encoded by microbial genomes (23).
Extracytoplasmic solute receptor-dependent uptake systems have been known as primary transporters for quite some time, which consist of a periplasmic binding protein, integral membrane proteins, and ABC (ATP-binding cassette) proteins (13, 16). This kind of system was once considered to be strictly limited to this ABC transporter family, with ATP hydrolysis as the mechanism of energy coupling, until the discovery of a new type of transporter designated TRAP (tripartite ATP-independent periplasmic) transporters (9, 14, 16, 25). This transporter was first delineated in the Dct system of Rhodobacter capsulatus as a type of secondary transporter which drives C4-dicarboxylate accumulation by an ion electrochemical gradient instead of ATP hydrolysis (9). From the available genome sequences, similar and hitherto-unrecognized TRAP transport systems are found to be widespread in bacteria and archea, but not eukaryotes (16).
Uptake of aromatic compounds is a prerequisite for metabolic degradation in microorganisms and can occur via passive diffusion of neutral compounds or active transport for charged compounds (6, 12). Several transporters are known to facilitate the movement of aromatic compounds across the membrane: BenK for benzoate (6), OphD for phthalate (5), PcaK for 4-hydroxybenzoate and protocatechuate (22), TfdK for 2,4-dichlorophenoxyacetate (18), StyE for styrene (21), and XylN for m-xylene (15). However, little is known about the transporter for 4-chlorobenzoate (4CBA), which is a metabolite in the microbial breakdown of certain chloroaromatic pollutants. Active transport of 4CBA has been suggested in the coryneform bacterium NTB-1 (11) and Arthrobacter sp. strain SB8 (31), and genes encoding putative transporters localized along with a 4CBA degradative gene cluster were found in Arthrobacter sp. strain SU (accession no. AF030397) (29), Arthrobacter sp. strain TM1 (accession no. AF042490) (10), Arthrobacter sp. strain FHP1 (accession no. AB041030), Alcaligenes sp. strain AL3007 (accession no. AF537222) (17), and Pseudomonas sp. strain DJ-12 (3).
Comamonas sp. strain DJ-12 (formerly Pseudomonas) has previously been reported as a 4CBA degrader containing an fcb gene cluster encoding enzymes responsible for hydrolytic dechlorination of 4CBA to 4-hydroxybenzoate (3). The genes encoding a coenzyme A (CoA) ligase (fcbA), a hydrolytic dehalogenase (fcbB), and a thioesterase (fcbC) are organized in the order fcbB-fcbA-fcbT1-fcbT2-fcbT3-fcbC. The fcbT1, fcbT2, and fcbT3 genes were previously postulated by us to encode substrate binding protein and small and large membrane proteins, respectively, of a TRAP transport family 4CBA transporter by homology to TRAP transport family proteins known to transport other polar compounds into the cell (3). The current work was performed to prove that the fcbT1T2T3 genes actually do encode a 4CBA transporter and to functionally characterize the ability to transport 4CBA and related compounds into the cell.
MATERIALS AND METHODS
Bacterial strains, plasmids, and cultivation.
Escherichia coli JM109 (35) and E. coli S17-1 (λpir) (24) were used as the recipient strains in the cloning experiments. Mineral salts basal (MSB) medium (32) was used as minimal medium, and LB broth (Difco) was used as complete medium. Ampicillin and kanamycin were added at 100 and 75 μg/ml, respectively, when necessary. Comamonas and E. coli transformants were cultivated at 30°C and 37°C, respectively.
The pBluescript SKII(+) (Stratagene) and pJP5603 suicide vector (24) were used for cloning and knockout mutations. The promoter probe vector pKRZ-1 (27) was employed for the promoter assay. E. coli HB101 harboring pRK2013 was used as a helper strain in mating experiments (8). Plasmids were isolated with the NucleoSpin plasmid Miniprep kit (BD Biosciences).
Isolation of total RNA and RT-PCR.
Comamonas sp. strain DJ-12 was grown in MSB with either 1 mM 4CBA or 5 mM succinate. To induce the corresponding genes, the cells were incubated at 30°C for at least 12 h. Total RNA was extracted with the Trizol reagent (Invitrogen) according to the manufacturer's recommended protocol. The extracted total RNA was further purified with the RNeasy mini kit (QIAGEN) and DNase I (QIAGEN) treatment according to the manufacturer's instructions. The reverse transcription-PCRs (RT-PCRs) were performed in 25-μl reaction volumes with 0.5 μg of the extracted RNA using the OneStep RT-PCR kit (QIAGEN). The thermocycler program was as follows: 50°C for 30 min, 95°C for 15 min, 30 cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 3 min), and 72°C for 10 min. The following primers were designed to amplify the fcb genes: for fcbBA, 5′-ATGTACGAAGCCATCGGTC-3′ (forward) and 5′-CTAACTTGACACCTGCTGC-3′ (reverse); for fcbBAT1, 5′-ATGTACGAAGCCATCGGTC-3′ (forward) and 5′-TCCTCATTGAGCCCTTCG-3′ (reverse); and for fcbT1T2T3C, 5′-CCTGAAGCTGACGCTCTG-3′ (forward) and 5′-TTAGCTACATAGGGCCAAG-3′ (reverse).
Sequence analysis.
Deduced amino acid sequences were analyzed using the Lasergene software (DNASTAR, Inc.) and compared with the GenBank database using programs based on the BLAST algorithm (1).
β-Galactosidase assays.
A 510-bp PCR product upstream of fcb operon harboring the putative promoter region was amplified using the primers fcbP-F (CGGTCGACACAGCACGCTGCATTC) and fcbP-R (GTACTCTAGAAATGCTCCTTGTGACG) containing SalI and XbaI restriction sites (underlined), respectively. The PCR product was obtained in a 50-μl reaction mixture using Premix Taq polymerase (Sigma). The reactions were initiated at 94°C (2 min); followed by 30 cycles of denaturing at 94°C (30 s), annealing at 55°C (30 s), and extension at 72°C (30 s); and ended with incubation at 72°C for 10 min. The resultant PCR product was purified from an agarose gel with the QIAEXII gel extraction kit (QIAGEN). Purified DNA fragments were digested with the two corresponding restriction enzymes and then ligated with pKRZ-1 treated with the same enzymes to construct pFCBP1. The latter construct was transferred into Comamonas sp. strain DJ-12 by triparental conjugation (8) with selection on LB agar (Difco) containing ampicillin and kanamycin.
DJ-12(pFCBP1) was cultivated overnight in MSB containing 10 mM succinate as a carbon source and kanamycin as selective pressure. The cells were subcultured into the same growth medium supplemented with 0.5 mM benzoate derivatives and cultivated for 8 h. The optical density at 600 nm (OD600) was determined, and 200 μl of the culture was used for a modified β-galactosidase assay (20). The cells were mixed with 800 μl of buffer Z (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 40 mM 2-mercaptoethanol) following permeabilization by adding 20 μl of chloroform and 30 μl of 0.1% sodium dodecyl sulfate. o-Nitrophenyl-β-d-galactopyranoside (100 μl of a 4-mg/ml stock) was added to initiate the reaction, and the mixture was incubated at 30°C for 30 min. The reaction was stopped by adding 250 μl of 1 M sodium carbonate to the mixture. Reported β-galactosidase activity values are presented in units as specified by Miller (20). The results given in this study were determined from three independent cultures.
Construction of a knockout mutant.
An fcbT1, -T2, and -T3 mutant was constructed by homologous recombination. A BamHI-NotI DNA fragment from pKC158 (3) containing the fcb gene cluster was subcloned into the pBluescript SKII(+) vector. Using the SacI recognition site originating from the vector, a BamHI-SacI insert fragment was ligated into the pJP5603 suicide vector and transformed into E. coli S17-1 (λpir). This construct (designated pMUT01) was digested with ClaI and self-ligated to remove the fcbT1T2T3 genes. The resultant pMUT02 was transformed into E. coli S17-1 (λpir) and transferred into Comamonas sp. strain DJ-12 by triparental filter mating. Single-crossover mutants were screened in solid LB medium with ampicillin and kanamycin. The recombinant strain was cultivated in liquid LB medium without selective pressure overnight. After continuous cultivation in the same medium for three subcultures, the cells were plated on solid LB medium with ampicillin. Double-crossover mutants were selected for the inability to grow on medium with kanamycin. The knockout strain was designated MUT1. Deletion of the ClaI fragment by double-crossover recombination was verified by colony PCR and Southern blotting. The same primers were used in PCR and to make a probe for the Southern blot: forward, 5′-ATGCGTATTCATCGTCGCCAG-3′; and reverse, 5′-TTAACGTCCCACGAACACATCG-3′. PCR amplification using intact cells was performed in a 10-μl reaction mixture. The PCR conditions were the same as those described above except for initiation at 94°C for 10 min to disrupt the cells and extension of the PCR cycle at 72°C for 2 min. BamHI-NotI-digested genomic DNA was used for the Southern blotting, which was performed as described previously (4).
Chemical analysis.
A cell culture collected in the incubation was filtered and analyzed with a high-performance liquid chromatography apparatus (Agilent 1100 series, German). 4-Chlorobenzoate was separated on a C18 column (4.6 mm in diameter; 250 mm in length; Spherisorb) at a flow rate of 1 ml/min with a mobile phase of methanol-water-acetic acid mixture (55:45:2) and monitored by absorbance at a wavelength of 254 nm.
4CBA transport assay.
Mid-log-phase cells of Comamonas sp. strain DJ-12 and MUT1 grown on LB broth were transferred into MSB minimal medium containing 5 mM succinate and 1 mM 4CBA. The cells were incubated for 12 h, harvested by centrifugation at 3,000 × g, washed three times with 50 mM sodium/potassium phosphate buffer (pH 6.8), and resuspended in the same buffer to an optical density of 1.0 at 600 nm. All assays were done at room temperature. Uptake was initiated by adding the cells to an equal volume of phosphate buffer containing 100 μM [ring-UL-14C]4CBA (130 mCi/mmol; Amersham Pharmacia Biotech.). Samples (0.1 ml) were removed from the reaction mixture at various times (10 s, 1 min, 2 min, 3 min, and 4 min) and filtered through a Nuclepore track-etched polycarbonate membrane (0.2-μm pore size; Whatman). The filters were washed before and after addition of the sample with 2 ml of phosphate buffer. Accumulated 4CBA inside the cells was determined by scintillation counting of the cells retained on the filters. Cell protein was determined by the method of Bradford (2).
Kinetic parameters for 4CBA uptake were determined by performing an uptake assay using different concentrations of 4CBA (0.05 to 150 μM). Reaction mixtures were prepared in triplicate, and samples were taken at 1 min and 2 min. The kinetic parameters Vmax and Km were obtained from nonlinear fit of data to the Michaelis-Menten equation using PRIZM software (version 3.02; GraphPad Software).
The effect of aromatic acid inhibition of 4CBA uptake was investigated using 50 μM of radiolabeled 4CBA and 1 mM of competing substrate. The transport assay mentioned above was initiated by adding an equal volume of cells to the reaction mixture containing 100 μM of 4CBA and 2 mM of competing substrate.
Sodium orthovanadate, 2,4-dinitrophenol (DNP), 1,3-dicyclohexylcarbodiimide (DCCD), and m-chlorophenylhydrazone (CCCP) were used for the energetic inhibition study (19, 26). DNP, CCCP, and DCCD were added to the cells at final concentrations of 2 mM, 0.2 mM, and 2 mM, respectively, 10 min prior to starting a transport assay. The cells were pretreated with 1 mM sodium orthovanadate for 15 min.
RESULTS AND DISCUSSION
Transcription of the fcb gene operon.
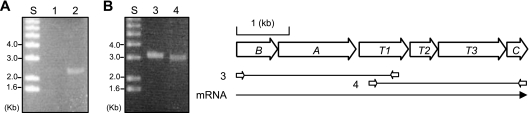
The nucleotide sequence of the fcb gene cluster encoding enzymes for the hydrolytic dehalogenation of 4CBA was previously reported by us from Comamonas sp. strain DJ-12 (3). In between the fcbA and fcbC genes are three genes recognizable as encoding a potential TRAP-type transporter (Fig. 1). These genes, designated fcbT1, fcbT2, and fcbT3, are not present in hydrolytic dehalogenation gene clusters found in other microorganisms (10, 17, 29, 30). Transcriptional analysis was accomplished to investigate the expression of the fcb gene cluster. When RT-PCR was performed with the same amount of total RNA extracted from Comamonas sp. strain DJ-12 grown on 4CBA or succinate, the fcbBA genes encoding 4CBA-CoA dehalogenase and 4CBA-CoA ligase were amplified only from the RNA extracted from cells induced by 4CBA (Fig. 1A). To examine whether the six open reading frames in the fcb gene cluster are transcribed as an operon, primer sets were designed to amplify the region in two overlapping PCR products. The expected RT-PCR products were as follows: fcbBAT1 and fcbT1T2T3C (3.2 kb and 3.0 kb, respectively). As shown in Fig. 1B, the expected two PCR products were amplified from total RNA extracted from Comamonas sp. strain DJ-12 induced with 4CBA. These RT-PCR experiments thus demonstrate that expression of the transporter genes is dependent on growth on 4CBA and that the fcbT1T2T3 genes are present on the same mRNA as the fcbBAC genes. The fcbBAT1T2T3C genes are thus coregulated and in the same operon.
FIG. 1.
Transcriptional analysis indicating inducible (A) and operonic (B) expression of the fcb gene cluster in Comamonas sp. strain DJ-12. Lanes 1 and 2 (A) show RT-PCR results amplified with total RNA extracted from cells grown on 10 mM succinate or 1 mM 4CBA, respectively. Lanes 3 and 4 (B) indicate RT-PCR products obtained using RNA prepared from cells induced with 1 mM 4CBA. fcbA, -B, and -C encode 4CBA-CoA ligase, 4CBA-CoA dehalogenase, and 4HBA-CoA thioesterase, respectively. fcbT1, -T2, and -T3 represent putative transporter genes. S, 1-kb DNA ladder.
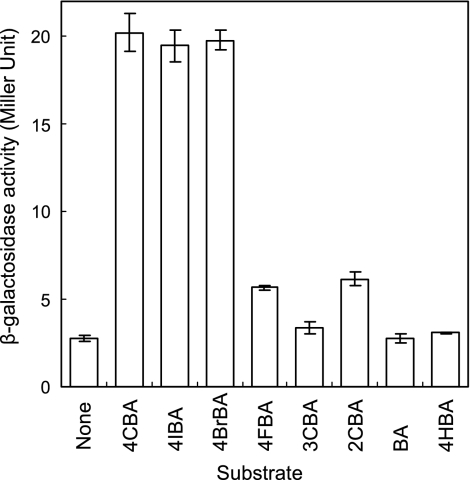
In order to verify the inducibility and the effect of different substrates, the upstream region of fcbB was fused with the lacZ reporter gene of the pKRZ-1 vector using SalI and XbaI as described in the Materials and Methods. The SalI-XbaI fragment includes the putative ribosome binding site and promoter. The resulting construct, pFCBP1, was transformed into the wild-type DJ-12 strain by triparental conjugation and used for the β-galactosidase assay (Fig. 2). DJ-12 with pFCBP1 was grown on succinate and exposed to different benzoate derivatives. Cells induced by 4CBA, 4-bromobenzoate, and 4-iodobenzoate showed the highest β-galactosidase activity. Interestingly, cells exposed to 2-chlorobenzoate and 4-fluorobenzoate have approximately double the β-galactosidase activity seen with the control with no substituted benzoate added. This demonstrates that 2-chlorobenzoate and 4-fluorobenzoate act as weak inducible substrates.
FIG. 2.
Expression of the fcb gene operon in response to benzoate derivatives. The enzyme activities are the average of triplicate experiments. Cells were grown on succinate and exposed to one of the following inducing compounds: 4CBA, 4-chlorobenzoate; 4IBA, 4-iodobenzoate; 4BrBA, 4-bromobenzoate; 4FBA, 4-fluorobenzoate; 3CBA, 3-chlorobenzoate; 2CBA, 2-chlorobenzoate; BA, benzoate; 4HBA, 4-hydroxybenzoate.
Disruption of the fcbT1T2T3 genes by homologous recombination.
An approximately 7-kb BamHI-NotI DNA fragment containing the fcb gene cluster was moved into the pJP5603 suicide vector as described in Materials and Methods. The construct was designated pMUT01. The two ClaI sites in the middle of fcbT1 and fcbT3 were used for disruption of fcbT1T2T3. The deletion of fcbT1T2T3 by ClaI digestion and self-ligation generates a hybrid open reading frame containing the 5′ end of fcbT1 and the 3′ end of fcbT3, so that expression of the downstream fcbC gene is not affected by the removal of the ClaI fragment. The resultant clone pMUT02 was transferred into Comamonas sp. strain DJ-12 by triparental filter mating. A mutated strain with a single recombinational crossover was selected on an LB plate with antibiotic pressure since the insertion of pMUT02 into the chromosome endowed kanamycin resistance on DJ-12. The recombinant strain was cultivated on liquid LB medium without selective pressure overnight. After being subcultured three times on the same medium, the culture was plated on solid LB medium. Double-crossover mutants were selected by inability to grow on LB plus kanamycin due to the removal of vector fragment by a second recombinational event. The selected potential mutants were screened by PCR and Southern hybridization for deletion of the transporter gene fragment.
4CBA uptake assay.
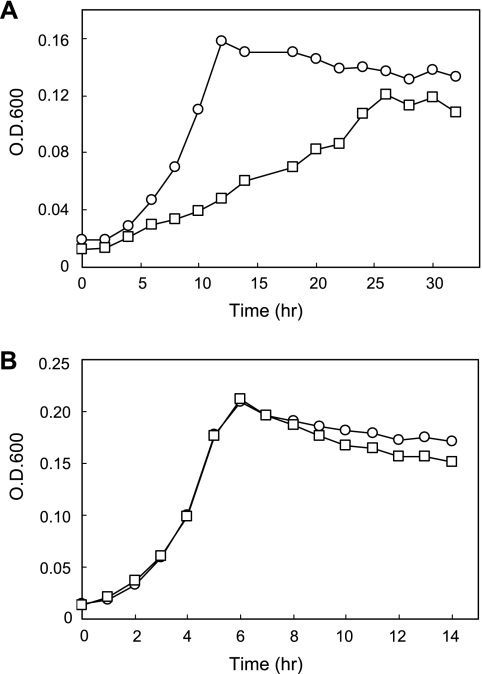
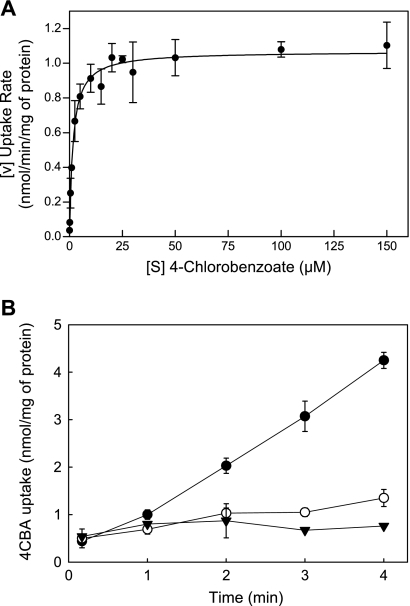
The ability to transport 4CBA was assayed in the wild-type strain DJ-12 and the knockout mutant strain MUT1. When grown in MSB medium containing 1 mM 4CBA, the knockout mutant MUT1 showed a much slower growth rate (generation time of 120.4 min) with the complete depletion of 4CBA within 12 h compared to the wild type (55.6 min), as shown in Fig. 3. On the other hand, the growth rates of both DJ-12 and MUT1 are identical (20.3-min doubling time) on MSB medium containing 1 mM 4-hydroxybenzoate. These data, combined with our previous bioinformatic analysis (3), indicate that the three fcbT-encoded proteins are involved in 4CBA transport into the cell. A 4CBA transport assay with DJ-12 and MUT1 positively proved this conclusion (Fig. 4). Saturation kinetics was established between uptake rate and different 4CBA concentrations with 4CBA-induced strain DJ-12 (Fig. 4A). Nonlinear fit of data to the Michaelis-Menten equation calculated a Vmax of 1.068 ± 0.0182 nmol min−1 mg of protein−1 and transport affinity (Km) of 1.648 ± 0.1723 μM. This indicates that 4CBA transport into the cell does not rely on passive diffusion by a concentration gradient but occurs by an enzymatic reaction.
FIG. 3.
Growth of Comamonas sp. strain DJ-12 (open circle) and MUT1 (open square) in minimal medium with 4-chlorobenzoate (A) and 4-hydroxybenzoate (B) as the sole carbon source. Optical density was measured at 600 nm.
FIG. 4.
Uptake of 4-chlorobenzoate by induced cells of Comamonas sp. strain DJ-12. (A) The curve shows a nonlinear regression fit of data to the Michaelis-Menten equation. (B) The uptake assay was performed with 4CBA-induced cells of Comamonas sp. strain DJ-12 (closed circle) and MUT1 (open circle) and uninduced DJ-12 (closed triangle). Each point in panels A and B is the average value from triplicate experiments. Error bars indicate standard deviation.
DJ-12 grown in the presence of 4CBA transports 4CBA into the cell at the rate of 1.08 ± 0.06 nmol min−1 mg of protein−1, as shown in Fig. 4B. In contrast, MUT1 grown under the same conditions (succinate plus 4CBA) transports 4CBA at the rate of 0.22 ± 0.06 nmol min−1 mg of protein−1. This is only slightly faster than DJ-12 cells grown only on succinate.
The slow rate of growth of the knockout mutant MUT1 on 4CBA and the extremely slow transport of 4CBA into MUT1 may be due either to the presence of a second inefficient transporter or simple diffusion of 4CBA into the cell driven by metabolic enzymes since 4CBA was completely depleted by MUT1. For instance, Chang and Zylstra (5) reported that a phthalate permease knockout mutant strain could take up phthalate at the same rate as the wild type, suggesting the existence of a second phthalate transport system. PcaK in Pseudomonas putida is characterized as a multifunctional transporter for 4-hydroxybenzoate as well as protocatechuate (22). Metabolic enzymes can accelerate the simple diffusion of the undissociated form of benzoate and 4-hydroxybenzoate across biological membranes (6, 34).
Transporter specificity.
The substrate specificity of the transporter was examined with Comamonas sp. strain DJ-12 through a substrate inhibition study which investigated the competitive uptake of radioactive 4CBA in the presence of related compounds. para-Substituted halogens inhibited transport the most in the order bromo > chloro > iodo > fluoro (Table 1). This indicates that the 4CBA transporter has strong substrate specificity for benzoates substituted in the 4 position. This is correlated with inducibility of the fcb operon by various substituted aromatic acids (Fig. 2). Interestingly, other substrates tested in this study also show an inhibitory effect on 4CBA transport. A 50 to 60% reduction in transport was exhibited with 3-chlorobenzoate, 2-chlorobenzoate, 4-hydroxybenzoate, 3-hydroxybenzoate, and benzoate. It is thus possible that this TRAP-type transporter has broad substrate affinity for many types of aromatic compounds.
TABLE 1.
Substrate inhibition of 4-chlorobenzoate uptake by Comamonas sp. strain DJ-12
| Competitora | % Inhibition of 4-chlorobenzoate uptakeb |
|---|---|
| 4-Bromobenzoate | 91.5 ± 3 |
| 4-Chlorobenzoate | 88.1 ± 3 |
| 4-Iodobenzoate | 86.3 ± 8 |
| 4-Fluorobenzoate | 76.0 ± 7 |
| 3-Chlorobenzoate | 61.0 ± 8 |
| 2-Chlorobenzoate | 55.2 ± 6 |
| 4-Hydroxybenzoate | 60.0 ± 7 |
| 3-Hydroxybenzoate | 52.7 ± 8 |
| Benzoate | 54.9 ± 4 |
| 2-Aminobenzoate | 16.5 ± 2 |
The concentrations of 4-chlorobenzoate and competing substrate were 50 μM and 1 mM, respectively.
Values are averages from three experiments.
Energetic inhibition.
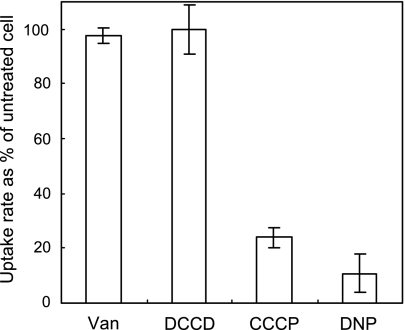
To determine the energy dependence of 4CBA uptake, Comamonas sp. strain DJ-12 was exposed to vanadate, DNP, CCCP, or DCCD before the transport assay was performed. DNP and CCCP are ionophores which destroy the formation of a proton motive force, whereas vanadate and DCCD disrupt ATP systhesis by acting on the ATPase complex. Ionophore and ATP synthesis blocking reagents are therefore used to distinguish between a primary and secondary transporter family by dissipating proton motive force or ATP (9, 14, 19, 26). As presented in Fig. 5, vanadate- or DCCD-treated cells retained almost 100% of the ability to transport 4CBA into the cell while DNP or CCCP exposure significantly decreased uptake rate in the cell. Although these compounds were used with rather high concentrations which may have side effects on the transporter or cell, the fact that an obvious difference was seen between the effects of CCCP or DNP and vanadate or DCCD suggests that transport of 4CBA into the cell is dependent on a proton motive force and not ATP. This is in agreement with what is known about other TRAP-type transporters (9, 33).
FIG. 5.
Effect of energetic inhibitors on 4-chlorobenzoate uptake in Comamonas sp. strain DJ-12. Van, sodium orthovanadate.
In addition, this result suggests that uptake of 4CBA in strain DJ-12 is dependent upon the transporter rather than the metabolic drag mechanism (34) caused by metabolic enzymes. Since ATP is required in CoA ligation onto 4CBA catalyzed by FcbA, which is the first step of dehalogenation (30), uptake of 4CBA should be severely inhibited by the vanadate or DCCD in case of the simple diffusion accelerated by the metabolic enzymes.
Conclusion.
To our knowledge, this is the first demonstration of 4CBA uptake via a TRAP-type transporter. The presence of a transport system for 4CBA has been suggested in the coryneform bacterium NTB-1 (11) and in Arthrobacter sp. strain SB8 (31). The uptake of 4CBA in coryneform bacterium NTB-1 was considered to be driven by a proton symport mechanism, and Shimao et al. (31) proposed that the transport of 4CBA requires energy in Arthrobacter sp. strain SB8. Interestingly, examination of gene clusters known to be involved in 4CBA dehalogenation in other organisms reveals the presence of a single gene encoding a major facilitator superfamily transporter predicted to transport 4CBA into the cell (accession no. AB041030, AF030397, and AF042490) (10, 29) or two-component transporter highly similar to TRAP family proteins (accession no. AF537222) (17). As documented in the present work, Comamonas sp. strain DJ-12 transports 4CBA into the cell through a TRAP-type transporter consisting of three components. It is thus readily apparent that different bacterial strains have recruited different genes to evolve mechanisms for 4CBA transport into the cell.
Acknowledgments
This work was supported by NSF CHE-0221978.
Footnotes
Published ahead of print on 13 October 2006.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Chae, J.-C., Y. Kim, Y. C. Kim, G. J. Zylstra, and C.-K. Kim. 2000. Genetic structure and functional implication of the fcb gene cluster for hydrolytic dechlorination of 4-chlorobenzoate from Pseudomonas sp. DJ-12. Gene 258:109-116. [DOI] [PubMed] [Google Scholar]
- 4.Chae, J.-C., C.-K. Kim, and G. J. Zylstra. 2005. Characterization of two small cryptic plasmids from Pseudomonas sp. strain S-47. Biochem. Biophys. Res. Commun. 338:1600-1606. [DOI] [PubMed] [Google Scholar]
- 5.Chang, H.-K., and G. J. Zylstra. 1999. Characterization of the phthalate permease OphD from Burkholderia cepacia ATCC 17616. J. Bacteriol. 181:6197-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collier, L. S., N. N. Nichols, and E. L. Neidle. 1997. benK encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain ADP1. J. Bacteriol. 179:5943-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driessen, A. J. M., B. P. Rosen, and W. N. Konings. 2000. Diversity of transport mechanism: common structural principles. Trends Biochem. Sci. 25:397-401. [DOI] [PubMed] [Google Scholar]
- 8.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forward, J. A., M. C. Behrendt, N. R. Wyborn, R. Cross, and D. J. Kelly. 1997. TRAP transporters: a new family of periplasmic solute transport systems encoded by the dctPQM genes of Rhodobacter capsulatus and by homologs in diverse gram-negative bacteria. J. Bacteriol. 179:5482-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartemann, K.-H., and R. Eichenlaub. 2001. Isolation and characterization of IS1409, an insertion element of 4-chlorobenzoate-degrading Arthrobacter sp. strain TM1, and development of a system for transposon mutagenesis. J. Bacteriol. 183:3729-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groenewegen, P. E. J., A. J. M. Driessen, W. N. Konings, and J. A. M. de Bont. 1990. Energy-dependent uptake of 4-chlorobenzoate in the coryneform bacterium NTB-1. J. Bacteriol. 172:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harwood, C. S., and J. Gibson. 1986. Uptake of benzoate by Rhodopseudomonas palustris grown anaerobically in light. J. Bacteriol. 165:504-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs, M. H. J., T. van der Heide, A. J. M. Driessen, and W. N. Konings. 1996. Glutamate transport in Rhodobacter sphaeroides is mediated by a novel binding protein-dependent secondary transport system. Proc. Natl. Acad. Sci. USA 93:12786-12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasai, Y., J. Inoue, and S. Harayama. 2001. The TOL plasmid pWW0 xylN gene product from Pseudomonas putida is involved in m-xylene uptake. J. Bacteriol. 183:6662-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly, D. J., and G. H. Thomas. 2001. The tripartite ATP-independent periplasmic (TRAP) transporters of bacteria and archaea. FEMS Microbiol. Rev. 25:405-424. [DOI] [PubMed] [Google Scholar]
- 17.Layton, A. C., J. Sanseverino, W. Wallace, C. Corcoran, and G. S. Sayler. 1992. Evidence for 4-chlorobenzoic acid dehalogenation mediated by plasmids related to pSS50. Appl. Environ. Microbiol. 58:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leveau, J. H. J., A. J. B. Zehnder, and J. R. van der Meer. 1998. The tfdK gene product facilitates uptake of 2,4-dichlorophenoxyacetate by Ralstonia eutropha JMP134(pJP4). J. Bacteriol. 180:2237-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Master, E. R., J. J. McKinlay, G. R. Stewart, and W. W. Mohn. 2005. Biphenyl uptake by psychrotolerant Pseudomonas sp. strain Cam-1 and mesophilic Burkholderia sp. strain LB400. Can. J. Microbiol. 51:399-404. [DOI] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 21.Mooney, A., N. D. O'Leary, and A. D. W. Dobson. 2006. Cloning and functional characterization of the styE gene, involved in styrene transport in Pseudomonas putida CA-3. Appl. Environ. Microbiol. 72:1302-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols, N. N., and C. S. Harwood. 1997. PcaK, a high-affinity permease for the aromatic compounds 4-hydroxybenzoate and protocatechuate from Pseudomonas putida. J. Bacteriol. 179:5056-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulsen, I. T., L. Nguyen, M. K. Sliwinski, R. Rabus, and M. H. Saier, Jr. 2000. Microbial genome analyses: comparative transport capabilities in eighteen prokaryotes. J. Mol. Biol. 301:75-100. [DOI] [PubMed] [Google Scholar]
- 24.Penfold, R. J., and J. M. Pemberton. 1992. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene 118:145-146. [DOI] [PubMed] [Google Scholar]
- 25.Rabus, R., D. L. Jack, D. J. Kelly, and M. H. Saier, Jr. 1999. TRAP transporters: an ancient family of extracytoplasmic solute-receptor-dependent secondary active transporters. Microbiology 145:3431-3445. [DOI] [PubMed] [Google Scholar]
- 26.Richarme, G., A. el Yaagoubi, and M. Kohiyama. 1993. The MglA component of the binding protein-dependent galactose transport system of Salmonella typhimurium is a galactose stimulated ATPase. J. Biol. Chem. 268:9473-9477. [PubMed] [Google Scholar]
- 27.Rothmel, R. K., D. L. Shinabarger, M. R. Parsek, T. L. Aldrich, and A. M. Chakrabarty. 1991. Functional analysis of the Pseudomonas putida regulatory protein CatR: transcriptional studies and determination of the CatR DNA-binding site by hydroxyl-radical footprinting. J. Bacteriol. 173:4717-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saier, M. H., Jr. 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64:354-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz, A., K.-H. Gartemann, J. Fiedler, E. Grund, and R. Eichenlaub. 1992. Cloning and sequence analysis of genes for dehalogenation of 4-chlorobenzoate from Arthrobacter sp. strain SU. Appl. Environ. Microbiol. 58:4068-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholten, J. D., K.-H. Chang, P. C. Babbitt, H. Charest, M. Sylvestre, and D. Dunaway-Mariano. 1991. Novel enzymic hydrolytic dehalogenation of a chlorinated aromatic. Science 253:182-185. [DOI] [PubMed] [Google Scholar]
- 31.Shimao, M., S. Onishi, S. Mizumori, N. Kato, and C. Sakazawa. 1989. Degradation of 4-chlorobenzoate by facultatively alkalophilic Arthrobacter sp. strain SB8. Appl. Environ. Microbiol. 55:478-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanier, R. Y., N. J. Palleroni, and M. Duodoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 33.Ullmann, R., R. Gross, J. Simon, G. Unden, and A. Kröger. 2000. Transport of C4-dicarboxylates in Wolinella succinogenes. J. Bacteriol. 182:5757-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong, C. M., M. J. Dilworth, and A. R. Glenn. 1994. Cloning and sequencing show that 4-hydroxybenzoate hydroxylase (PobA) is required for uptake of 4-hydroxybenzoate in Rhizobium leguminosarum. Microbiology 140:2775-2786. [DOI] [PubMed] [Google Scholar]
- 35.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]