Abstract
The expression of virulence determinants in Pseudomonas aeruginosa is coordinately regulated in response to both the social environment—commonly referred to as quorum sensing—and to environmental cues. In this study we have dissected the various independent regulation levels for pyocyanin production, which is influenced by the homoserine lactone- and Pseudomonas quinolone signal (PQS)-mediated quorum-sensing systems as well as by iron and phosphate availability. We demonstrate that the phosphate regulon is involved in the transcriptional activation of rhlR and the augmentation of PQS and pyocyanin production under phosphate limitation. However, we also observed an enhancement of rhlR transcription under low-iron medium conditions and after the addition of PQS that was independent of the phosphate regulon. These results highlight the complexity of secondary metabolite production in P. aeruginosa via environmental cues and the quorum-sensing system.
Bacterial organisms that elaborate traits tailored to their surroundings have better chances of surviving the pressures of unfavorable environmental conditions and host defenses. The outstanding capability of Pseudomonas aeruginosa for adaptation is reflected by the large number of putative transcriptional regulators (53), as bacterial differentiation is often controlled by transcription factors whose activity is influenced by local cues. Moreover, it has been recognized that environmental signals (33) as well as the social surrounding control bacterial virulence factor production. Cell-density-dependent gene regulation is commonly referred to as quorum sensing (QS) (20). QS is based on the release of soluble communicator molecules that trigger the transcription of QS-dependent genes when the bacterial population has reached a certain cell density. Many of these genes are involved in bacterial pathogenicity (9, 19, 39, 40, 50). Three chemically distinct signal molecules have been identified so far in P. aeruginosa. Two of these are acyl-homoserine lactones (AHL): a butyryl-homoserine lactone and a 3-oxo-dodecanoyl homoserine lactone, which together with their corresponding transcriptional activator proteins (R proteins) comprise the two hierarchically organized QS systems las and rhl (8, 27, 38, 41, 44) and control the expression of over 200 genes (23, 48, 57). The third signal molecule is 2-heptyl-3-hydroxy-4-quinolone (43). This Pseudomonas quinolone signal (PQS) interacts with the las and the rhl systems. While the las system seems to induce the production of PQS, exogenous PQS up-regulates the expression of the rhl system (15, 16, 31). In P. aeruginosa the impact of the rhl QS on the biosynthesis of the secondary metabolites pyocyanin and rhamnolipids is well documented (42). However, the production of these secondary metabolites also seems to be dependent on environmental cues (3, 62). A link between QS and iron homeostasis was suggested previously (5, 11, 21, 24, 25, 52, 60). Moreover, PQS was shown to exhibit an iron-chelating activity, and PQS-dependent rhlR induction seems to be at least in part a consequence of iron depletion (6).
Recent studies on the transcriptional regulation of RhlR revealed that the rhlR promoter region harbors four different transcription start sites (32). Whereas under rich medium conditions the expression of RhlR is dependent on LasR, under phosphate-limiting conditions various transcriptional activators, including Vfr, RhlR, and the sigma factor σ54, participate in the expression of RhlR from multiple promoters. Moreover, it was demonstrated that rhlR expression can be enhanced under low-phosphate medium conditions, albeit phosphate limitation was shown to reduce AHL levels while allowing rhamnolipid production (3). These results indicate that QS involves a very complex genetic regulatory circuit, and its fine-tuning is regulated by both environmental and cell-density-dependent cues, which seems to be important not only for the pathogenesis but also for the adaptation of the pathogen to given environmental conditions (32). In this study we demonstrate that the physiological sensing of phosphate as an environmental factor is important in rhlR gene transcription and in PQS and pyocyanin production. Moreover, our results suggest that PhoB links environmental and cell-density-dependent cues to secondary metabolite production in P. aeruginosa, thus establishing a molecular genetic basis that couples the Pho regulon and quorum sensing.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The clinical P. aeruginosa strain SCV 20265, isolated from the respiratory tract of a cystic fibrosis patient who attended the Cystic Fibrosis Clinic at Hanover Medical School, Hanover, Germany, was used in this study (22). This P. aeruginosa strain produced large amounts of hydroxy-alkyl-quinolones (HAQs), including PQS (7), was an efficient pyocyanin producer, and therefore was especially suitable for monitoring pyocyanin and PQS expression under various medium conditions. P. aeruginosa was routinely cultured at 37°C on Columbia or Luria-Bertani (LB) agar. A transposon mutant of SCV 20265 (phoB mutant) that was generated using the transposon construction vector EZ:TN pMOD-2 (Epicenter) was grown in LB medium supplemented with 50 μg/ml gentamicin. This mutant had been previously identified within an (unpublished) screen for low pyocyanin production and harbored a transposon insertion within the phoB gene located upstream of phoR on the same operon. The expression of rhlR was studied by the determination of β-galactosidase activity in bacteria harboring the plasmid pMAL.V, which contains a lacZ transcriptional fusion of rhlR and was provided by courtesy of A. Lazdunski (28). For the complementation of the phoB gene locus, phoB was amplified from chromosomal DNA by using flanking amplimers (5′-AAA AAA GCT TAT GGT TGG CAA GAC AAT CCT CA-3′ and 5′-AAA AGG ATC CTC AGC TCT TGG TGG AGA AAC G-3′) designed against the 5′ upstream and 3′ downstream regions adjacent to the relevant coding regions. The amplicons were cloned into the XhoI-BamHI sites of pUCP20T (54) to construct pUCP20:phoB.
Bacteria were cultured at 37°C and 180 rpm in 100-ml Erlenmeyer flasks, wide neck, in 20 ml LB medium and in 250-ml Erlenmeyer flasks, wide neck, in 40 ml of a synthetic medium according to the protocol of Frank and DeMoss (18). The medium contained either 0.8 mM or 4.0 mM dipotassium hydrogen phosphate and was supplemented with 2.5 μM or 50 μM ferric citrate, respectively. Where indicated, synthetic PQS (7) was added at a concentration of 40 μM.
Measurement of β-galactosidase activity.
Miller assays were carried out as described previously (34). Briefly, 100- to 200-μl samples of the bacterial culture grown to an optical density at 600 nm of 0.6 to 1 were added to the reaction mix and vortexed. The reaction mix consisted of 800 to 900 μl Z buffer, 10 μl 0.1% sodium dodecyl sulfate, and 10 μl of chloroform. A 200-μl volume of o-nitrophenyl-β-d-galactopyranoside (4 mg/ml 0.1 M K2HPO4) was added to the reaction mixture and incubated until there was a color change or for 30 min if there was no obvious color change.
Extraction of extracellular P. aeruginosa HAQ metabolites and TLC.
HAQ metabolites were extracted from P. aeruginosa broth cultures with dichloromethane as described previously (7). Briefly, the bacterial cultures were extracted with 2 volumes of dichloromethane by vigorous shaking. After centrifugation at 3,500 × g for 10 min, the lower organic layer was evaporated. Thin-layer chromatography (TLC) was performed using a silica gel 60 F254 TLC plate which had been previously soaked for 30 min in 5% KH2PO4 and activated at 85°C for 1.5 h. The extracted P. aeruginosa material was dissolved in dichloromethane and separated by TLC using 95:5 dichloromethane-methanol as solvent. Fluorescent spots were visualized under UV light and photographed. Synthesized PQS and 2-heptyl-4-hydroxy-quinolin (HHQ) were used as standards.
Assay for pyocyanin production.
Pyocyanin production was determined as described previously (17). Briefly, the bacterial supernatant was extracted with chloroform and then reextracted with 0.2 N HCl to give a pink solution. The absorbance was measured at 520 nm, and the pyocyanin content was calculated as described elsewhere (17).
Search for putative PHO box sequences in the P. aeruginosa genome.
A position weight matrix (PWM) model was built from 33 aligned heptameric PHO half-sites using the data from the PRODORIC database (35). The PHO box was afterwards modeled using this PWM for a bipartite pattern with a variable spacer length between 2 and 5 bp. The scoring was done by using the information theoretical approach (46) and by summing up the individual scores of the two half-site PWMs to an overall score. We additionally refined the method by a linear correction of noise (47) due to the high GC content of the P. aeruginosa genome. The genome-wide search was performed using the Virtual Footprint software (36). Sequence logos from the matches derived were created using WebLogo (12).
RESULTS AND DISCUSSION
The role of iron in bacterial pathogenicity is well established, as the low concentration of iron present in the host is an important signal to enhance the expression of a wide variety of bacterial toxins and other virulence determinants (29, 33). More recently, phosphate has also been recognized as an important nutrient and environmental signal that regulates virulence in bacteria, and a cross talk between the phosphate regulons and virulence has been suggested in various bacterial species (2, 45, 49, 56, 61).
Phosphate limitation stimulates the expression of rhlR in P. aeruginosa in a phoB-dependent manner.
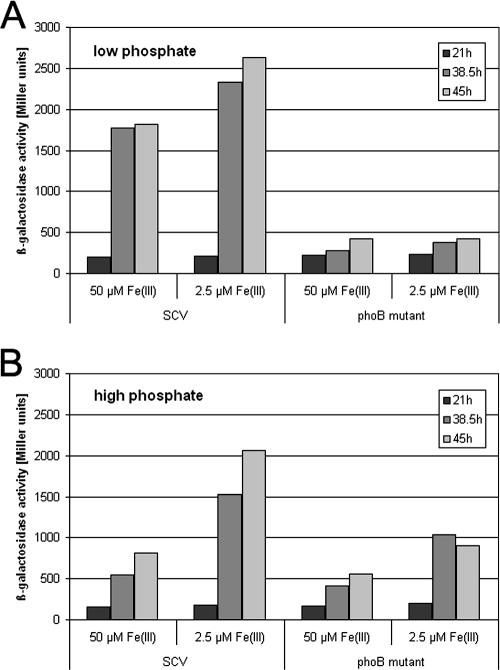
With the aim to reevaluate rhlR expression in P. aeruginosa under defined medium conditions, we introduced a rhlR-lacZ transcriptional fusion in the low-copy-number plasmid pMP220 (pMAL.V) into P. aeruginosa SCV 20265 and a phoB mutant background and determined the β-galactosidase activity in bacteria grown in low-phosphate (0.8 mM phosphate) or high-phosphate (4 mM phosphate) medium supplemented with 2.5 μM or 50 μM ferric citrate, respectively. As demonstrated in Fig. 1, under low-phosphate medium conditions (50 μM ferric citrate) we found an increased β-galactosidase activity in the wild-type cultures compared to the controls grown under high-phosphate medium conditions (paired t test, P = 0.0036). The highest β-galactosidase activity was found under low-phosphate and low-iron medium conditions. In contrast to the increased rhlR expression of the wild type under low-phosphate conditions, we did not observe an induction of rhlR transcription in the phoB mutant (Fig. 1). However, low-iron medium induced rhlR expression even in the phoB mutant under phosphate-replete conditions (Fig. 1), albeit not significantly (paired t test, P = 0.24).
FIG. 1.
rhlR promoter activity at three different time points in SCV 20265 and the phoB mutant cultivated in low-phosphate (0.8 mM) (A) and high-phosphate (4 mM) (B) medium supplemented with 50 μM and 2.5 μM ferric citrate, respectively. This graph is representative of at least three independent experiments.
Under high-phosphate medium conditions, the overall levels of β-galactosidase activity of the phoB mutant did not reach the level of those detected in the wild-type background. This PhoB/PhoR dependency of rhlR transcription even under high-phosphate medium conditions implicates that PhoB activation might not be restricted to low-phosphate medium conditions. A functional basis for a concept of cross-regulation in the PHO regulon has been proposed before in Escherichia coli, where a regulatory coupling has been suggested to exist between the PHO regulon and genes for enzymes in central metabolism for incorporation of phosphate into ATP (59).
Search for putative PHO box sequences in the P. aeruginosa genome.
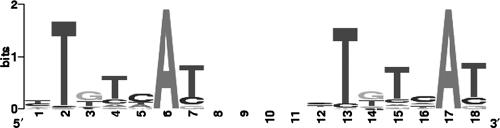
Our data showing a phoB/phoR-dependent rhlR induction under phosphate depletion led us to survey the intergenic regions of the P. aeruginosa genome for consensus sequences with similarities to the Escherichia coli PHO box. In E. coli, the phosphate-dependent regulation of the phosphate regulon is controlled by the two-component regulatory system PhoR-PhoB (58), comprising the response regulator PhoB and its partner sensor kinase, PhoR. Each phosphate-regulated Pho regulon promoter is preceded by an upstream activation site with a consensus PHO box sequence for transcriptional activation by phosphorylated PhoB. The PHO box consists of two 7-bp direct repeats separated by a 4-bp segment: CTGTCAT-A(A/T)A(T/A)-CTGT(C/A)A(C/T) (4, 30, 55). However, the spacer length between the two heptamers seems to be variable (26). In P. aeruginosa a very similar Pho regulon system exists (1). Thereby, PhoB and PhoR constitute an operon with PhoB being promoter proximal. A well-conserved putative PHO box was previously identified in the regulatory region of phoB (1) and upstream of the P. aeruginosa ABC phosphate transporter (pstC, pstA, pstB, and phoU) (37). In this study we applied a global approach and scanned the intergenic regions extending 300 bp upstream of the translation start site of the whole P. aeruginosa genome. We identified 237 putative PHO boxes, including the two published PHO boxes preceding pstC (37) and the phoB gene (1). This number represents 417 putative downstream genes without consideration of operon structures. Among the genes preceded by putative PHO boxes we identified phzA2/phzA1 and rhlR, rhlI, and lasR as well as pqsR. The complete list of genes preceded by a putative PHO box is shown in Table S1 in the supplemental material. The most frequent category (apart from hypothetical genes) consists of genes known or predicted to be involved in transcriptional regulation. Genes in the category membrane proteins, transport of small molecules, and putative enzymes are also numerous. A graphical representation of aligned sequences with a 4-bp spacer is depicted in Fig. 2 as a sequence logo. The match found most frequently exhibited a spacer length of 4 bp and showed the highest information content.
FIG. 2.
PhoB sequence logo derived from genome-wide matches with the preferred spacer length of 4 bp.
HAQ production is enhanced under low-phosphate medium conditions.
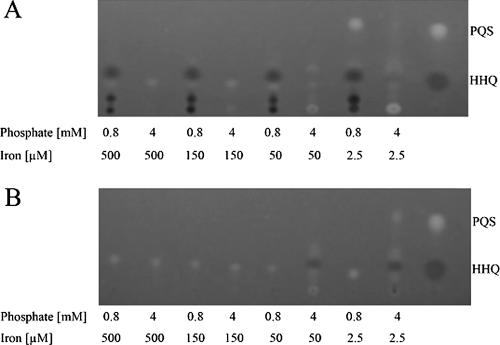
Apart from the two AHL-mediated QS systems (las and rhl), a third interbacterial communication system, the PQS system, is important in P. aeruginosa pathogenesis (10). PQS signaling seems to be independent of the rhl QS regulon (13) and has been described to influence secondary metabolite production in P. aeruginosa (16, 51). The putative PHO boxes preceding rhlR/I, lasR, and pqsR suggest that phosphate availability has an influence on all three QS systems, including PQS signaling. Thus, we analyzed the impact of low-phosphate medium conditions on HAQ biosynthesis. As shown in Fig. 3, HAQ production was enhanced under low-phosphate medium conditions, and this induced HAQ production was abolished in a phoB mutant background. Moreover, decreasing iron concentrations induced PQS/HAQ production in the wild type and the phoB mutant background (Fig. 3). We also detected an increased pyochelin production (bright fluorescent spot at the place of depot [6]) under low-iron and high-phosphate concentrations.
FIG. 3.
Thin-layer chromatography of dichloromethane extracts of the SCV 20265 (A) and the isogenic phoB mutant (B) grown to stationary phase. Bacterial cultures were extracted after cultivation in low-phosphate (0.8 mM) and high-phosphate (4 mM) medium supplemented with 500 μM, 150 μM, 50 μM, and 2.5 μM ferric citrate. Synthetic PQS and HHQ served as controls. This TLC is representative of at least three independent experiments.
PQS activates rhlR transcription in a phoB-independent manner and enhances pyocyanin production in a phoB-dependent manner.
While PQS appears to act as an intercellular signal, the mechanism by which PQS controls gene expression remains elusive. However, it is clear that a PQS-controlled regulatory pathway must act at several different levels (16). The effect of PQS has been shown previously to be partially mediated via up-regulation of the rhl system (16). However, a rhl-independent system has been suggested to exist, as PQS induces the rhl QS system but it does not mediate its regulatory activity through lasRI, rhlRI, or the production of AHL. RhlR and butyryl-homoserine lactone levels during entry into stationary phase were shown not to be significantly altered in mutants defective in PQS production or response, and yet these mutants failed to induce pyocyanin production (16).
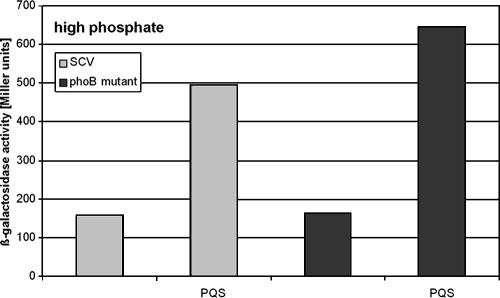
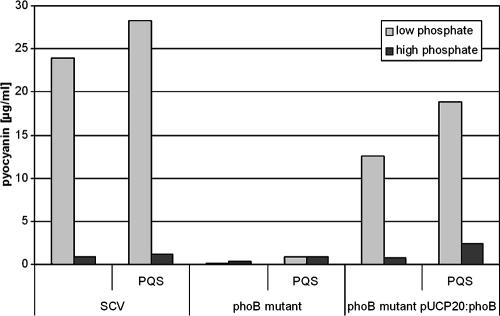
To further dissect the various independent regulation levels for rhl QS- and PQS-induced genes, we tested whether the enhancement of pyocyanin production by the addition of PQS is indirect via an induction of the rhl QS system or whether there are direct effects that are independent from the rhl system. PQS has previously been described to induce the rhl QS system, probably by depleting iron from the growth medium (6). Since in a phoB mutant rhlR expression is induced under low-iron medium conditions, we were interested in whether PQS activates rhlR transcription in P. aeruginosa in a phoB-independent manner. Indeed, as demonstrated in Fig. 4, PQS addition to the wild type and the phoB mutant enhanced rhlR expression under high-phosphate medium conditions. In addition, as shown in Fig. 5, PQS stimulates the production of pyocyanin in P. aeruginosa SCV 20265. However, this effect cannot be attributed to the direct induction of the rhl system alone, since the addition of PQS did not induce high pyocyanin levels under high-phosphate medium conditions or in a phoB mutant background (Fig. 5). Thus, although PQS activates rhlR transcription in P. aeruginosa in a PhoB-independent manner, phoB mutants cannot be stimulated by PQS to produce high levels of pyocyanin. Since a putative PHO box was also identified preceding the two phenazine biosynthetic operons (phzA2/phzA1), it seems that pyocyanin production is, apart from the regulatory influence of the rhl system, under the strong regulatory influence of PhoB and that this influence is enhanced in the presence of PQS. Recent work of Dietrich et al. (14) provided evidence that pyocyanin itself functions as a signal molecule and up-regulates genes encoding the MexGHI-OmpD pump. Interestingly, we found a putative PHO box preceding mexG, implicating that the pump is also under the regulatory influence of PhoB. To further confirm our suggestion that pyocyanin production is influenced by PhoB, we monitored pyocyanin production in the phoB mutant background after complementation with phoB in trans with and without the addition of PQS (Fig. 5). Under low-phosphate medium conditions, complementation of phoB could partially restore pyocyanin production. The complemented mutant probably did not reach the pyocyanin levels of the wild type due to a lack of a (fully) functional PhoR. Under high-phosphate medium conditions the phoB mutant, the phoB-complemented strain, and the wild type did not produce high levels of pyocyanin, albeit in all three strains the expression level could be slightly enhanced (significant induction could only be observed for the phoB mutant [paired t test, P = 0.02]) by the addition of PQS probably via a PQS-dependent rhlR activation.
FIG. 4.
rhlR promoter activity in SCV 20265 and the isogenic phoB mutant grown in high-phosphate (4 mM) medium supplemented with 50 μM ferric citrate with or without the addition of 40 μM PQS. This graph is representative of at least three independent experiments.
FIG. 5.
Pyocyanin production of SCV 20265 grown for 96 h under high- and low-phosphate medium conditions and supplemented with 50 μM ferric citrate with or without the addition of 40 μM PQS. This graph is representative of at least three independent experiments.
Conclusion.
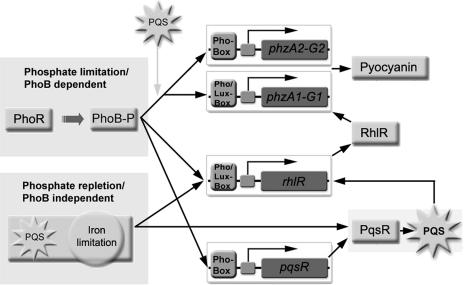
Our results amount to the elucidation of the complex relationship between the phosphate regulon and QS in P. aeruginosa (Fig. 6): in response to low phosphate and possibly yet-to-be-determined other (environmental) factors, PhoB is activated, and phosphorylated PhoB subsequently enhances PhoB-dependent gene transcription. We found an enhanced pyocyanin production probably directly by a phoB-dependent transcriptional activation of phzA1/phzA2 and indirectly via an augmentation of rhlR and pqsR expression, both of which positively influence pyocyanin production. PQS (possibly due to its iron-chelating capability) and low-iron medium conditions also stimulate rhlR transcription and the expression of HAQs in a phoB-independent manner under high-phosphate medium conditions.
FIG. 6.
Model of the complex regulatory circuit of secondary metabolite production in P. aeruginosa.
It appears that QS in P. aeruginosa is a very complex and fine-tuned regulatory circuit important for the pathogenesis of P. aeruginosa (32). The identification of PhoB as a response regulator that links environmental and cell-density-dependent cues sheds light on the molecular mechanisms by which environmental signals trigger the regulatory events required for QS. Research in this area and molecular details on how PQS signaling is dependent on PhoB will contribute significantly to the understanding of the molecular mechanism underlying bacterial adaptation involving both perception of the environment and cell density.
Supplementary Material
Acknowledgments
We thank Michael Morr for synthesizing PQS, Victor Wray for the critical reading of the manuscript, and Jürgen Wehland for his continuous encouraging support.
V.J. is a recipient of a Wilhelm Hirte stipend of the Hanover Biomedical Research School. Financial support of the Helmholtz-Gemeinschaft is gratefully acknowledged.
Footnotes
Published ahead of print on 6 October 2006.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Anba, J., M. Bidaud, M. L. Vasil, and A. Lazdunski. 1990. Nucleotide sequence of the Pseudomonas aeruginosa phoB gene, the regulatory gene for the phosphate regulon. J. Bacteriol. 172:4685-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoyama, T., M. Takanami, K. Makino, and A. Oka. 1991. Cross-talk between the virulence and phosphate regulons of Agrobacterium tumefaciens caused by an unusual interaction of the transcriptional activator with a regulatory DNA element. Mol. Gen. Genet. 227:385-390. [DOI] [PubMed] [Google Scholar]
- 3.Bazire, A., A. Dheilly, F. Diab, D. Morin, M. Jebbar, D. Haras, and A. Dufour. 2005. Osmotic stress and phosphate limitation alter production of cell-to-cell signal molecules and rhamnolipid biosurfactant by Pseudomonas aeruginosa. FEMS Microbiol. Lett. 253:125-131. [DOI] [PubMed] [Google Scholar]
- 4.Blanco, A. G., M. Sola, F. X. Gomis-Ruth, and M. Coll. 2002. Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure 10:701-713. [DOI] [PubMed] [Google Scholar]
- 5.Bollinger, N., D. J. Hassett, B. H. Iglewski, J. W. Costerton, and T. R. McDermott. 2001. Gene expression in Pseudomonas aeruginosa: evidence of iron override effects on quorum sensing and biofilm-specific gene regulation. J. Bacteriol. 183:1990-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bredenbruch, F., R. Geffers, M. Nimtz, J. Buer, and S. Häussler. 2006. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ. Microbiol. 8:1318-1329. [DOI] [PubMed] [Google Scholar]
- 7.Bredenbruch, F., M. Nimtz, V. Wray, M. Morr, R. Müller, and S. Häussler. 2005. Biosynthetic pathway of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines. J. Bacteriol. 187:3630-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camara, M., P. Williams, and A. Hardman. 2002. Controlling infection by tuning in and turning down the volume of bacterial small-talk. Lancet Infect. Dis. 2:667-676. [DOI] [PubMed] [Google Scholar]
- 10.Cao, H., G. Krishnan, B. Goumnerov, J. Tsongalis, R. Tompkins, and L. G. Rahme. 2001. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc. Natl. Acad. Sci. USA 98:14613-14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelis, P., and S. Aendekerk. 2004. A new regulator linking quorum sensing and iron uptake in Pseudomonas aeruginosa. Microbiology 150:752-756. [DOI] [PubMed] [Google Scholar]
- 12.Crooks, G. E., G. Hon, J. M. Chandonia, and S. E. Brenner. 2004. WebLogo: a sequence logo generator. Genome Res. 14:1188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deziel, E., S. Gopalan, A. P. Tampakaki, F. Lepine, K. E. Padfield, M. Saucier, G. Xiao, and L. G. Rahme. 2005. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol. Microbiol. 55:998-1014. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich, L. E., A. Price-Whelan, A. Petersen, M. Whiteley, and D. K. Newman. 2006. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 61:1308-1321. [DOI] [PubMed] [Google Scholar]
- 15.Diggle, S. P., P. Cornelis, P. Williams, and M. Camara. 2006. 4-Quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives. Int. J. Med. Microbiol. 296:83-91. [DOI] [PubMed] [Google Scholar]
- 16.Diggle, S. P., K. Winzer, S. R. Chhabra, K. E. Worrall, M. Camara, and P. Williams. 2003. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol. 50:29-43. [DOI] [PubMed] [Google Scholar]
- 17.Essar, D. W., L. Eberly, A. Hadero, and I. P. Crawford. 1990. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 172:884-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank, L. H., and R. D. DeMoss. 1959. On the biosynthesis of pyocyanine. J. Bacteriol. 77:776-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuqua, C., and E. P. Greenberg. 1998. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1:183-189. [DOI] [PubMed] [Google Scholar]
- 20.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 21.Hassett, D. J., J. F. Ma, J. G. Elkins, T. R. McDermott, U. A. Ochsner, S. E. West, C. T. Huang, J. Fredericks, S. Burnett, P. S. Stewart, G. McFeters, L. Passador, and B. H. Iglewski. 1999. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 34:1082-1093. [DOI] [PubMed] [Google Scholar]
- 22.Häussler, S., I. Ziegler, A. Lottel, F. von Gotz, M. Rohde, D. Wehmhohner, S. Saravanamuthu, B. Tümmler, and I. Steinmetz. 2003. Highly adherent small-colony variants of Pseudomonas aeruginosa in cystic fibrosis lung infection. J. Med. Microbiol. 52:295-301. [DOI] [PubMed] [Google Scholar]
- 23.Hentzer, M., H. Wu, J. B. Andersen, K. Riedel, T. B. Rasmussen, N. Bagge, N. Kumar, M. A. Schembri, Z. Song, P. Kristoffersen, M. Manefield, J. W. Costerton, S. Molin, L. Eberl, P. Steinberg, S. Kjelleberg, N. Hoiby, and M. Givskov. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juhas, M., L. Wiehlmann, B. Huber, D. Jordan, J. Lauber, P. Salunkhe, A. S. Limpert, F. von Gotz, I. Steinmetz, L. Eberl, and B. Tümmler. 2004. Global regulation of quorum sensing and virulence by VqsR in Pseudomonas aeruginosa. Microbiology 150:831-841. [DOI] [PubMed] [Google Scholar]
- 25.Kim, E. J., W. Wang, W. D. Deckwer, and A. P. Zeng. 2005. Expression of the quorum-sensing regulatory protein LasR is strongly affected by iron and oxygen concentrations in cultures of Pseudomonas aeruginosa irrespective of cell density. Microbiology 151:1127-1138. [DOI] [PubMed] [Google Scholar]
- 26.Krol, E., and A. Becker. 2004. Global transcriptional analysis of the phosphate starvation response in Sinorhizobium meliloti strains 1021 and 2011. Mol. Genet. Genomics 272:1-17. [DOI] [PubMed] [Google Scholar]
- 27.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 28.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 29.Litwin, C. M., and S. B. Calderwood. 1993. Role of iron in regulation of virulence genes. Clin. Microbiol. Rev. 6:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makino, K., H. Shinagawa, M. Amemura, and A. Nakata. 1986. Nucleotide sequence of the phoB gene, the positive regulatory gene for the phosphate regulon of Escherichia coli K-12. J. Mol. Biol. 190:37-44. [DOI] [PubMed] [Google Scholar]
- 31.McKnight, S. L., B. H. Iglewski, and E. C. Pesci. 2000. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 182:2702-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medina, G., K. Juarez, R. Diaz, and G. Soberon-Chavez. 2003. Transcriptional regulation of Pseudomonas aeruginosa rhlR, encoding a quorum-sensing regulatory protein. Microbiology 149:3073-3081. [DOI] [PubMed] [Google Scholar]
- 33.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Munch, R., K. Hiller, H. Barg, D. Heldt, S. Linz, E. Wingender, and D. Jahn. 2003. PRODORIC: prokaryotic database of gene regulation. Nucleic Acids Res. 31:266-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munch, R., K. Hiller, A. Grote, M. Scheer, J. Klein, M. Schobert, and D. Jahn. 2005. Virtual Footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics 21:4187-4189. [DOI] [PubMed] [Google Scholar]
- 37.Nikata, T., Y. Sakai, K. Shibat, J. Kato, A. Kuroda, and H. Ohtake. 1996. Molecular analysis of the phosphate-specific transport (pst) operon of Pseudomonas aeruginosa. Mol. Gen. Genet. 250:692-698. [DOI] [PubMed] [Google Scholar]
- 38.Ochsner, U. A., and J. Reiser. 1995. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:6424-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 41.Pearson, J. P., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1995. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:1490-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pesci, E. C., J. B. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruberg, S., A. Puhler, and A. Becker. 1999. Biosynthesis of the exopolysaccharide galactoglucan in Sinorhizobium meliloti is subject to a complex control by the phosphate-dependent regulator PhoB and the proteins ExpG and MucR. Microbiology 145:603-611. [DOI] [PubMed] [Google Scholar]
- 46.Schneider, T. D., G. D. Stormo, L. Gold, and A. Ehrenfeucht. 1986. Information content of binding sites on nucleotide sequences. J. Mol. Biol. 188:415-431. [DOI] [PubMed] [Google Scholar]
- 47.Schreiber, M., and C. Brown. 2002. Compensation for nucleotide bias in a genome by representation as a discrete channel with noise. Bioinformatics 18:507-512. [DOI] [PubMed] [Google Scholar]
- 48.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slater, H., M. Crow, L. Everson, and G. P. Salmond. 2003. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and -independent pathways. Mol. Microbiol. 47:303-320. [DOI] [PubMed] [Google Scholar]
- 50.Smith, R. S., and B. H. Iglewski. 2003. Pseudomonas aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 51.Soberon-Chavez, G., F. Lepine, and E. Deziel. 2005. Production of rhamnolipids by Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 68:718-725. [DOI] [PubMed] [Google Scholar]
- 52.Stintzi, A., K. Evans, J. M. Meyer, and K. Poole. 1998. Quorum-sensing and siderophore biosynthesis in Pseudomonas aeruginosa: lasR/lasI mutants exhibit reduced pyoverdine biosynthesis. FEMS Microbiol. Lett. 166:341-345. [DOI] [PubMed] [Google Scholar]
- 53.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 54.Sweizer, H. P., M. Classen, and D. Hogan. 1996. Improved methods for gene analysis in pseudomonads, p. 229-237. In T. Nakawawa et al. (ed.), Molecular biology of pseudomonads. ASM Press, Washington, D.C.
- 55.Tommassen, J., M. Koster, and P. Overduin. 1987. Molecular analysis of the promoter region of the Escherichia coli K-12 phoE gene. Identification of an element, upstream from the promoter, required for efficient expression of PhoE protein. J. Mol. Biol. 198:633-641. [DOI] [PubMed] [Google Scholar]
- 56.von Kruger, W. M., L. M. Santos Lery, M. R. Soares, d. N.-M. Saloum, Batista E Silva, C. M., da Costa Neves-Ferreira, A. G., J. Perales, and P. M. Bisch. 2006. The phosphate-starvation response in Vibrio cholerae O1 and phoB mutant under proteomic analysis: disclosing functions involved in adaptation, survival and virulence. Proteomics 6:1495-1511. [DOI] [PubMed] [Google Scholar]
- 57.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wanner, B. L. 1993. Gene regulation by phosphate in enteric bacteria. J. Cell Biochem. 51:47-54. [DOI] [PubMed] [Google Scholar]
- 59.Wanner, B. L., and M. R. Wilmes-Riesenberg. 1992. Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in control of the phosphate regulon in Escherichia coli. J. Bacteriol. 174:2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whiteley, M., K. M. Lee, and E. P. Greenberg. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:13904-13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winans, S. C. 1990. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J. Bacteriol. 172:2433-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, Y., and R. M. Miller. 1992. Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant). Appl. Environ. Microbiol. 58:3276-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.