Abstract
The virulence of Pseudomonas aeruginosa and other surface pathogens involves the coordinate expression of a wide range of virulence determinants, including type IV pili. These surface filaments are important for the colonization of host epithelial tissues and mediate bacterial attachment to, and translocation across, surfaces by a process known as twitching motility. This process is controlled in part by a complex signal transduction system whose central component, ChpA, possesses nine potential sites of phosphorylation, including six histidine-containing phosphotransfer (HPt) domains, one serine-containing phosphotransfer domain, one threonine-containing phosphotransfer domain, and one CheY-like receiver domain. Here, using site-directed mutagenesis, we show that normal twitching motility is entirely dependent on the CheY-like receiver domain and partially dependent on two of the HPt domains. Moreover, under different assay conditions, point mutations in several of the phosphotransfer domains of ChpA give rise to unusual “swarming” phenotypes, possibly reflecting more subtle perturbations in the control of P. aeruginosa motility that are not evident from the conventional twitching stab assay. Together, these results suggest that ChpA plays a central role in the complex regulation of type IV pilus-mediated motility in P. aeruginosa.
Pseudomonas aeruginosa is a rod-shaped bacterium that is an opportunistic pathogen of plants and animals, including immunocompromised patients such as cystic fibrosis sufferers (14). The bacterium is equipped with a large arsenal of secreted and cell-associated virulence factors, including type IV pili, which are filamentous appendages located at the poles of the bacterium that facilitate attachment to host epithelial cells, and a form of surface translocation called twitching motility (TM) (16).
Twitching motility occurs on wet surfaces and is an important factor for host infection and colonization as well as other forms of complex colonial behavior (16). Twitching motility occurs by the extension and retraction of type IV pili and is distinct from swimming motility (as in Escherichia coli and P. aeruginosa), which is mediated by the rotation of polar flagella, and from orthodox “swarming motility” (as in Proteus mirabilis), which is mediated by the coordinated action of peritrichous flagella (23).
“Swarming motility” is, however, a loosely defined term that has been used somewhat indiscriminately to describe various forms of organized bacterial motility. A form of “swarming motility” in P. aeruginosa has been reported but differs from that described for other bacteria in that it appears to demonstrate a reliance on type IV pili rather than flagella (11). However, there is contradictory evidence, with another report indicating that flagella are essential for swarming (22), which is consistent with the usual basis of swarming in other species.
In P. aeruginosa, around 40 genes whose products are required for TM, 16 of which code for proteins that are involved in transcriptional regulation and chemosensory control, have now been identified (16, 33). The chemosensory system that controls TM is encoded predominantly by the pilGHIJK (5-7) and chpABC (33) gene clusters. Together, the proteins encoded by these genes appear to comprise a complex chemosensory signal transduction pathway that shares many modules with, but is significantly more complex than, the chemosensory systems that control flagellum rotation in bacteria and the Frz system that controls social gliding motility in Myxococcus xanthus, with which it shares many similarities (16, 28). The lynchpin of this chemosensory system appears to be ChpA, a complex signal transduction protein containing nine potential sites of phosphorylation, including six histidine-containing phosphotransfer (HPt) domains, one serine-containing phosphotransfer (SPt) domain, one threonine-containing phosphotransfer (TPt) domain, and the CheY-like receiver domain (33). Here, we begin to dissect the function of these domains in motility by site-directed mutagenesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Escherichia coli strain DH5α (recA endA1 gyrA96 hsdR17 thi-1 supE44 relA1 φ80dlacΖΔM15) was used in all genetic manipulations and in the preparation of DNA sequencing templates. Escherichia coli strain S17-1 was used as the donor strain in bacterial conjugations. The P. aeruginosa strain used was PAO1 strain ATCC 15692 (American Type Culture Collection). The MTP cosmid library (10) was used in the subcloning of the chp region. The preparation of E. coli competent cells and transformation protocols were performed according to methods described previously (25). The preparation of P. aeruginosa competent cells was performed as described previously (17). Plasmids used in this study are listed in Table 1. E. coli and P. aeruginosa liquid cultures were maintained in Luria-Bertani (LB) broth, and solid medium was prepared by adding 0.5 to 1.5% agar (Becton Dickinson BBL agar grade A). The following antibiotic concentrations were used for the selection of E. coli: 12.5 μg/ml tetracycline for plasmid selection and 40 μg/ml tetracycline for cosmid selection, 100 μg/ml ampicillin, 25 μg/ml chloramphenicol, and 50 μg/ml kanamycin. The concentrations of antibiotics for the selection of P. aeruginosa were as follows: 250 μg/ml carbenicillin, 20 μg/ml rifampin, 250 μg/ml chloramphenicol, and 200 μg/ml tetracycline.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| DH5α | recA endA1 gyrA96 hsdR17 thi-1 supE44 relA1 φ80dlacΖΔM15 | Lab collection |
| S17-1 | thi pro hsdR recA chr::RP4-2 | 29 |
| GM2163 | F− ara-14 leuB6 fhuA31 lacY1 tsx78 glnV44 galK2 galT22 mcrA dcm-6 hisG4 rfbD1 rpsL136 (Strr) dam13::Tn9 (Cmr) xylA5 mtl-1 thi-1 mcrB1 hsdR2 | New England Biolabs |
| PAO1 | Wild type P. aeruginosa strain ATCC 15692 | Lab collection |
| PAO1ΔchpA | PAO1 with Tcr insertion in chpA | This study |
| Plasmids | ||
| pBluescript II KS | E. coli cloning vector with Ap selection | Stratagene |
| pOK12 | E. coli cloning vector with Km selection | 31 |
| pUC21 | E. coli cloning vector with Ap selection | 31 |
| pUCP19 | E. coli-P. aeruginosa cloning vector | 27 |
| pUCPSK | P. aeruginosa-E. coli shuttle vector | 32 |
| pMMB206 | Pseudomonas controlled expression level vector with Cm selection | 19 |
| pMOB3 | Counterselectable sacB/sacR genes from Bacillus subtilis | 26 |
| pSM-TET | Source of Tetr cartridge | 18 |
| MTP26 | Cosmid containing P. aeruginosa PAO1 chromosomal DNA from bp 448166-472109 | 10 |
| pAL1 | pBluescript II KS with modified multiple cloning site | This study |
| pAL5 | 3,168-bp SacII/EcoRV fragment of chpA from MTP26 in pAL1 | This study |
| pAL6 | 458-bp H912i PCR product in pUC21 | This study |
| pAL7 | 3,168-bp SacII/EcoRV fragment of chpA containing H1088 in pAL1 | This study |
| pAL8 | 1,297-bp S1231i/H1410i PCR product in pUC21 | This study |
| pAL9 | 611-bp H1570i PCR product in pUC21 | This study |
| pAL10 | 872-bp BamHI/KpnI fragment of chpA containing H1717 in pUC21 | This study |
| pAL11 | 1,396-bp EcoRV/KpnI fragment of chpA from MTP26 in pAL5 | This study |
| pAL13 | 1,561-bp EcoRV/AatII fragment of chpA from MTP26 containing H54 in pOK12 | This study |
| pAL14 | ChpA H54Q in pAL13 | This study |
| pAL16 | ChpA H912Q in pAL6 | This study |
| pAL17 | ChpA H1088Q in pAL7 | This study |
| pAL18 | ChpA S1231A in pAL8 | This study |
| pAL19 | ChpA H1410Q in pAL8 | This study |
| pAL20 | ChpA H1570Q in pAL9 | This study |
| pAL37 | 2,839-bp BamHI/ClaI fragment of chpA from MTP26 containing D2406N in pUC21 | This study |
| pAL40 | 4,291-bp SacI/ClaI chpA fragment in pUCPSK | This study |
| pAL41 | Full-length chpA under Plac control in pUCPSK | This study |
| pAL42 | ChpA D2406N in pAL37 | This study |
| pAL49 | Tcr cassette from pSM-TET (EcoRI/MluI) inserted into MfeI/AscI pAL11 | This study |
| pAL52 | 5-kb NotI fragment from pMOB3 ligated into pAL49 | This study |
| pAL57 | 7,972-bp NotI chpA fragment from MTP26 in pAL1 | This study |
| pAL64 | pAL41 with an internal double FLAG tag inserted between the native NotI/NruI sites of chpA | This study |
| pAL77 | 3,862-bp ClaI/SacI chpA fragment from pAL41 in pUCP19 | This study |
| pAL78 | Full-length chpA against Plac in pUCP19 | This study |
| pAL79 | 8-kb ClaI/SacI fragment from MTP26 ligated into pMMB206 with PtaclacUV | This study |
| pAL82 | 3,503-bp StuI fragment of chpA cut from pAL57 and ligated into pAL14 | This study |
| pAL83 | pAL79 with ChpA H54Q (mutation in HPt1 domain) | This study |
| pAL86 | pAL79 with ChpA H912Q (mutation in HPt2 domain) | This study |
| pAL87 | pAL79 with ChpA H1088Q (mutation in HPt3 domain) | This study |
| pAL88 | pAL79 with ChpA S1231A (mutation in SPt domain) | This study |
| pAL89 | pAL79 with ChpA H1410Q (mutation in HPt4 domain) | This study |
| pAL90 | pAL79 with ChpA H1570Q (mutation in HPt5 domain) | This study |
| pAL92 | pAL79 with ChpA D2406N (mutation in CheY domain) | This study |
| pAL79FLAG | pAL79 with a double FLAG tag inserted into the NotI/KpnI sites of chpA | This study |
| pAL86FLAG | pAL86 with a double FLAG tag inserted into the NotI/KpnI sites of chpA | This study |
| pAL87FLAG | pAL87 with a double FLAG tag inserted into the NotI/KpnI sites of chpA | This study |
| pAL92FLAG | pAL92 with a double FLAG tag inserted into the NotI/KpnI sites of chpA | This study |
Construction of PAO1ΔchpA.
The chpA deletion mutant PAO1ΔchpA was constructed using standard allelic exchange techniques. The plasmid pBluescript II KS was digested with SacI/KpnI, and a synthesized multiple cloning site was ligated in to form pAL1. A 3,168-bp SacII/EcoRV fragment of chpA from cosmid MTP26 (10) was cloned in to give pAL5. A 1,396-bp EcoRV/KpnI fragment from MTP26 was then also ligated in to give pAL11. This plasmid was then digested with MfeI/AscI, and a Tetr resistance cassette from pSM-TET (18) (EcoRI/MluI) was inserted to give pAL49. A NotI fragment from pMOB3 (that carries the sacB/sacR genes for counterselection) (26) was then inserted into pAL49 to give pAL52. This construct was then transformed into the E. coli donor strain S17-1 in preparation for mating into P. aeruginosa. Following conjugation, the transconjugates were selected on 10% sucrose medium containing 200 μg/ml tetracycline, which forces the excision of the plasmid while leaving the homologously recombined inactivated gene in the chromosome. The genotype of the mutants was confirmed by Southern hybridization analysis.
Construction of chpA point mutants.
Site-directed mutagenesis was performed using either the Stratagene QuikChange site-directed mutagenesis kit (catalog no. 200518) or a sequential PCR method (24). Oligonucleotide primer sequences are provided in the supplemental material.
Construction of chpA expression vectors.
Cosmid MTP26 (10) was digested with SacI and ClaI, and the resulting fragments were separated by size on a 0.7% agarose gel. A 4,291-bp SacI/ClaI fragment was excised, purified, and ligated into pUCPSK (32) to give pAL40. A 3,739-bp SacI fragment was then ligated in to give pAL41, a full-length chpA clone regulated by the Plac promoter. A second chpA expression vector that would orientate chpA against the Plac promoter was then constructed in pUCP19. A 3,862-bp ClaI/SacI fragment was cut from pAL41 and ligated into AccI/SacI-digested pUCP19 to give pAL77. A 3,739-bp SacI fragment was then excised from pAL41 and ligated into pAL77 to give a full-length chpA clone orientated against the Plac promoter, pAL78. A third chpA expression vector was then created using a low-copy-number controlled expression vector, pMMB206, that contains the lacI repressor (19), which is essential for isopropyl-β-d-thiogalactopyranoside (IPTG) expression studies in Pseudomonas. This allows for the titration of ChpA activity by controlling the expression level. Vector pMMB206 (which utilizes a PtaclacUV5 promoter) was chosen in preference to pMMB207 (which uses a Ptac promoter) primarily because of the relative strengths of the promoters (19). The PtaclacUV5 promoter has previously been reported to reach only 6.5% of the expression level of the Ptac promoter in Pseudomonas species (19), reducing the chance of detrimental effects from the overexpression of the 270-kDa signal transduction protein. Partial EcoRI/HindIII digestion was performed on pAL78 to give an 8,064-bp fragment. This was ligated into EcoRI/HindIII-digested pMMB206 to give pAL79 (see Fig. S1 in the supplemental material).
The mutated phosphotransfer sites were then individually shuttled into pAL79 as described below to create full-length chpA expression constructs with point-mutated phosphotransfer domains. The mutated alleles H912Q, H1088Q, S1231A, H1410Q, and H1570Q were subcloned from pAL16, pAL17, pAL18, pAL19, and pAL20, respectively, into pAL79 using the unique restriction enzyme sites for MfeI and KpnI to give pAL86, pAL87, pAL88, pAL89 and pAL90, respectively (Table 1).
The mutated His54 site was subcloned into pAL79 in a three-step process. Plasmids pAL14 (which contains H54Q) and pAL57 were prepared from E. coli strain GM2163 to allow digestion at Dcm methylation-sensitive sites. A 3,503-bp StuI fragment was cut from pAL57 and cloned into StuI-digested pAL14, giving pAL82. This step increased the length of P. aeruginosa DNA contained in the vector and added a SacI site while retaining the mutated codon. A SacI fragment was then cut from pAL82 and ligated into SacI-digested pAL79, giving pAL83.
The mutated CheY site (D2406N) was cloned by first digesting pAL79 with HindIII, followed by blunting the 5′ overhang of the DNA ends with T4 DNA polymerase and then a second digestion with NotI. A 682-bp fragment containing the mutated codon was excised from pAL42 with AfeI/NotI and then ligated into pAL79, creating pAL92 (Table 1).
Twitching motility assay.
The subsurface twitching motility assay was carried out as described previously (28). Following stab inoculation of the P. aeruginosa strain to be tested through a 1% agar plate and overnight incubation at 37°C, the zone of motility between the agar and the petri dish interface was visualized by compressing the agar and then staining using 0.05% (wt/vol) Coomassie brilliant blue R250 stain (40% methanol, 10% acetic acid) as described previously (2).
“Swarming motility” assay.
Swarm agar was prepared according to a method described previously by Kohler et al. (11). Medium was based on M9 salts without NH4Cl, supplemented with 0.2% glucose (wt/vol), 0.05% glutamate or aspartate (wt/vol) (as the sole nitrogen source), 2 mM MgSO4, and trace elements (available upon request) and solidified with 0.5% agar (Becton Dickinson BBL agar grade A). After solidification, plates were briefly dried and then inoculated with a single colony from a fresh LB plate by pipette tip. Incubation was performed at 37°C for 16 h.
PilA Northern blot analysis.
RNA preparations were carried out using the QIAGEN RNeasy Mini kit. Northern blot analysis of pilA mRNA levels was performed as described previously (34), except that Ultrahybe was used as the hybridization solution.
Rhamnolipid assay.
The amount of rhamnolipid was quantitated using the orcinol method (20). Briefly, 500 μl of cell-free supernatant was extracted twice with 1 ml of diethyl ether. The ether samples were pooled and evaporated to dryness, and 200 μl of water was added. To 100 μl of each sample, 900 μl of a solution containing 0.19% orcinol (in 54% H2SO4) was added, heated to 80°C for 15 min, and cooled. The A421 was then measured, with higher rhamnolipid levels giving a higher absorbance.
ChpA Western analysis.
Detection of FLAG-tagged ChpA was performed as follows. P. aeruginosa transformants were inoculated (1:50) into fresh 5-ml LB broth aliquots (in 50-ml tubes) from cultures grown overnight and were incubated at 37°C with shaking for 4 h. After the addition of 0.3 mM IPTG, cultures were grown for 1 h, and 2 ml of culture was then pelleted and resuspended in 400 μl of loading buffer. To reduce viscosity, whole-cell proteins were prepared by passage through a 27-gauge needle three times and centrifugation at 45,000 rpm for 1 h to pellet chromosomal DNA. Samples were then boiled for 5 min. Proteins were then displayed on 8% sodium dodecyl sulfate-polyacrylamide gels (12) and electroblotted onto an Immobilon-P transfer membrane (Millipore Corp., Bedford, MA) in a Tris-glycine system described previously (30). Membranes were blocked with 3% skim milk and probed with a 1:500 dilution of primary anti-FLAG M2 monoclonal antibody (Sigma Aldrich) in 3% skim milk powder (0.1% Tween 20) in Tris-buffered saline. Membranes were then incubated with a 1:10,000 dilution of rabbit anti-mouse immunoglobulin G conjugated with horseradish peroxidase (Bio-Rad) in 3% skim milk powder (0.1% Tween 20) in Tris-buffered saline followed by detection by enhanced chemiluminescence using the Supersignal chemiluminescence kit (Pierce).
RESULTS
ChpA point mutant twitching phenotypes.
Given the large size and complex structure of ChpA, our experimental strategy was to create a chromosomal deletion mutant of chpA in PAO1 (PAO1ΔchpA) and to complement this mutant in trans with point-mutated alleles of full-length chpA expressed from the low-copy-number plasmid pAL79, which contained the inducible PtaclacUV5 promoter (19). Eight phosphotransfer domains (HPt1 to HPt6, SPt, and CheY) were targeted for mutagenesis using the Stratagene QuikChange kit, following a cassette mutagenesis and segment replacement strategy (Fig. 1; see also Fig. S1 in the supplemental material). Clones that showed the correct mutagenesis of the desired codon were obtained for seven of the domains, with only the mutation of HPt6 being unsuccessful, despite numerous attempts. DNA sequencing confirmed that all mutated alleles were correct.
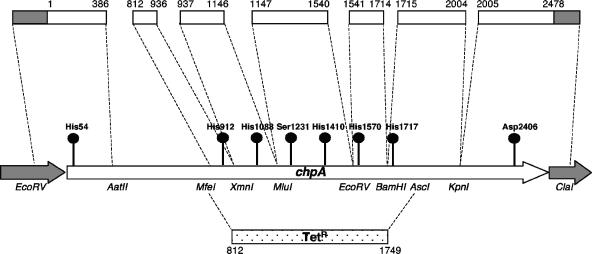
FIG. 1.
Diagram of chpA open reading frame detailing the location of phosphotransfer active-site codons (circles), a Tetr insertional deletion spanning codons 812 to 1749 of chpA, and fragments of chpA (clear boxes) that were subcloned to isolate the phosphotransfer codons for mutagenesis. The numbers above the subcloned fragments indicate the codon regions of chpA contained in the fragment. The restriction enzyme sites used for the cassette substitution of the mutated alleles are listed. Full details of the mutagenesis and cloning strategies used to create the mutants referred to in this study may be found in Fig. S1 and Table S1 in the supplemental material, Table 1, and Materials and Methods.
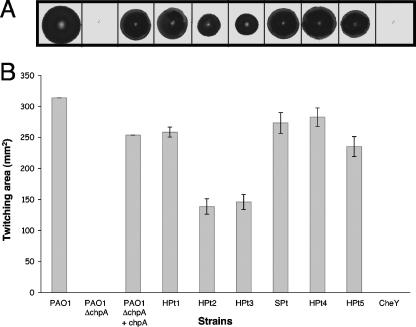
Cassettes containing the mutated domains were then substituted into chpA under the control of the inducible promoter PtaclacUV in pAL79 (Table 1; also see Fig. S1 in the supplemental material) and transformed into PAO1ΔchpA to study the motility phenotypes of the various phosphotransfer domain mutations. The appropriate level of wild-type chpA expression was empirically determined by titrating the IPTG concentration to obtain wild-type levels of twitching motility for PAO1ΔchpA plus pAL79 (wild-type chpA) in the traditional twitching stab assay (16, 28). A concentration of 0.3 mM IPTG was found to give optimal complementation. The strains containing point mutations in the active sites of HPt1, SPt, HPt4, and HPt5 as well the complemented chpA mutant (PAO1ΔchpA plus chpA) exhibit TM zones that are indistinguishable from those of the wild type. The chpA deletion mutant (PAO1ΔchpA) and the CheY point mutant both appear to completely lack TM, while the HPt2 and HPt3 point mutants both display reduced TM (Fig. 2).
FIG. 2.
(A) Subsurface twitching motility assay of P. aeruginosa strains PAO1, PAO1ΔchpA, PAO1ΔchpA plus chpA, HPt1, HPt2, HPt3, SPt, HPt4, HPt5, and CheY. The shorthand notation for HPt1, HPt2, HPt3, SPt, HPt4, HPt5, and CheY is used to designate PAO1ΔchpA containing plasmids pAL83, pAL86, pAL87, pAL88, pAL89, pAL90, and pAL92, respectively, encoding chpA alleles with mutations in the corresponding domain. Twitching assays were performed by the subsurface stab method followed by Coomassie blue staining, as described previously (33). (B) Graph depicting twitching zone areas for the above-described strains. The average zone expansion from three independent experiments is displayed, with error bars representing the standard deviations from the means.
To confirm that the reduction in TM observed in the three mutants was not due to the instability of the mutated protein, we performed a Western blot analysis to visualize the level of ChpA protein in each strain. To circumvent the lack of a ChpA antibody, we modified the point mutant expression vectors (pAL79, pAL86, pAL87, and pAL92) to include the coding region for an internal double FLAG tag in frame at the C-terminal end of the sequences encoding ChpA and its variants (pAL79FLAG, pAL86FLAG, pAL87FLAG, and pAL92FLAG, respectively) (see Table 1 for details). A Western analysis was then performed using the anti-FLAG M2 monoclonal antibody (Fig. 3), which confirmed that the introduction of the point mutations into ChpA did not affect protein stability and therefore that the observed phenotypic effects are most likely due to the cessation of phosphotransfer through the domains in question.
FIG. 3.
Western analysis using anti-FLAG M2 monoclonal antibody to assess ChpA protein levels. Lanes: 1, PAO1ΔchpA(pAL79); 2, PAO1ΔchpA(pAL79FLAG); 3, PAO1ΔchpA(pAL86FLAG); 4, PAO1ΔchpA(pAL87FLAG); 5, PAO1ΔchpA(pAL92FLAG). Bacterial samples were harvested from exponentially growing cultures 1 h after induction with 0.3 mM IPTG.
pilA expression in chpA point mutants.
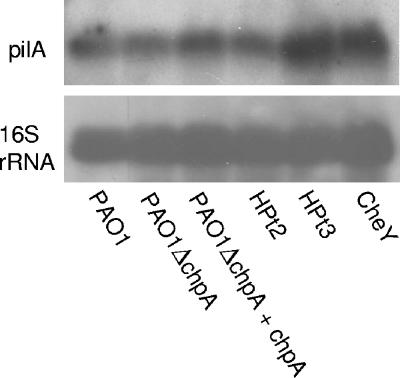
Having shown that (at least) three of the phosphorylation domains of ChpA were involved in the regulation of TM, we then examined whether this regulation is mediated through the transcriptional control of pilA. Northern analysis showed that mutations in the three domains involved in twitching motility (HPt2, HPt3, and CheY) have no discernible effect on the level of pilA mRNA (Fig. 4). This suggests that all three domains are directly involved in the chemosensory control of TM, presumably by controlling the rate of extension and retraction of the pilus (16, 28).
FIG. 4.
Northern analysis of pilA transcript for ChpA point mutants. The top and bottom panels show the autoradiographs from the pilA and 16S rRNA probes, respectively. The shorthand notation for HPt2, HPt3, and CheY is used to designate PAO1ΔchpA containing plasmids (pAL86, pAL87, and pAL92, respectively) encoding chpA alleles with mutations in the corresponding domain. The 16S rRNA probe was used to quantitate the total amount of RNA loaded.
Swarming motility phenotypes.
Recent studies have indicated that what has been described as “swarming motility” in P. aeruginosa is dependent on flagella, type IV pili, and the biosurfactant rhamnolipid (11). Under the conditions of the swarming assay, using a modified M9 medium with 0.2% glucose (wt/vol) and 0.05% glutamate (wt/vol) as sole carbon and nitrogen sources, respectively, supplemented with 0.3 mM IPTG, PAO1 produced the branching dendritic pattern that is normally characteristic of swarming motility (8, 11), while PAO1ΔpilA showed no zone of expansion over the agar surface (Fig. 5), confirming the requirement of type IV pili for the process that we and others (11) observed under these conditions. It is important that we could observe this type of motility, which we will henceforth refer to as “pilus-mediated swarming,” only with Becton Dickinson BBL agar grade A and not with some other types of agar, suggesting the presence of stimulatory or inhibitory materials. The latter is indicated by the fact that the ability to detect this type of motility could be restored for some agars by dialysis of the agar prior to plate preparation, but this was not always the case. This problem may also account for some of the different gross manifestations of motility described in the literature.
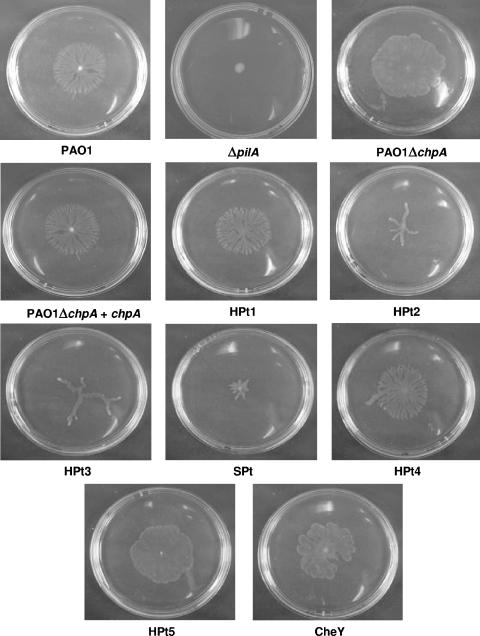
FIG. 5.
Swarming motility phenotypes of chpA point mutants. The photographs are representative of three separate experiments. Swarm plates contained a modified M9 medium with 0.2% glucose (wt/vol) and 0.05% glutamate or aspartate (wt/vol) as sole carbon and nitrogen sources, respectively, and 0.5% agar (Becton Dickinson BBL agar grade A), supplemented with 0.3 mM IPTG and the appropriate antibiotics. Single colonies were inoculated onto the surface of the agar and incubated at 37°C for 16 h (11).
Under the conditions of our assay, the PAO1ΔchpA mutant produced a zone of expansion on the agar surface similar to if not greater than that of PAO1, but the colony did not possess the characteristic branching pattern. This suggests that none of the central HPt domains are required for this form of motility per se, and it is a moot point whether this motility is another manifestation of TM or in some way distinct from it, although we favor the former. It is unlikely that the truncated ChpA protein retains residual activity, as the deletion is not in frame and gives a phenotype similar to that of the mutant lacking the CheY effector domain (Fig. 5). Complementation of PAO1ΔchpA with wild-type chpA restored the branching colony morphology, confirming that the mutation in chpA is responsible for the altered swarming phenotype seen in the PAO1ΔchpA mutant. These data suggest that chpA is not required for the form of motility observed under these conditions (although pilA is) but that it does affect its characteristics, presumably by influencing chemosensory directional control.
The HPt1 and HPt4 point mutants produce a swarming colony morphology identical to that of the wild type and appear to have no effect on this process, at least at a gross level. Interestingly, the two HPt domains that regulate twitching motility, HPt2 and HPt3, both show an unusual swarming phenotype, where thick finger-like projections replace the normal dendritic pattern. The HPt5 point mutant produces a colony morphology that is identical to that of the PAO1ΔchpA mutant, suggesting that the phenotype seen in the chpA deletion mutant is from inactivation of the HPt5 domain. The SPt point mutant produces a small irregular-shaped colony that appears to be restricted in its ability to swarm. The point mutant in the CheY domain exhibited an altered motility phenotype that resembles that of the PAO1ΔchpA mutant except that some simplified branching is observed (Fig. 5). Interestingly, neither of these mutants showed any motility in the conventional twitching stab assay but did exhibit active motility in the swarming assay, indicating that ChpA is not required for pilA-dependent motility per se but rather that lack of chemosensory control of this process affects the former more than the latter. Additionally, although surfactants have been reported to affect swarming motility in P. aeruginosa (11) and other species (8), we did not detect any significant alteration in the level of rhamnolipid production in the chpA mutants under the conditions of the assay (data not shown).
DISCUSSION
The results of the twitching complementation assay indicate that the HPt2, HPt3, and CheY domains of ChpA are directly involved in the signal transduction pathways that control TM. Given that the mechanism of TM is the extension and retraction of pili (16, 28), it is tempting to speculate that one of the HPt domains initiates the signal for extension, while the other initiates the signal for retraction, through PilH and PilG, respectively (both of which contain CheY domains), according to their predicted roles (5, 6, 33). However, there is a difference in twitching phenotypes between PilG/PilH and the HPt2/3 domains. A pilG mutant has little to no surface pili and is nontwitching, while a pilH mutant is hyperpiliated and is an aberrant twitcher (1, 5, 6). A pilH mutant also displays an aberrant swarming phenotype (data not shown) that resembles a ΔchpA mutant but not the individual HPt2 and HPt3 mutants (Fig. 5). Therefore, it would seem unlikely that either HPt domain is interacting exclusively with PilG or PilH. An alternative hypothesis that is in agreement with the twitching and swarming phenotypes is that both HPt domains (and perhaps other ChpA-HPt domains) can interact with either, or both, PilG and PilH. The notion that HPt domains can service multiple response regulators is supported by the chemotaxis phosphorelay of Rhodobacter sphaeroides (21) and the ArcB/ArcA/OmpR phosphorelay of E. coli (15).
Interestingly, the PAO1ΔchpA mutant also exhibited wild-type levels of pilA transcription as observed by Northern analysis (Fig. 4). This is in contrast to our previous finding that some C-terminal transposon mutants of chpA in strain PAK resulted in reduced levels of pilA mRNA, although this effect was variable (33). One explanation may be that there are significant strain differences between PAO and PAK in relation to ChpA, and indeed, we have been unable to complement a PAKΔchpA mutant with PAO1 chpA (A. J. Leech, unpublished observation). It is also possible that the difference in pilA transcription between the PAO1ΔchpA mutant and the C-terminal PAK transposon mutants may be caused by a dominant negative effect of truncated, nearly full-length ChpA protein in the transposon mutants (33).
The complete absence of surface translocation in the pilA deletion mutant (Fig. 5) indicates that what has been previously described as swarming motility is dependent on type IV pili, in agreement with the findings reported previously by Kohler et al. (11), and may be either a different manifestation of twitching motility at the gross colony morphology level under the assay conditions employed or a distinct motility that also requires type IV pili, analogous to the requirement of flagella (polar and peritrichous, respectively) for swimming and swarming motility in other bacteria. It is not known whether the surface deployment of type IV pili is altered under the “swarming” motility assay conditions in P. aeruginosa. The finger-like rafts seen for wild-type pilus-mediated swarming motility are, superficially at least, characteristic of swarming motility in other bacteria, although they resemble (on a larger scale) the motile rafts that are distinctive for the leading edge of a twitching zone (4, 6, 9).
The chemosensory system that controls type IV pilus-mediated motility in M. xanthus (frz) contains many modules that are similar to the chp system of P. aeruginosa (16). While there are a number of orthologs between the two systems (mimicking the Che proteins of E. coli and Salmonella enterica serovar Typhimurium), the closest match to ChpA in terms of domain type and organization (i.e., a CheA-CheY hybrid) is FrzE, although ChpA is substantially more complex. Mutational studies that assess motility phenotypes, similar to the ones completed here, have been carried out on frzE. There appear to be analogies between the phenotypes observed in the two studies. The swarm morphologies of the HPt2/3 mutations (which seem to lack the ability to divide the swarm stream) are similar to frzA, frzE in-frame, or frzE(D709E) mutations, which cause a hyporeversal phenotype in social gliding motility (3, 13), while the CheY-ChpA mutation (which seems to divide the swarm stream continuously) results in swarm morphologies similar to an frzE(D709A) mutation, which gives a hyperreversal phenotype in social gliding motility (13).
Regardless of the semantics of terminology for the observed motility, it is clear that the “swarming” assay is a sensitive indicator of perturbations in type IV pilus-mediated motility resulting from point mutations in phosphotransfer domains of ChpA. The sensitivity of the assay makes it potentially a good system to probe the subtleties of the chemosensory control of twitching/swarming motility in P. aeruginosa as well as perhaps to study such motility in other species. It is also apparent that the role of the different phosphotransfer domains, and the signals that they respond to and impart, remains to be elucidated. The mutants described here provide useful tools to enable future studies to dissect this complex system.
Supplementary Material
Acknowledgments
This work was supported by National Health and Medical Research Council of Australia (NHMRC) grant number 143054.
Footnotes
Published ahead of print on 29 September 2006.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alm, R. A., and J. S. Mattick. 1997. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene 192:89-98. [DOI] [PubMed] [Google Scholar]
- 2.Alm, R. A., and J. S. Mattick. 1995. Identification of a gene, pilV, required for type 4 fimbrial biogenesis in Pseudomonas aeruginosa, whose product possesses a pre-pilin-like leader sequence. Mol. Microbiol. 16:485-496. [DOI] [PubMed] [Google Scholar]
- 3.Blackhart, B. D., and D. R. Zusman. 1985. “Frizzy” genes of Myxococcus xanthus are involved in control of frequency of reversal of gliding motility. Proc. Natl. Acad. Sci. USA 82:8767-8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley, D. E. 1980. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can. J. Microbiol. 26:146-154. [DOI] [PubMed] [Google Scholar]
- 5.Darzins, A. 1994. Characterization of a Pseudomonas aeruginosa gene cluster involved in pilus biosynthesis and twitching motility: sequence similarity to the chemotaxis proteins of enterics and the gliding bacterium Myxococcus xanthus. Mol. Microbiol. 11:137-153. [DOI] [PubMed] [Google Scholar]
- 6.Darzins, A. 1993. The pilG gene product, required for Pseudomonas aeruginosa pilus production and twitching motility, is homologous to the enteric, single-domain response regulator CheY. J. Bacteriol. 175:5934-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darzins, A. 1995. The Pseudomonas aeruginosa pilK gene encodes a chemotactic methyltransferase (CheR) homologue that is translationally regulated. Mol. Microbiol. 15:703-717. [DOI] [PubMed] [Google Scholar]
- 8.Fraser, G. M., and C. Hughes. 1999. Swarming motility. Curr. Opin. Microbiol. 2:630-635. [DOI] [PubMed] [Google Scholar]
- 9.Henrichsen, J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36:478-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, B., C. B. Whitchurch, L. Croft, S. A. Beatson, and J. S. Mattick. 2000. A minimal tiling path cosmid library for functional analysis of the Pseudomonas aeruginosa PAO1 genome. Microb. Comp. Genomics 5:189-203. [DOI] [PubMed] [Google Scholar]
- 11.Kohler, T., L. K. Curty, F. Barja, C. van Delden, and J. C. Pechere. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signalling and requires flagella and pili. J. Bacteriol. 182:5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 13.Li, Y., V. H. Bustamante, R. Lux, D. Zusman, and W. Shi. 2005. Divergent regulatory pathways control A and S motility in Myxococcus xanthus through FrzE, a CheA-CheY fusion protein. J. Bacteriol. 187:1716-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 15.Matsubara, M., S. I. Kitaoka, S. I. Takeda, and T. Mizuno. 2000. Tuning of the porin expression under anaerobic growth conditions by His-to-Asp cross-phosphorelay through both the EnvZ-osmosensor and ArcB-anaerosensor in Escherichia coli. Genes Cells 5:555-569. [DOI] [PubMed] [Google Scholar]
- 16.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56:289-314. [DOI] [PubMed] [Google Scholar]
- 17.Mattick, J. S., M. M. Bills, B. J. Anderson, B. Dalrymple, M. R. Mott, and J. R. Egerton. 1987. Morphogenetic expression of Bacteroides nodosus fimbriae in Pseudomonas aeruginosa. J. Bacteriol. 169:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mongkolsuk, S., P. Vattanaviboon, S. Rabibhadana, and P. Kiatpapan. 1993. Versatile gene cassette plasmids to facilitate the construction of generalized and specialized cloning vectors. Gene 124:131-132. [DOI] [PubMed] [Google Scholar]
- 19.Morales, V. M., A. Backman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-47. [DOI] [PubMed] [Google Scholar]
- 20.Ochsner, U. A., A. K. Koch, A. Fiechter, and J. Reiser. 1994. Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J. Bacteriol. 176:2044-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter, S. L., and J. P. Armitage. 2002. Phosphotransfer in Rhodobacter sphaeroides chemotaxis. J. Mol. Biol. 324:35-45. [DOI] [PubMed] [Google Scholar]
- 22.Rashid, M. H., and A. Kornberg. 2000. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97:4885-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauprich, O., M. Matsushita, C. J. Weijer, F. Siegert, S. E. Esipov, and J. A. Shapiro. 1996. Periodic phenomena in Proteus mirabilis swarm colony development. J. Bacteriol. 178:6525-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reikofski, J., and B. Y. Tao. 1992. Polymerase chain reaction (PCR) techniques for site-directed mutagenesis. Biotechnol. Adv. 10:535-547. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Schweizer, H. P. 1992. Allelic exchange in Pseudomonas aeruginosa using novel ColE1-type vectors and a family of cassettes containing a portable oriT and the counter-selectable Bacillus subtilis sacB marker. Mol. Microbiol. 6:1195-1204. [DOI] [PubMed] [Google Scholar]
- 27.Schweizer, H. P. 1991. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene 97:109-121. [DOI] [PubMed] [Google Scholar]
- 28.Semmler, A. B., C. B. Whitchurch, and J. S. Mattick. 1999. A re-examination of twitching motility in Pseudomonas aeruginosa. Microbiology 145:2863-2873. [DOI] [PubMed] [Google Scholar]
- 29.Simon, R., U. Reifer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. BioTechnology 1:784-791. [Google Scholar]
- 30.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vieira, J., and J. Messing. 1991. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene 100:189-194. [DOI] [PubMed] [Google Scholar]
- 32.Watson, A. A., R. A. Alm, and J. S. Mattick. 1996. Construction of improved vectors for protein production in Pseudomonas aeruginosa. Gene 172:163-164. [DOI] [PubMed] [Google Scholar]
- 33.Whitchurch, C. B., A. J. Leech, M. D. Young, D. Kennedy, J. L. Sargent, J. J. Bertrand, A. B. Semmler, A. S. Mellick, P. R. Martin, R. A. Alm, M. Hobbs, S. A. Beatson, B. Huang, L. Nguyen, J. C. Commolli, J. N. Engel, A. Darzins, and J. S. Mattick. 2004. Characterization of a complex chemosensory signal transduction system which controls twitching motility in Pseudomonas aeruginosa. Mol. Microbiol. 52:873-893. [DOI] [PubMed] [Google Scholar]
- 34.Whitchurch, C. B., and J. S. Mattick. 1994. Characterization of a gene, pilU, required for twitching motility but not phage sensitivity in Pseudomonas aeruginosa. Mol. Microbiol. 13:1079-1091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.