Abstract
Lysine decarboxylase expression by Vibrio vulnificus, which is up-regulated by CadC in response to acid stress, is also induced by SoxR in response to superoxide stress. SoxR binds to the promoter region of the cadBA operon, coding for a lysine-cadaverine antiporter (CadB) and a lysine decarboxylase (CadA). The induction of cadBA transcription by SoxR is independent of CadC. Cadaverine, which neutralizes the external medium, also appears to scavenge superoxide radicals, since increasing cellular cadaverine by elevating the gene dosage of cadBA significantly diminished the induction of Mn-containing superoxide dismutase under methyl viologen-induced oxidative stress. Consistently, a lack of cadaverine caused by mutation in cadA resulted in low tolerance to oxidative stress compared with that of the wild type.
Vibrio vulnificus is a pathogen that may cause food-borne gastroenteritis. Resistance to acid stress is an important virulence factor of many enteric bacteria, including V. vulnificus, Vibrio cholerae, and Escherichia coli. Multiple effects of low external pH on physiological responses have been documented for E. coli (for a review, see reference 3). When exposed to low pH, one of the most striking responses is the induction of amino acid decarboxylases which form amines from their respective substrates (for a review, see reference 33). Glutamate decarboxylase and lysine decarboxylase are typical examples that have been studied in E. coli. Lysine decarboxylase is also required for the acid tolerance of V. vulnificus and V. cholerae (17, 26). The neutralization of external pH by amine is known to protect cells from acid stress. Polyamines are also associated with protection of cells from the toxic effects of oxygen (4, 6, 20, 34).
The transcription of cadBA, which codes for a lysine-cadaverine antiporter and an inducible lysine decarboxylase, respectively, is activated in acid environments. CadC, a membrane-bound protein whose gene lies upstream from cadBA, has been identified as a positive regulator of cadBA expression (18, 21). A lack of cadaverine caused by mutation in cadA resulted in low tolerance to low pH (26). A cadC mutant of V. vulnificus, whose lysine decarboxylase activity is significantly decreased at low pH, also showed low tolerance to low pH (25, 27).
Previously, we found that the cellular superoxide level is elevated when V. vulnificus is exposed to low pH (13). The transcription of sodA, coding for Mn-containing superoxide dismutase (MnSOD), is activated by SoxR in acid environments. Accordingly, mutations in soxR or sodA resulted in low tolerance to low pH. An increase of cytosolic SOD activity through MnSOD induction is essential for V. vulnificus to withstand the acid challenge (13).
Although cadBA expression by V. vulnificus is largely regulated by CadC, the lysine decarboxylase activity of a cadC mutant was still induced at low pH, to yield 25% of the wild-type level. This result indicates another regulatory mechanism for the enzyme induction, which is independent of CadC. Since superoxide stress was shown to build up in an acid environment, it was determined whether lysine decarboxylase of V. vulnificus is induced under superoxide stress. Indeed, cadBA transcription is increased by methyl viologen (MV) treatment, but no such response was observed in soxR mutant. SoxR binds to the promoter region of the cadBA operon. Thus, lysine decarboxylase expression by V. vulnificus, which is activated by CadC in response to acid stress, is also induced by SoxR in response to superoxide stress. Consistently, a cadC soxR double mutant barely showed lysine decarboxylase activity in an acid environment. CadC and SoxR regulate cadBA expression independently. Cadaverine appears to scavenge superoxide radicals, since MnSOD induction under superoxide stress was significantly reduced by an increase of cadaverine formation in V. vulnificus. The antioxidant role of cadaverine was further corroborated by the higher sensitivity of a cadA mutant to MV-induced oxidative stress compared with the wild type.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. V. vulnificus was grown at 30°C in Luria-Bertani (LB) medium (28) supplemented with 2% (wt/vol) NaCl (LBS) (pH 7.5). E. coli was grown at 37°C in LB medium. When appropriate, antibiotics were added at concentrations described previously (13). Cell growth measurement and growth transition to LBS (pH 5.0) were carried out as described previously (13). The same transfer to LBS (pH 7.5) containing 3 mM MV was performed to examine cadBA expression in response to superoxide stress. The experiments were repeated at least three times, yielding similar results; the averages from three independent experiments are shown.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Bacterial strains | ||
| V. vulnificus | ||
| AR | ATCC 29307, Rifr | 10 |
| JR203 | ATCC 29307, cadA::nptI Kmr | 26 |
| JR309 | AR, ΔcadC | 25 |
| SR1 | AR, soxR::aph Kmr | 13 |
| CSR1 | JR309, ΔcadC soxR::aph Kmr | This study |
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZ ΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 8 |
| S17-1 | C600::RP4 2-(Tc::Mu) (Km::Tn7) thi pro hsdR hsdM+ recA | 30 |
| S17-1λpir | λpir lysogen of S17-1 | 30 |
| BL21(DE3) | E. coli B F−dcm ompT hsdS(rB− mB−) gal λ(DE3) | Stratagene |
| Plasmids | ||
| pDM4 | ori R6K Mob RP4; Cmr | 19 |
| pDMSXR | pDM4 + 3.1-kb fragment containing soxR::aph; Kmr Cmr | 13 |
| pRK415 | ori IncP Mob RP4 lacZα; Tcr | 11 |
| pRKCAT | pRK415 with cat; Smr/Spr Tcr | 13 |
| pCB367 | pRK415 + cadB::cat fusion; 456-bp cadBA DNA from −367 to +89; Smr/Spr Tcr | This study |
| pCB097 | pRK415 + cadB::cat fusion; 186-bp cadBA DNA from −97 to +89; Smr/Spr Tcr | This study |
| pRKCadBA | pRK415 + 3.8-kb cadBA DNA from −10; Tcr | This study |
| pRKCadC | pRK415 + 2.1-kb DNA containing cadC; Tcr | This study |
| pRKSoxR | pRK415 + 0.6-kb DNA containing soxR; Tcr | This study |
| pMAL-p2E | ori M13, MBP fusion vector; Apr | NEB |
| pMAL-SXR | pMAL-p2E + 0.6-kb DNA containing soxR; Apr | This study |
Conjugation.
pRK415- and pDM4-derived plasmids were transformed into E. coli S17-1 and S17-1λpir, respectively, and were subsequently mobilized into V. vulnificus by conjugation as described previously (13).
Lysine decarboxylase activity and cadaverine determination.
The lysine decarboxylase activity of V. vulnificus and the cadaverine level in culture medium were determined as described previously (13, 15, 26). The enzyme reaction was monitored by measuring the absorbance at 340 nm. Specific activities were calculated as 1,000 × A340 per min (units) per A600 (15).
Detection and quantification of SOD activity.
Preparation of cell extracts, electrophoresis on a native polyacrylamide (12%) gel, and staining of SOD activity were performed as described previously (2). The relative SOD activities between samples were also quantified by scanning the gel with the Tina 2.0 program of the BIO-Imaging analyzer (Fuji, Japan). Proteins were determined by a modified Lowry method using bovine serum albumin as a standard (16).
Construction of cadB::cat fusion and CAT assay.
A 456-bp DNA fragment extending from position −367 to +89 (+1 is the 5′ end of the cadBA transcript [27]) was PCR amplified using forward primer F1 and reverse primer R1; F1 (5′-CCTAGCTGCAGCGCATTT-3′ [mutated sequence is underlined, unless noted otherwise]) contains a PstI site (boldface) and R1 (5′-CGACACCTCTAGAGGCAA-3′) contains an XbaI site (boldface). Another 186-bp DNA fragment spanning from position −97 to +89 was PCR amplified using forward primer F2 and reverse primer R1 described above; F2 (5′-AATCTGCAGTTCGAA-3′) contains a PstI site (boldface). The PCR products were digested with PstI and XbaI and cloned into the PstI/XbaI sites of pRKCAT containing cat (13) to generate cadB::cat fusion constructs of pCB367 and pCB097 (Table 1). The plasmids contain transcription-translation stop Ω DNA (Smr/Spr) (24) at the border between the vector and cadB upstream DNA. The recombinant plasmids were mobilized into V. vulnificus by conjugation as described above. Chloramphenicol (Cm) acetyltransferase (CAT) assay was performed as described previously (13, 29). Activity was expressed as nanomoles of Cm acetylated minute−1 milligram of protein−1 (29).
RNA isolation and primer extension analysis.
Total RNA was isolated from V. vulnificus as described previously (12). The primer 5′-CTCGCCAGTGTAGAAG-3′, representing the coding strand of cadB between codons 31 and 35, was labeled with [γ-32P]ATP by using T4 polynucleotide kinase (Promega, Madison, WI). The products of the extension reaction were analyzed on an 8.3 M urea-8% polyacrylamide sequencing gel. The nucleotide sequence was determined by the dideoxy termination reaction with a Thermo Sequenase cycle sequencing kit (Amersham Pharmacia Biotech, Piscataway, NJ).
Construction of cadC soxR double mutant.
pDM4-derived recombinant plasmid pDMSXR (13) was used for soxR disruption in V. vulnificus cadC mutant JR309 (25). The plasmid was transformed into E. coli S17-1λpir and mobilized into JR309 as described previously (13). Conjugants carrying a single crossover were obtained by selecting colonies on LBS containing Cm, kanamycin (Km), and rifampin (Rif). The cadC soxR double mutants showing indications of double crossover (Cms Kmr Rifr) were isolated on LBS agar plates supplemented with 10% sucrose. The DNA replacement was confirmed by Southern hybridization analysis (28).
Overexpression and purification of SoxR by using a construct fused to maltose-binding protein (MBP).
In order to clone soxR into the expression vector pMAL-p2E (New England Biolabs, Beverly, MA), the initiation and stop codons of SoxR were modified to have EcoRI and HindIII sites, respectively. Forward primer F3 (5′-GGAAGCGAATTCGACATC-3′ [mutated sequence is underlined, unless otherwise noted]), having the SoxR initiation codon changed into an EcoRI site (boldface), was used in PCR with reverse primer R2 (5′-GATAAAGCTTAGCTGGCTAAC-3′), containing a HindIII site (boldface) that had been mutated from its stop codon. The PCR product was digested with EcoRI and HindIII and cloned into pMAL-p2E to generate pMAL-SXR. Sequence analysis confirmed an in-frame insertion into the plasmid.
When E. coli BL21(DE3) containing pMAL-SXR grew to an A600 of between 0.6 and 0.7, isopropyl-β-d-thiogalactopyranoside (IPTG) was added at 0.1 mM for the overexpression of MBP-SoxR fusion protein. Cells were harvested 2 h after IPTG induction, disrupted by sonication, and centrifuged at 4°C to obtain cell extracts. An MBP fusion protein of approximately 59 kDa was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% polyacrylamide) and purified using amylose resin (New England Biolabs, Beverly, MA) as described previously (28). V. vulnificus SoxR (17 kDa on SDS-PAGE) was further purified after digestion of the fusion protein with enterokinase, followed by MBP binding to amylose resin (28). The purified SoxR was assessed for purity and size by SDS-PAGE.
Gel mobility shift assay.
The DNA fragments to be run for the gel mobility shift assay were labeled with [γ-32P]ATP by using T4 polynucleotide kinase (Promega, Madison, WI). The DNA probes (approximately 104 cpm) were incubated for 10 min at 25°C with various amounts of purified SoxR in a previously described buffer comprising 12.5 mM Tris-HCl (pH 7.5), 5% glycerol, 62.5 mM KCl, 0.75 mM dithiothreitol, 5 mM MgCl2, and 1 μg of poly(dI-dC) (Amersham Pharmacia Biotech, Piscataway, NJ) (12, 14). The reaction mixtures were analyzed using 5% nondenaturing polyacrylamide gels as described previously (12, 14).
Survival under superoxide stress.
Tolerance to oxidative stress was examined essentially in the same way as described for tolerance to low pH (13). Cells were grown to late logarithmic phase (A600, ∼4.0) in LBS (pH 7.5), and an aliquot (0.5 ml) was then harvested and inoculated into LBS (pH 7.5) supplemented with 3 mM MV. The initial A600 was close to 0.1. Cells were incubated for 4 h for the induction of lysine decarboxylase and then harvested and washed with phosphate-buffered saline (PBS) (pH 7.5) (28), followed by suspension to a final concentration of 105 CFU ml−1 in the same buffer containing 3 mM MV. A control experiment was performed with PBS (pH 7.5) without MV. Cell suspensions were incubated at 30°C with shaking (13). Samples were taken intermittently for 90 min, and viable counts (CFU/ml) were determined by plating dilutions of cells on LBS (pH 7.5) agar plates. Survival was expressed as the percentage of the initial CFU. The experiments were repeated three times, yielding similar results; data shown are representative of triplicate experiments.
RESULTS
Lysine decarboxylase expression by V. vulnificus, which is up-regulated under acid stress, is also induced under superoxide stress.
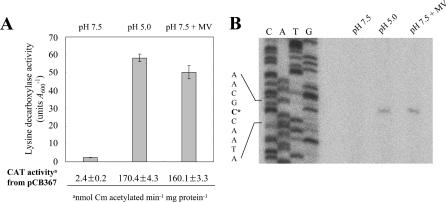
The lysine decarboxylase induction of V. vulnificus in an acid environment is regulated at the level of cadBA transcription (27). CadC, a putative transmembrane protein, activates the transcription of cadBA in a pH-dependent manner (25, 27). Since superoxide stress was shown to build up at low pH (13), it was determined whether lysine decarboxylase induction was affected by oxidative stress. V. vulnificus grown exponentially was inoculated into fresh LBS (pH 7.5) supplemented with 3 mM MV or fresh LBS adjusted to pH 5.0 as described in Materials and Methods. Cells began to grow after lag phases of approximately 4 h in both transitions, whereas no lag occurred after inoculation into LBS (pH 7.5), as described previously (13). Cells were measured for lysine decarboxylase activity after transition, and the maximum ones are shown (Fig. 1A). As previously observed (13), lysine decarboxylase activity was highly induced during the lag period after the transfer to low pH (Fig. 1A). Remarkably, similar induction of the enzyme activity was also observed during the lag after transfer to medium (pH 7.5) supplemented with MV, whereas no induction was detected without MV (Fig. 1A). cadA expression was also examined using the cadB::cat transcriptional fusion construct pCB367 (Fig. 1A and 2), since cadBA genes of V. vulnificus are transcribed monocistronically (27). The plasmid contains a 367-bp regulatory DNA upstream from the 5′ end of the cadBA transcript (27), which also includes a CadC-binding domain centered at position −233.5 (+1 is the 5′ end of the cadBA transcript, unless stated otherwise). The CAT activity from the cadB::cat fusion construct pCB367 in the wild-type cells, which was up-regulated in an acid environment, was also induced in the presence of MV (Fig. 1A). Hydrogen peroxide (100 μM) treatment did not result in any induction of lysine decarboxylase expression (data not shown). The results argue that lysine decarboxylase expression by V. vulnificus, which is regulated at the level of cadBA transcription, is highly induced in response to superoxide stress.
FIG. 1.
Lysine decarboxylase expression by V. vulnificus under acid stress and superoxide stress. (A) Lysine decarboxylase activity of wild-type cells (AR) after growth transition to either LBS (pH 7.5) supplemented with 3 mM MV or LBS adjusted to pH 5.0. The growth transition to LBS (pH 7.5) was included as a control. Lysine decarboxylase activities were measured every hour, and only the maximum activities, which were observed 4 h, 2 h, and 4 h after transfer to LBS (pH 7.5), LBS (pH 5.0), and LBS (pH 7.5) supplemented with 3 mM MV, respectively, are shown. CAT activity from pCB367 containing a cadB::cat transcriptional fusion construct is shown. The error bars correspond to the standard deviations of the means, and CAT activity data represent means ± standard deviations. (B) Mapping of the 5′ end of the cadBA transcript of wild-type cells (AR) under acid stress and superoxide stress. Cells were harvested at 4 h, 2 h, and 4 h, after transfer to LBS (pH 7.5), LBS (pH 5.0), and LBS (pH 7.5) supplemented with 3 mM MV, respectively. The same 32P-labeled oligonucleotide was used to generate the sequence ladder (lanes C, A, T, and G). The DNA sequence of the noncoding strand is illustrated on the left, with the 5′ end of the transcript marked with an asterisk.
FIG. 2.
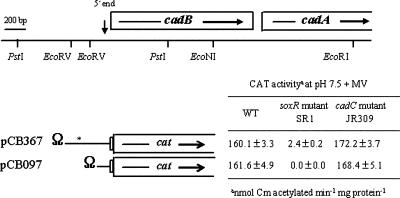
CAT activity from a cadB::cat fusion. pCB367 has the 456 bp of cadBA DNA extending from −367 to +89 (+1 is the 5′ end of the cadBA transcript), whereas pCB097 harbors the 186 bp of cadBA DNA extending from −97 to +89. Transcription-translation stop Ω DNA (Smr/Spr) (24) is inserted at the border between the vector and cadB regulatory DNA. The CadC-binding domain is shown with an asterisk. The plasmids were mobilized into the wild type (AR) (WT), soxR mutant SR1, and cadC mutant JR309, and CAT activities were determined 4 h after growth transition to LBS (pH 7.5) supplemented with 3 mM MV. CAT activity data represent means ± standard deviations.
We examined whether cadBA transcription under superoxide stress employs the same promoter as that for transcription under acid stress (27). The 5′ end of the cadBA transcript from the total RNA of cells grown in the presence of MV was mapped at 54 nucleotides upstream from the CadB initiation codon (Fig. 1B), which is the same as determined with cells grown in an acid environment (Fig. 1B) (27). The results suggest the same promoter is used for the expression of cadBA under both acid stress and superoxide stress. No extension product was observed without MV at pH 7.5 (Fig. 1B).
Lysine decarboxylase induction in response to superoxide stress is regulated by SoxR.
When V. vulnificus was treated with other superoxide generators, such as menadione (4 mM) and plumbagin (20 μM), the induced activities of lysine decarboxylase were similar to that observed with MV (3 mM) (data not shown). Lysine decarboxylase expression by V. vulnificus increased in proportion to the level of MV treatment; enzyme activities of 2.1, 23.4, 52.5, and 88.5 (units A600−1) were observed after treatment with MV at 0, 1, 3, and 6 mM, respectively. Thus, the level of superoxide stress determines the induced level of lysine decarboxylase.
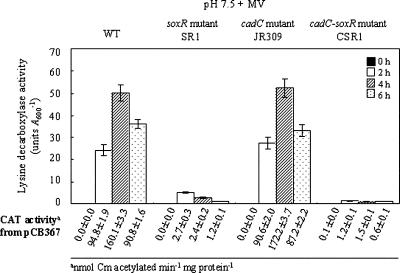
It was determined whether CadC and/or the redox-responsive transcriptional regulator SoxR regulates cadBA transcription in response to superoxide stress. cadC mutant JR309 showed induction of lysine decarboxylase activity like that of the wild type after MV treatment (Fig. 3). Neither soxR mutant SR1 nor cadC soxR double mutant CSR1, however, showed such induction (Fig. 3). The lysine decarboxylase induction of mutants SR1 and CSR1 in response to superoxide stress was fully complemented with soxR DNA, whereas cadC DNA, which complemented cadC mutant JR309 (27), did not restore the enzyme induction in CSR1 (Table 2). The CAT activity from pCB367 reflects the lysine decarboxylase activity (Fig. 3). Therefore, SoxR induces cadBA transcription in response to superoxide stress, and the regulation is independent of CadC.
FIG. 3.
Expression of lysine decarboxylase of the wild type (AR) (WT), soxR mutant SR1, cadC mutant JR309, and cadC soxR double mutant CSR1 after growth transition to LBS (pH 7.5) supplemented with 3 mM MV. Lysine decarboxylase activity was examined with cells harvested at 0 h, 2 h, 4 h, and 6 h after transfer. CAT activity from pCB367 was determined at the time points indicated. The error bars correspond to the standard deviations of the means, and CAT activity data represent means ± standard deviations.
TABLE 2.
Complementation of lysine decarboxylase induction of V. vulnificus mutants with cadC and soxR DNA under superoxide stress
| Strain | Plasmid | Lysine decarboxylase activitya (units A600−1) |
|---|---|---|
| Wild-type AR | pRK415 | 55.5 ± 2.9 |
| soxR mutant SR1 | pRK415 | 1.1 ± 0.1 |
| pRKSoxR | 59.7 ± 2.2 | |
| cadC-soxR mutant CSR1 | pRK415 | 1.2 ± 0.1 |
| pRKSoxR | 52.1 ± 4.8 | |
| pRKCadC | 5.0 ± 0.3 |
Enzyme activity was measured 4 h after growth transition to LBS (pH 7.5) supplemented with 3 mM MV. Data represent means ± standard deviations.
The cadBA upstream DNA responsible for SoxR regulation was narrowed down using another cadB::cat transcriptional fusion construct, pCB097 (Fig. 2). Its cad DNA contains 97 bp of regulatory DNA upstream from the 5′ end of the transcript and does not include the CadC-binding region. The plasmid was mobilized into the wild-type strain, cadC mutant JR309, and soxR mutant SR1, and CAT activities were measured under superoxide stress. The CAT activity from pCB097 was nearly the same as that from pCB367, and there were essentially no differences in the activity levels between the wild type and cadC mutant JR309. However, the CAT activities from the plasmids were barely detected in soxR mutant SR1. The results further corroborates that the SoxR-dependent cadBA transcription is independent of CadC, and the 97-bp regulatory DNA of pCB097 appears to be sufficient for SoxR regulation.
SoxR directly binds to the regulatory DNA of the cadBA operon.
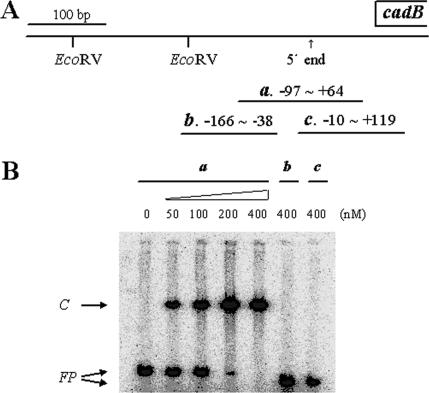
We examined whether SoxR regulates cadBA transcription by directly binding to the regulatory DNA of the operon or indirectly, possibly via another regulator such as SoxS, as observed in E. coli. No SoxS homolog, however, has been found in V. vulnificus. The gel mobility of the 161-bp cadB regulatory DNA (Fig. 4A, probe a) extending from position −97 to +64 was shifted by the purified SoxR from V. vulnificus (Fig. 4B). The retarded band was intensified in proportion to the amount of SoxR used. Neither the 128-bp DNA (from −166 to −38; probe b) nor the 129-bp DNA (from −10 to +119; probe c) was retarded by SoxR (Fig. 4A and B). Thus, the DNA region between −38 and −10 is required for SoxR binding. This result is consistent with that suggested by the CAT assays with pCB367 and pCB097 (Fig. 2).
FIG. 4.
Gel mobility shift assay of cadB regulatory DNA with purified SoxR. (A) Facing sets of 17-mers were used for PCR to obtain DNA probes a (from position −97 to +64), b (from −166 to −38), and c (from −10 to +119), which were then labeled with [γ-32P]ATP. (B) Gel mobility shift assays were performed as described in Materials and Methods. Probe a was incubated with increasing concentrations of purified SoxR (0, 50, 100, 200, and 400 nM), whereas probes b and c were incubated with 400 nM purified SoxR protein. Free probes (FP) and binding complex (C) are indicated with arrows.
Cellular cadaverine appears to acts as a superoxide scavenger.
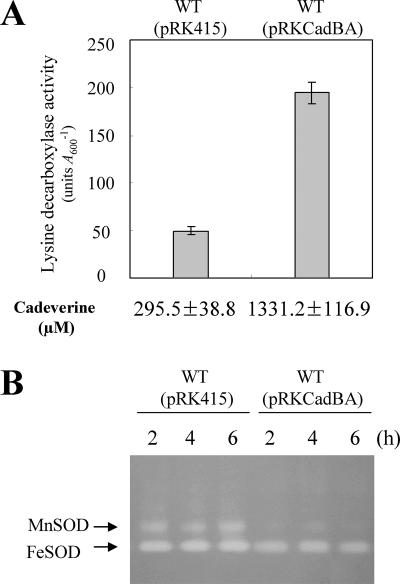
Polyamines are associated with many biochemical processes, such as the regulation of gene expression, the stabilization of chromatin, and the prevention of DNA damage (6, 33, 35). Polyamines, which may directly scavenge oxygen radicals (7), have been also known to protect cells from the toxic effects of reactive oxygen such as hydrogen peroxide, singlet oxygen, and oxygen radicals (4, 6, 20, 34). We examined whether cadaverine acts as a superoxide scavenger in V. vulnificus. The gene dosage of cadBA was increased by maintaining plasmid pRKCadBA in trans in wild-type cells, and the resulting cells were subjected to growth transition to LBS (pH 7.5) supplemented with 3 mM MV. A 3.8-kb insert DNA of pRKCadBA, which had been PCR amplified with a set of facing primers flanking cadBA, does not contain its own promoter, since the forward primer was the same as that used for probe c (Fig. 4). Thus, the lysine decarboxylase activity of wild-type cells containing pRKCadBA, whose insert was cloned in the same orientation as the lac promoter of pRK415, was not affected by MV treatment (data not shown) and was approximately four times as much as that of the control cells containing pRK415 (Fig. 5A).
FIG. 5.
Lysine decarboxylase, cadaverine excretion, and cytosolic SOD activities of wild-type (WT) cells (AR) harboring pRKCadBA in trans after growth transition to LBS (pH 7.5) supplemented with 3 mM MV. (A) The lysine decarboxylase activity of wild-type cells harboring either pRKCadBA or pRK415 was examined 4 h after transfer to LBS (pH 7.5) supplemented with 3 mM MV. Cadaverine in the culture supernatant was determined at the same time points. The error bars correspond to the standard deviations of the means, and cadaverine concentrations represent means ± standard deviations. (B) The cytosolic SOD activity of wild-type cells harboring either pRKCadBA or pRK415 was examined at 2 h, 4 h, and 6 h after the same transfer. The same amount (50 μg) of protein was loaded in each lane.
Cadaverine concentrations in cell-free supernatants from cultures of wild-type cells containing pRKCadBA also increased approximately fourfold compared with that for control cells (Fig. 5A). The cadaverine in the culture supernatant should reflect its content in the cells. The MnSOD activity from the cells containing pRKCadBA was only 10 to 15% of that from the control cells under superoxide stress (Fig. 5B). Thus, the increase of cellular cadaverine of V. vulnificus decreased the induction of MnSOD under MV-induced oxidative stress (Fig. 5B), suggestive of radical scavenge by cadaverine in cells.
Although the increase of cadaverine formation in V. vulnificus decreased MnSOD induction under superoxide stress (Fig. 5), addition of exogenous cadaverine (2 mM) to the cultures of wild-type V. vulnificus and the cadA mutant did not affect MnSOD expression under superoxide stress (data not shown). The results implied that unlike the polyamine-deficient mutant of E. coli, where cadaverine is taken up into the cells by CadB at neutral pH (31), exogenous cadaverine may not go into V. vulnificus under the conditions examined in this work. It remains to be determined whether the cadaverine uptake would occur only in a polyamine-deficient mutant or whether there are any intrinsic differences in cadaverine uptake between E. coli and V. vulnificus.
Cadaverine is needed for superoxide stress tolerance of V. vulnificus.
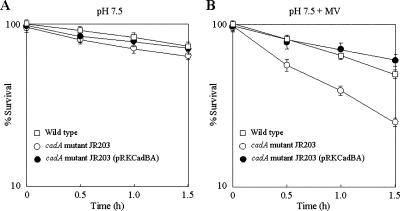
We examined whether cadaverine formation affects V. vulnificus survival under oxidative stress. The cadA mutant JR203 was more susceptible to MV-induced oxidative stress than the wild type (Fig. 6B), whereas no significant difference in survival between the wild type and the mutant was observed without MV (Fig. 6A). The low tolerance of the cadA mutant to MV-induced stress was complemented with pRKCadBA (Fig. 6B). Thus, cadaverine is needed for tolerance to superoxide stress in V. vulnificus.
FIG. 6.
Tolerance of a cadA mutant to superoxide stress. Cells grown in LBS (pH 7.5) supplemented with 3 mM MV were transferred to PBS (pH 7.5) containing 3 mM MV (B). Transfer to PBS (pH 7.5) without MV was included as a control (A). Viable cell counts of the wild type (AR) (WT) (□), cadA mutant JR203 (○), and cadA mutant JR203 containing pRKCadBA (•) were determined, and survival was expressed as percentage of the initial CFU. The error bars correspond to the standard deviations of the means.
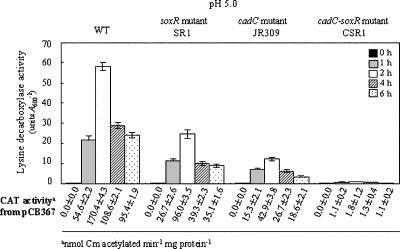
Lysine decarboxylase induction at low pH is controlled by CadC and SoxR.
When V. vulnificus is exposed to low pH, superoxide stress is generated in addition to the acid stress (13). Accordingly, it was determined whether the lysine decarboxylase expression by V. vulnificus in an acid environment is also regulated by SoxR. Indeed, the soxR mutant SR1 showed only 50% of the lysine decarboxylase activity of wild-type cells after a growth transition to pH 5.0, whereas the cadC mutant JR309 showed approximately 25% of the wild-type activity under the same conditions (Fig. 7). Thus, SoxR induces the enzyme expression in an acid environment, although CadC-mediated induction appears to be more significant. The enzyme activity was barely detected in the cadC soxR double mutant CSR1. The CAT activity from pCB367 also reflects the lysine decarboxylase activity. Therefore, the transcriptional induction of cadBA of V. vulnificus is regulated by both CadC and SoxR in acid environments.
FIG. 7.
Expression of lysine decarboxylase of the wild type (AR) (WT), soxR mutant SR1, cadC mutant JR309, and cadC soxR double mutant CSR1 after growth transition to LBS (pH 5.0). Lysine decarboxylase activity was examined with cells harvested 0 h, 1 h, 2 h, 4 h, and 6 h after transfer. CAT activity from pCB367 was determined at the time points indicated. The error bars correspond to the standard deviations of the means, and CAT activity data represent means ± standard deviations.
DISCUSSION
As a mechanism to withstand acid challenge, the lysine decarboxylase of V. vulnificus is induced by CadC in response to acid stress. The production and excretion of cadaverine are essential for neutralization of the external pH, thus protecting cells from acid stress (17, 21, 26, 31). In this work we found that cadaverine also acts as an antioxidant to scavenge superoxide radicals. Furthermore, SoxR was shown to induce cadBA transcription, possibly by direct binding to the regulatory DNA of cadBA.
soxR and soxS, coding for two separate transcriptional activators, constitute a two-step activation cascade, in which SoxR activates soxS expression in response to superoxide and the increased level of SoxS induces target genes to protect cells from oxidative damage (1). The −10/−35 spacer of the soxS promoter, where a dimeric form of SoxR binds, constitutes the overlong 19-bp distance, in comparison with spacers of 17 ± 1 bp for most E. coli promoters. The topology of the soxS promoter is compensated by the conformational change of SoxR, which is accompanied by the oxidation of [2Fe-2S] clusters upon exposure to superoxide stress (5, 9, 22; for a review, see reference 23). The −10/−35 spacer of the V. vulnificus cadBA promoter is 20 bp (27), whereas that of its sodA promoter is 17 bp (13). Although both sodA and cadBA of V. vulnificus are induced by SoxR, only the cadBA promoter showed a gel electrophoretic mobility shift by the protein (Fig. 4); no such result was observed with the sodA promoter (13). The results indicate the direct involvement of SoxR in activating cadBA transcription.
However, the cadBA transcription by V. vulnificus at low pH is still significant in soxR mutant SR1, which means that CadC can activate cadBA transcription without SoxR. Considering that the CadC-binding domain is centered at −233.5, there should be a way for CadC to interact with the cadBA promoter. A putative integration host factor (IHF)-binding site was found between the CadC-binding domain and the cadBA promoter. The cadBA transcription by wild-type cells at low pH was reduced by approximately 40% in the IHF mutant (J.-S. Kim and J. K. Lee, unpublished results). The results suggest a possible interaction of CadC with RNA polymerase bound on the cadBA promoter through IHF-mediated DNA bending (for a review, see reference 32). The molecular basis for the interaction between CadC and RNA polymerase remains to be examined.
Unlike that of V. vulnificus, MnSOD of E. coli is not induced at low pH (13). When E. coli was treated with MV (3 mM), no significant induction of lysine decarboxylase was observed (Kim and Lee, unpublished results). Thus, the results in this work further support the intrinsic differences in acid tolerance response between E. coli and V. vulnificus.
When a spin trap, 5,5′-dimethyl-1-pyrroline-N-oxide (DMPO), is included in the xanthine oxidase reaction, the most widely used biochemical source of superoxide, DMPO reacts with superoxide to reveal a signal characteristic of a DMPO-OH spin adduct, with a quartet signal showing intensity ratios of 1:2:2:1 and hyperfine coupling constants of αN = αβH = 14.89 G in electron paramagnetic resonance spectroscopy. The signal was significantly reduced by addition of E. coli MnSOD or cadaverine in the reaction mixture (Kim and Lee, unpublished results), thus confirming that cadaverine is capable of scavenging superoxide radicals. Consistently, the increase of cellular cadaverine decreased the expression of MnSOD under oxidative stress (Fig. 5), and a V. vulnificus mutant deficient in cadA is more susceptible to reactive oxygen than the wild type (Fig. 6). Thus, the results shown in this work further corroborate the antioxidant role of cadaverine.
Taken together, expression of V. vulnificus lysine decarboxylase, which is activated by CadC in response to acid stress, is also induced by SoxR in response to superoxide stress. Since superoxide stress is generated in acid environments, the lysine decarboxylase expression by V. vulnificus at low pH is also induced by SoxR. The cadaverine thus formed not only neutralizes the external medium (13, 26) but also scavenges superoxide radicals in V. vulnificus.
Acknowledgments
This work was supported by the 21C Frontier Microbial Genomics and Applications Center Program (MG05-0309-1-0) from the Ministry of Science and Technology, Korea.
Footnotes
Published ahead of print on 29 September 2006.
REFERENCES
- 1.Amabile-Cuevas, C. F., and B. Demple. 1991. Molecular characterization of the soxRS genes of Escherichia coli: two genes control a superoxide stress regulon. Nucleic Acids Res. 19:4479-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44:276-287. [DOI] [PubMed] [Google Scholar]
- 3.Boot, I. R., P. Cash, and C. O'Byrne. 2002. Sensing and adapting to acid stress. Antonie Leeuwenhoek 81:33-42. [DOI] [PubMed] [Google Scholar]
- 4.Chattopadhyay, M. K., C. W. Tabor, and H. Tabor. 2003. Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc. Natl. Acad. Sci. USA 100:2261-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudu, P., and B. Weiss. 1996. SoxR, a [2Fe-2S] transcription factor, is active only in its oxidized form. Proc. Natl. Acad. Sci. USA 93:10094-10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha, H. C., D. J. P. Yager, P. A. Woster, and R. A. Casero, Jr. 1998. Structural specificity of polyamines and polyamine analogues in the protection of DNA from strand breaks induced by reactive oxygen species. Biochem. Biophys. Res. Commun. 244:298-303. [DOI] [PubMed] [Google Scholar]
- 7.Ha, H. C., N. S. Sirisoma, P. Kuppusamy, J. L. Zweier, P. M. Woster, and R. A. Casero, Jr. 1998. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 95:11140-11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 9.Hidalgo, E., and B. Demple. 1997. Spacing of promoter elements regulates the basal expression of the soxS gene and converts SoxR from a transcriptional activator into a repressor. EMBO J. 16:1056-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong, K. C., H. S. Jeong, J. H. Rhee, S. E. Lee, S. S. Chung, A. M. Starks, G. M. Escudero, P. A. Gulig, and S. H. Choi. 2000. Construction and phenotypic evaluation of a Vibrio vulnificus vvpE mutant for elastolytic protease. Infect. Immun. 68:5096-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmid for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 12.Kim, J. S., J. H. Jang, J. W. Lee, S. O. Kang, K. S. Kim, and J. K. Lee. 2000. Identification of cis site involved in nickel-responsive transcriptional repression of sodF gene coding for Fe- and Zn-containing superoxide dismutase of Streptomyces griseus. Biochim. Biophys. Acta. 1493:200-207. [DOI] [PubMed] [Google Scholar]
- 13.Kim, J.-S., M.-H. Sung, D.-H. Kho, and J. K. Lee. 2005. Induction of manganese-containing superoxide dismutase is required for acid tolerance in Vibrio vulnificus. J. Bacteriol. 187:5984-5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, J.-S., S.-O. Kang, and J. K. Lee. 2003. The protein complex composed of nickel-binding SrnQ and DNA binding motif-bearing SrnR of Streptomyces griseus represses sodF transcription in the presence of nickel. J. Biol. Chem. 278:18455-18463. [DOI] [PubMed] [Google Scholar]
- 15.Lemonnier, M., and L. David. 1998. Expression of the second lysine decarboxylase gene of Escherichia coli. Microbiology 144:751-760. [DOI] [PubMed] [Google Scholar]
- 16.Markwell, M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87:206-210. [DOI] [PubMed] [Google Scholar]
- 17.Merrell, D. S., and A. Camilli. 1999. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol. Microbiol. 34:836-849. [DOI] [PubMed] [Google Scholar]
- 18.Merrell, D. S., and A. Camilli. 2000. Regulation of Vibrio cholerae genes required for acid tolerance by a member of the “ToxR-like” family of transcriptional regulators. J. Bacteriol. 182:5342-5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milton, D. L., R. O'Toole, P. Horstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minton, K. W., H. Tabor, and C. W. Tabor. 1990. Paraquat toxicity is increased in Escherichia coli defective in the synthesis of polyamines. Proc. Natl. Acad. Sci. USA 87:2851-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neely, M. N., and E. R. Olson. 1996. Kinetics of expression of the Escherichia coli cad operon as a function of pH and lysine. J. Bacteriol. 178:5522-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunoshiba, T., E. Hidalgo, C. F. Amabile-Cuevas, and B. Demple. 1992. Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J. Bacteriol. 174:6054-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pomposiello, P. J., and B. Demple. 2001. Redox-operated genetic switches: the SoxR and OxyR transcription factors. Trends Biotechnol. 19:109-114. [DOI] [PubMed] [Google Scholar]
- 24.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 25.Rhee, J. E., H.-M. Ju, U. Park, B. C. Park, and S. H. Choi. 2004. Identification of the Vibrio vulnificus cadC and evaluation of its role in acid tolerance. J. Microbiol. Biotechnol. 14:1093-1098. [Google Scholar]
- 26.Rhee, J. E., J. H. Rhee, P. Y. Ryu, and S. H. Choi. 2002. Identification of the cadBA operon from Vibrio vulnificus and its influence on survival to acid stress. FEMS Microbiol. Lett. 208:245-251. [DOI] [PubMed] [Google Scholar]
- 27.Rhee, J. E., K. S. Kim, and S. H. Choi. 2005. CadC activates pH-dependent expression of the Vibrio vulnificus cadBA operon at a distance through direct binding to an upstream region. J. Bacteriol. 187:7870-7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Shaw, W. V. 1975. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 43:737-755. [DOI] [PubMed] [Google Scholar]
- 30.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:37-45. [Google Scholar]
- 31.Soksawatmaekhin, W., A. Kuraishi, K. Sakata, K. Kashiwagi, and K. Igarashi. 2004. Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol. Microbiol. 51:1401-1412. [DOI] [PubMed] [Google Scholar]
- 32.Swinger, K., and P. Rice. 2004. IHF and HU: flexible architects of bent DNA. Curr. Opin. Struct. Biol. 14:28-35. [DOI] [PubMed] [Google Scholar]
- 33.Tabor, C. W., and H. Tabor. 1985. Polyamines in microorganisms. Microbiol. Rev. 49:81-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tkachenko, A., L. Nesterova, and M. Pshenichnov. 2001. The role of the natural polyamine putrescine in defense against oxidative stress in Escherichia coli. Arch. Microbiol. 176:155-157. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida, M., K. Kashiwagi, A. Shigemasa, S. Taniguchi, K. Yamamoto, H. Makinoshima, A. Ishihama, and K. Igarashi. 2004. A unifying model for the role of polyamines in bacterial cell growth, the polyamine modulon. J. Biol. Chem. 279:46008-46013. [DOI] [PubMed] [Google Scholar]