Abstract
The phototrophic purple bacterium Rhodobacter capsulatus encodes two similar but functionally not identical molybdenum-dependent regulator proteins (MopA and MopB), which are known to replace each other in repression of the modABC genes (coding for an ABC-type high-affinity Mo transport system) and anfA (coding for the transcriptional activator of Fe-nitrogenase genes). We identified further Mo-regulated (mor) genes coding for a putative ABC-type transport system of unknown function (MorABC) and a putative Mo-binding protein (Mop). The genes coding for MopA and the ModABC transporter form part of a single transcriptional unit, mopA-modABCD, as shown by reverse transcriptase PCR. Immediately upstream of mopA and transcribed in the opposite direction is mopB. The genes coding for the putative MorABC transporter belong to two divergently transcribed operons, morAB and morC. Expression studies based on lacZ reporter gene fusions in mutant strains defective for either MopA, MopB, or both revealed that the regulators substitute for each other in Mo-dependent repression of morAB and morC. Specific Mo-dependent activation of the mop gene by MopA, but not MopB, was found to control the putative Mo-binding protein. Both MopA and MopB are thought to bind to conserved DNA sequences with dyad symmetry in the promoter regions of all target genes. The positions of these so-called Mo boxes relative to the transcription start sites (as determined by primer extension analyses) differed between Mo-repressed genes and the Mo-activated mop gene. DNA mobility shift assays showed that MopA and MopB require molybdenum to bind to their target sites with high affinity.
Molybdenum serves as a cofactor for many redox enzymes catalyzing basic reactions in the nitrogen, sulfur, and carbon cycles. There are two distinct types of molybdoenzymes. Mo-nitrogenase, which catalyzes the reduction of N2 to ammonia, has a unique molybdenum-iron-sulfur cofactor called FeMo-co. All other molybdoenzymes, such as nitrate reductase, dimethyl sulfoxide (DMSO) reductase, and xanthine dehydrogenase, contain a cofactor (called Mo-co) in which a mononuclear Mo atom is coordinated to the sulfur atoms of a pterin.
Many bacteria actively take up molybdate by use of a high-affinity ABC-type transport system comprising three proteins (25). ModA, the molybdate-binding protein, is localized in the periplasm in gram-negative bacteria or attached to the outer side of the cytoplasmic membrane in gram-positive bacteria. ModB is the membrane integral channel protein, and ModC is the cytoplasmic ATPase. In Escherichia coli, the modABC genes constitute a single operon, whose expression requires Mo starvation (8). When the intracellular Mo content is high, a molybdate-dependent regulator, ModE, binds to the modABC promoter and represses transcription of the Mo transport operon. ModE consists of two functionally distinct domains, an N-terminal DNA-binding domain and a C-terminal molybdate-binding domain (9). In the presence of molybdenum, ModE binds to a region of the modABC promoter with a dyad symmetric element (the so-called Mo box) that overlaps the transcription start (3).
ModABC-like Mo transport systems and ModE-like regulators are widespread in bacteria. In addition to E. coli, these proteins have been characterized in greater detail for Anabaena variabilis (30), Azotobacter vinelandii (20), Bradyrhizobium japonicum (6), Rhodobacter capsulatus (29), and Staphylococcus carnosus (21).
Molybdate binding involves a conserved domain of about 70 amino acids, the Mop domain (for a review, see reference 22). Mop domains occur in three classes of cytoplasmic proteins with distinct functions. Molbindins (Mop proteins), which are implicated in Mo homeostasis within the cell, consist solely of Mop domains present either as single Mop domains or tandem Mop repeats. The Mo-dependent regulatory protein ModE contains a C-terminal tandem Mop repeat, and a single Mop domain occurs in the C-terminal domain of ModC.
While E. coli contains only a single copy of modE, the phototrophic purple bacterium Rhodobacter capsulatus codes for two ModE-like regulator proteins (MopA and MopB) (29). In addition, R. capsulatus can synthesize a Mo-dependent nitrogenase (Mo-nitrogenase) and an alternative iron-only nitrogenase (Fe-nitrogenase), the latter being expressed only in the absence of molybdenum (for a review, see reference 17). MopA and MopB replace each other in Mo repression of anfA (which codes for the transcriptional activator of Fe-nitrogenase genes) and the mopA-modABCD genes (13).
In the present study, we compared expression of known Mo-controlled genes (anfA and mopA-modABCD) and newly identified Mo-regulated genes (morABC, coding for a putative ABC-type transporter, and mop, coding for a putative Mo homeostasis protein) in R. capsulatus by genetic means. Mo-dependent transcription control depends on direct interaction of MopA and MopB with their respective target promoters as shown by DNA mobility shift assays. While MopA and MopB replaced each other in repressing transcription of anfA, mopA-modABCD, morAB, and morC, only MopA was required for activation of mop gene expression.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Methods for conjugational plasmid transfer between E. coli S17-1 and R. capsulatus, the selection of mutants, rich medium (PY), molybdenum-free minimal medium (AK-NL), growth conditions, and antibiotic concentrations were as previously described (see reference 26 and references therein).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | Host for plasmid amplification | 10 |
| BL21(DE3) | Host for expression of MopAHis and MopBHis | Novagen, Darmstadt, Germany |
| S17-1 | RP4-2 (Tc::Mu) (Km::Tn7) integrated in the chromosome | 27 |
| R. capsulatus | ||
| B10S | Spontaneous Smr mutant of R. capsulatus B10 | 12 |
| KS94A | anfA::[Sp] insertion mutant of B10S | 29 |
| R423AI | mopA::[Gm>] insertion mutant of B10S | 13 |
| R423BI | mopB::[Gm>] insertion mutant of B10S | 13 |
| R423CI | Δ(mopA mopB)::[Gm] deletion mutant of B10S | 13 |
| R438II | mopA::[<Gm] polar insertion mutant of B10S | 29 |
| Plasmids | ||
| pAW12 | pBluescript KS derivative carrying ΔmorABC::[Km] | This study |
| pBluescript KS | High-copy expression vector; Ap | Stratagene, Amsterdam, The Netherlands |
| pBSL86 | Km cassette flanked by polylinker; Ap | 1 |
| pET22b(+) | High-copy His tag expression vector; Ap | Novagen, Darmstadt, Germany |
| pJW32 | pET22b(+) derivative carrying mopAhis | This study |
| pJW33 | pET22b(+) derivative carrying mopBhis | This study |
| pJW42 | pUC18 derivative carrying a mop promoter fragment | This study |
| pJW45 | pUC18 derivative carrying the morA-morC intergenic region | This study |
| pJW59 | pML5 derivative carrying a mop-lacZ transcriptional fusion | This study |
| pKS131A | pPHU236 derivative carrying an anfA-lacZ translational fusion | 13 |
| pML5 | Mobilizable lac fusion broad-host-range vector; Tc | 14 |
| pMOT15 | pML5 derivative carrying a morA-lacZ transcriptional fusion | This study |
| pMOT16 | pML5 derivative carrying a morC-lacZ transcriptional fusion | This study |
| pPHU236 | Mobilizable lac fusion broad-host-range vector; Tc | 11 |
| pSL21I | pML5 derivative carrying a modA-lacZ transcriptional fusion | This study |
| pSL21II | pML5 derivative carrying a mopB-lacZ transcriptional fusion | This study |
| pWKR459 | mob Tc Km | 7 |
Ap, ampicillin; Gm, gentamicin; Km, kanamycin; Tc, tetracycline; Sm, streptomycin; Sp, spectinomycin.
Construction of R. capsulatus morABC mutant strain AW12.
A 3.5-kb DNA fragment carrying the R. capsulatus mor gene region (see Fig. 2) was PCR amplified using primer pair PAW3-U/PAW3-L (Table 2). A 2.5-kb BamHI-XhoI fragment from the PCR amplification product was cloned into vector plasmid pBluescript KS. Subsequently, a 1.5-kb SmaI fragment encompassing the entire morA gene, the morA-morC intergenic region, and large parts of morB and morC was replaced by a 1.2-kb SmaI kanamycin cartridge from pBSL86. Finally, insertion of a 8.8-kb XhoI fragment (containing a tetracycline resistance gene and the mob locus of PR4) from pWKR459 led to the mobilizable hybrid plasmid pAW12. Conjugational transfer of pAW12 from E. coli S17-1 into R. capsulatus and selection for marker rescue were carried out as described earlier (12, 29).
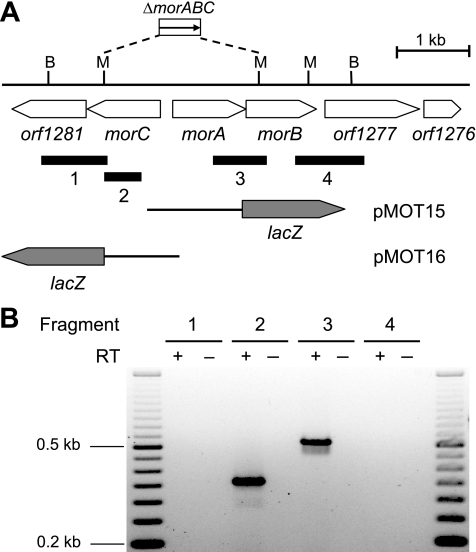
FIG. 2.
Transcriptional analysis of the R. capsulatus mor gene region. (A) Physical and genetic maps of the mor gene region. The physical map is given for BamHI and SmaI (B and M, respectively). Black bars below the genetic map indicate DNA fragments 1 to 4 emerging from RT-PCR (see Materials and Methods and panel B). The corresponding primer pairs used for RT-PCR are listed in Table 2. The morABC deletion mutant AW12 contains a kanamycin resistance cassette (not drawn to scale). Hybrid plasmids pMOT15 and pMOT16, carrying transcriptional morA-lacZ or morC-lacZ fusions, respectively, are based on the mobilizable broad-host-range plasmid pML5. (B) Transcriptional analysis of the morAB and morC operons by RT-PCR. Total RNA was isolated from R. capsulatus cells grown under Mo-limiting conditions. Either RNA samples were treated with reverse transcriptase to synthesize cDNA (+) or, as a negative control, reverse transcriptase was omitted (−). A 50-bp DNA ladder (Fermentas, St. Leon-Rot, Germany) was used as a length standard.
TABLE 2.
Primers used for RT-PCR and PCR amplification of selected DNA fragments
| Primer | Oligonucleotide sequences (5′→3′) | Relevant characteristics |
|---|---|---|
| PAW3-U | ACGGGGAAGCGCGGGGGAAAGAGG | Amplification of mor gene region, 3,548 bp |
| PAW3-L | GCGCGACAGAAAGCCGAACAGC | |
| PJW1-U | CACCGTTGCACCGCCCACAGT | RT-PCR (modC-modD), 694 bp (fragment 1 in Fig. 1) |
| PJW1-L | TGCCCCCACCGACACCACGATTCT | |
| PJW2-U | GGTGATCTGCCGCCCCTCCTG | RT-PCR (modB-modC), 606 bp (fragment 2 in Fig. 1) |
| PJW2-L | GGTCGTCGGCTCGGTCATCTATTC | |
| PJW3-U | CCAGCCCCGCGAAGGTGAAGGA | RT-PCR (modA-modB), 688 bp (fragment 3 in Fig. 1) |
| PJW3-L | TGACAAGGGCGCGGTGCTGAAAAC | |
| PJW4-U | GACGCATCGGCCGAAAGAAAGAC | RT-PCR (mopA-modA), 757 bp (fragment 4 in Fig. 1) |
| PJW4-L | CGCGCCGGAAAAAGCCCTCAAC | |
| PJW5-U | GCGCCGTGCCATTGAAA | RT-PCR (morC-orf1281), 639 bp (fragment 1 in Fig. 2) |
| PJW5-L | GGCGCTTGATCCCGACACC | |
| PJW6-U | ACGGCAAGGCGGGGCGGCAGTAT | RT-PCR (morA-morB), 519 bp (fragment 3 in Fig. 2) |
| PJW6-L | CCAGCACGATCGGCGGAAACACCA | |
| PJW7-U | GCTTGGCGCGGGGCTCTT | RT-PCR (morB-orf1277), 672 bp (fragment 4 in Fig. 2) |
| PJW7-L | CGGGGCTGACGCAAATCC | |
| PJW8-U | CGGTCTGGTGCGGATGGGGTCTTC | RT-PCR (orf413-mop), 624 bp (fragment 1 in Fig. 3) |
| PJW8-L | TCGGCGGCGGCTTCGTTGGTGAT | |
| PJW9-U | CAATATTTGGCGGGCAAGGTCAC | RT-PCR (mop-orf411), 559 bp (fragment 3 in Fig. 3) |
| PJW9-L | GCGCGAAGCAAGGCAGGAGA | |
| PJW9-U | CAATATTTGGCGGGCAAGGTCAC | RT-PCR (mop), 117 bp (fragment 2 in Fig. 3) |
| PJW8-L | TCGGCGGCGGCTTCGTTGGTGAT | |
| PJW12-U | GGGCGGCCGTTCCTGTTCCT | mop promoter fragment (509 bp) in pJW42 |
| PJW12-L | TCGGCGGCGGCTTCGTTGGTGAT | |
| PJW18 | GAAGGCCCCGTCAGCACCAGAAAT | Primer extension (morC) |
| PJW19 | TCGGCGGCGGCTTCGTTGGTGAT | Primer extension (mop) |
| PJW27-U | AACATATGAACGAACAGCCCCTCATCG | Amplification of mopA coding region |
| PJW27-L | TTCTCGAGGGGCATCGCCAGGATGACATG | |
| PJW28-U | AACATATGACGGACGGTGTGCGCGGGG | Amplification of mopB coding region |
| PJW28-L | TTCTCGAGGGGCAGGGCCAGGATCACATG | |
| PJW29-U | CCTCGGCGGTCTCGTGGCTTGTCATCA | morA-morC intergenic region (1,139 bp) in pJW45 |
| PJW29-L | ACTGCCGCCCCGCCTTGCCGTAAAT | |
| PJW36-U | CCTCGGCGGTCTCGTGGCTTGTCATC | RT-PCR (morC), 363 bp (fragment 2 in Fig. 2) |
| PJW36-L | CGCGGTCGCTGGGCTTTGTCTTTCA | |
| PJW49-U | GGCACTGACCGACCTTTTGACC | DNA mobility shift, 222 bp (mop promoter) |
| PJW49-L | AGAATATTGCGTGCGCTGAGTTT | |
| PJW52-U | ACGGGCAGGCGCGGGGTTCT | DNA mobility shift, 236 bp (anfA promoter) |
| PJW52-L | CGGTAAAGCGTCGGCAGCAGGTTCA | |
| PJW53-U | GCATCCCAGGCGGTCTTGTAGG | DNA mobility shift, 272 bp (mopA promoter) |
| PJW53-L | ATGAGGCCGCGGGTGATAACG | |
| PJW54-U | CAGCCCGACATCGAGCGTGAAC | DNA mobility shift, 244 bp (morC/A promoter) |
| PJW54-L | CGGCAGAGGCGGAAAGGAGAAGA | |
| PJW55-U | ACTGCGCCGCGATCCCCGAGAC | DNA mobility shift, 251 bp (anfA internal fragment) |
| PJW55-L | CGCCGCAATCACCCGCACATCA |
β-Galactosidase assays.
R. capsulatus strains carrying reporter fusions between Mo-regulated genes and the promoterless E. coli lacZ gene, namely, anfA-lacZ, mopA-modA-lacZ, morA-lacZ, morC-lacZ, and mop-lacZ (Table 1), were grown in Mo-free AK-NL minimal medium containing either 9.5 mM serine (nitrogen-limiting conditions) or 20 mM ammonium (nitrogen-sufficient conditions). When required, 10 μM Na2MoO4 was added. Following growth to late exponential phase, β-galactosidase activities were determined by the sodium dodecyl sulfate-chloroform method (19).
RNA isolation, transcriptional analysis by RT-PCR, and primer extension.
R. capsulatus wild-type cultures were grown in Mo-free AK-NL minimal medium containing 9.5 mM serine as the sole nitrogen source. When required, 10 μM Na2MoO4 was added. Total RNA was isolated using the Micro-to-Midi total RNA purification system according to the instructions of the manufacturer (Invitrogen, Karlsruhe, Germany). Specific transcripts were analyzed with the ThermoScript reverse transcriptase PCR (RT-PCR) system (Invitrogen). To analyze transcription of the mopA-modABCD, morAB, morC, and mop operons, the primers shown in Table 2 were used for cDNA synthesis and/or second-strand synthesis and subsequent PCR amplification steps. Primer extension was carried out as described previously (4) using synthetic oligonucleotide primers (Table 2) to map the transcription start sites of morC and mop, respectively.
Overexpression of His-tagged R. capsulatus MopA and MopB proteins in E. coli.
The mopA and mopB coding regions were PCR amplified with primer pairs PJW27-U/PJW27-L and PJW28-U/PJW28-L (encompassing recognition sites for NdeI and XhoI, respectively) (Table 2), using R. capsulatus chromosomal DNA as a template. Subsequently, the 0.8-kb NdeI-XhoI fragments with either mopA or mopB were cloned into expression vector pET22b(+), resulting in hybrid plasmids pJW32 (mopAhis) and pJW33 (mopBhis), respectively. The plasmids were transformed into E. coli strain BL21(DE3), which served as a host for overexpression of the tagged R. capsulatus MopA and MopB proteins (MopAHis and MopBHis). Purification of the recombinant proteins was carried out as described previously (23).
DNA mobility shift assays.
DNA fragments encompassing Mo-regulated promoters were obtained by PCR amplification with appropriate primer pairs (Table 2), using chromosomal DNA as a template. Amplification products were purified using a NucleoSpin Extract II kit (Macherey-Nagel, Düren, Germany) prior to 32P labeling of 5′ ends with T4 polynucleotide kinase (Fermentas, St. Leon-Rot, Germany). Different amounts (up to 150 pmol) of either MopAHis or MopBHis in buffer B (40 mM NaH2PO4 [pH 8.0], 500 mM NaCl) in a total volume of 16 μl were preincubated at room temperature. When required, 250 nmol Na2MO4 was added at the beginning of the preincubation phase. After 10 min, a mixture consisting of 1 μl 32P-labeled DNA (5 fmol/μl), 1 μl poly(dI-dC) (1 μg/μl), and 2 μl binding buffer (25 mM HEPES [pH 8.0], 50 mM K-glutamate, 50 mM MgSO4, 1 mM dithiothreitol, 0.1 mM EDTA, 0.05% Igepal CA-630) was added to the protein samples. After incubation at 30°C for 20 min, samples were separated on 6% polyacrylamide gels before 32P-labeled bands were documented using a Hyperscreen X-ray film (Fuji Photo Film Europe, Düsseldorf, Germany).
RESULTS AND DISCUSSION
Genetic organization of selected molybdenum-regulated genes in R. capsulatus.
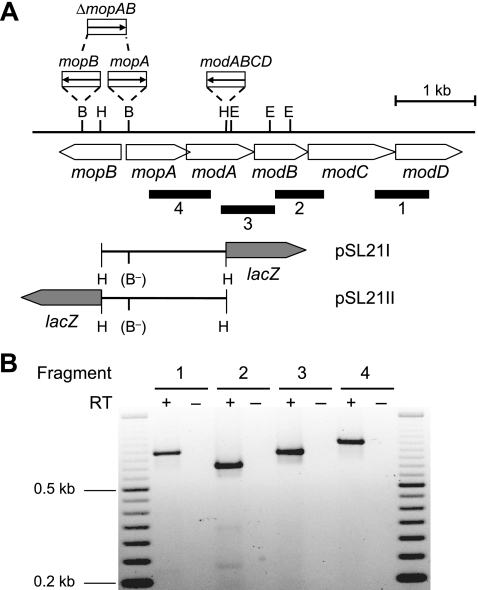
Genes coding for two ModE-like Mo-dependent regulators (mopA and mopB) and a Mo transport system (modABC) were previously identified downstream of the structural genes of Mo-nitrogenase, nifHDK (29). These genes are organized in two divergently transcribed operons, mopA-modABCD and mopB. Cotranscription of mopA-modABCD was demonstrated by RT-PCR (see Materials and methods) (Fig. 1). Total RNA was isolated from R. capsulatus wild-type cells grown in Mo-free minimal medium. After reverse transcription, selected primer pairs (Table 2; Fig. 1) were used to PCR amplify DNA fragments overlapping the gene borders of mopA-modA, modA-modB, modB-modC, and modC-modD. The presence of amplification products was completely dependent on the addition of reverse transcriptase to the reaction mixtures, indicating that the RNA was not contaminated with DNA. The presence of PCR products based on all four primer pairs strongly suggested that mopA-modABCD comprise a single transcription unit.
FIG. 1.
Transcriptional analysis of the R. capsulatus mopA- modABCD gene region. (A) Physical and genetic maps of the mop-mod gene region. The physical map is given for BamHI, EcoRI, and HindIII (B, E, and H, respectively). Black bars below the genetic map indicate DNA fragments 1 to 4 emerging from RT-PCR (see Materials and Methods and panel B). The corresponding primer pairs used for RT-PCR are listed in Table 2. Mutant strains defective for either mopA (R423AI), mopB (R423BI), mopA and mopB (R423CI), or modABCD (R438II) contain gentamicin resistance cassettes, with the directions of transcription of the Gm resistance gene symbolized by arrows. Hybrid plasmids pSL21I and pSL21II, carrying transcriptional modA-lacZ and mopB-lacZ fusions, respectively, are based on the mobilizable broad-host-range plasmid pML5. In these reporter plasmids, the BamHI sites were destroyed (indicated by B−) by cutting with BamHI, filling in protruding ends, and blunt-end religation, leading to a frameshift within the mopA coding region. Neither the Gm cassette nor the lacZ gene is drawn to scale. (B) Transcriptional analysis of the mopA-modABCD operon by RT-PCR. Total RNA was isolated from R. capsulatus cells grown under Mo-limiting conditions. Either RNA samples were treated with reverse transcriptase to synthesize cDNA (+) or, as a negative control, reverse transcriptase was omitted (−). A 50-bp DNA ladder (Fermentas, St. Leon-Rot, Germany) was used as a length standard.
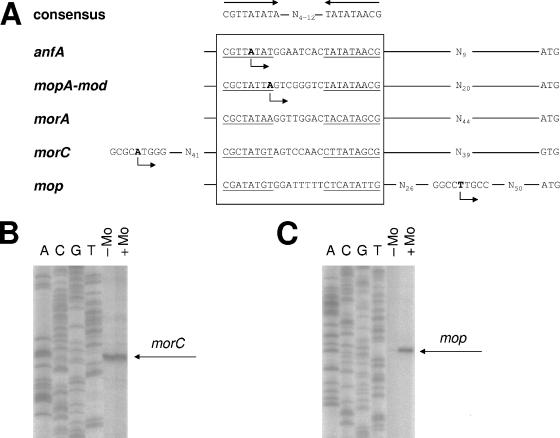
Using the ModABC proteins as query to screen the R. capsulatus genome database (www.ergo-light.com), we identified a related ABC-type transport system encoded by open reading frames Rc1279, Rc2331, and Rc1280. Since this study revealed that expression of these genes is repressed by molybdenum (see below), we propose new designations, namely, morA, morB, and morC (for Mo-regulated genes) (Fig. 2). In addition, a mop-like gene (Rc412) coding for a small protein similar to the Mop domain of ModC was identified 2.3 kb downstream of nifB1 coding for a protein involved in biosynthesis of the cofactor of Mo-nitrogenase, FeMo-co. As was the case for anfA and the mopA-modABCD operon (13), putative Mo boxes (binding sites for MopA and/or MopB) were identified in the intergenic region between morAB and morC as well as in the mop promoter region (Fig. 5A), giving a first hint for Mo regulation of both the putative transporter MorABC and the putative Mo homeostasis protein Mop.
FIG. 5.
DNA sequence comparison of Mo-regulated promoters (A) and transcription start site mapping of the morC (B) and mop (C) genes. DNA sequences of Mo boxes are compared to the consensus as defined by Kutsche et al. (13). The morA-morC intergenic region contains a single Mo box, which is thought to control expression of the divergently transcribed mor operons (Fig. 2). For clarity, two complementary sequences (morA and morC) of the same Mo box from this region are shown. The transcription start sites of anfA and the mopA-modABCD operon were taken from Kutsche et al. (13). To determine the transcription start sites of the other genes, primer extension was carried out with total RNA from R. capsulatus cells grown either under Mo-limiting conditions (−Mo) or in the presence of 10 μM Na2MoO4 (+Mo). Primers PJW18 and PJW19 (binding to the 5′ regions of morC and mop, respectively) were used for reverse transcription. The corresponding sequencing reactions (A, C, G, and T) with plasmids pJW45 (morC) and pJW42 (mop) served as length standards. No transcription start site was mapped for morA.
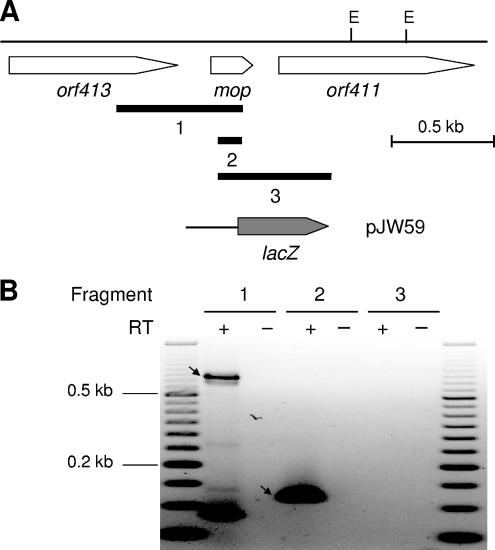
The genetic organization of the mor and mop gene regions was analyzed by RT-PCR essentially as described above for the mopA-modABCD operon. These studies revealed that morAB formed part of a bicistronic operon, while morC formed a monocistronic transcription unit (Fig. 2). While mod and mor gene expression was repressed by molybdenum, transcription of the mop gene was Mo activated (Table 3) (see below). Therefore, in contrast to studies on mod and mor, for analysis of mop gene organization, total RNA from cultures grown in the presence of molybdenum was used. While mop expression studies clearly demonstrated the presence of a Mo-activated promoter within the orf413-mop intergenic region (Table 3) (see below), RT-PCR studies identified an amplification product overlapping the gene border of orf413-mop (Fig. 3). These findings are most likely explained by the presence of two promoters driving expression of the mop gene, one immediately upstream of the mop coding region and the second either upstream of or within the coding region of orf413.
TABLE 3.
Expression of Mo-controlled lacZ reporter fusions in R. capsulatus wild-type and mutant strains
| Strain(plasmid) | Genetic background | Reporter fusion | β-Galactosidase activitya
|
|||
|---|---|---|---|---|---|---|
| +Mo/−N | −Mo/−N | +Mo/+N | −Mo/+N | |||
| B10S(pKS131A) | Wild type | anfA-lacZ | 1 ± 1 | 63 ± 10 | 1 ± 1 | 0 ± 0 |
| R423AI(pKS131A) | mopA | anfA-lacZ | 1 ± 1 | 73 ± 11 | 1 ± 1 | 0 ± 0 |
| R423BI(pKS131A) | mopB | anfA-lacZ | 0 ± 0 | 64 ± 10 | 0 ± 0 | 0 ± 0 |
| R423CI(pKS131A) | Δ(mopAB) | anfA-lacZ | 72 ± 18 | 67 ± 9 | 2 ± 2 | 1 ± 1 |
| B10S(pSL21I) | Wild type | modA-lacZ | 11 ± 1 | 70 ± 7 | 6 ± 2 | 4 ± 3 |
| R423AI(pSL21I) | mopA | modA-lacZ | 7 ± 9 | 88 ± 5 | 7 ± 2 | 3 ± 2 |
| R423BI(pSL21I) | mopB | modA-lacZ | 6 ± 5 | 78 ± 11 | 13 ± 6 | 6 ± 0 |
| R423CI(pSL21I) | Δ(mopAB) | modA-lacZ | 87 ± 9 | 89 ± 13 | 0 ± 0 | 2 ± 2 |
| B10S(pSL21II) | Wild type | mopB-lacZ | 21 ± 3 | 22 ± 2 | 18 ± 2 | 18 ± 1 |
| R423AI(pSL21II) | mopA | mopB-lacZ | 18 ± 3 | 18 ± 4 | 18 ± 4 | 23 ± 7 |
| R423BI(pSL21II) | mopB | mopB-lacZ | 16 ± 1 | 22 ± 1 | 16 ± 2 | 18 ± 4 |
| R423CI(pSL21II) | Δ(mopAB) | mopB-lacZ | 17 ± 4 | 20 ± 4 | 21 ± 2 | 20 ± 2 |
| B10S(pMOT15) | Wild type | morA-lacZ | 43 ± 3 | 369 ± 28 | 37 ± 3 | 117 ± 3 |
| R423AI(pMOT15) | mopA | morA-lacZ | 51 ± 4 | 441 ± 52 | 48 ± 3 | 67 ± 27 |
| R423BI(pMOT15) | mopB | morA-lacZ | 120 ± 19 | 408 ± 44 | 70 ± 11 | 171 ± 7 |
| R423CI(pMOT15) | Δ(mopAB) | morA-lacZ | 452 ± 66 | 435 ± 63 | 175 ± 19 | 202 ± 21 |
| B10S(pMOT16) | Wild type | morC-lacZ | 438 ± 27 | 733 ± 76 | 148 ± 14 | 387 ± 39 |
| R423AI(pMOT16) | mopA | morC-lacZ | 411 ± 48 | 791 ± 84 | 147 ± 13 | 368 ± 10 |
| R423BI(pMOT16) | mopB | morC-lacZ | 454 ± 32 | 785 ± 61 | 196 ± 18 | 456 ± 34 |
| R423CI(pMOT16) | Δ(mopAB) | morC-lacZ | 891 ± 63 | 862 ± 56 | 388 ± 36 | 494 ± 41 |
| B10S(pJW59) | Wild type | mop-lacZ | 551 ± 51 | 40 ± 15 | 316 ± 40 | 64 ± 5 |
| R423AI(pJW59) | mopA | mop-lacZ | 24 ± 3 | 25 ± 20 | 41 ± 5 | 43 ± 3 |
| R423BI(pJW59) | mopB | mop-lacZ | 382 ± 18 | 18 ± 4 | 333 ± 22 | 22 ± 2 |
| R423CI(pJW59) | Δ(mopAB) | mop-lacZ | 44 ± 13 | 36 ± 11 | 57 ± 17 | 43 ± 5 |
R. capsulatus strains were grown under phototrophic conditions in AK-NL minimal medium either without addition of Mo (−Mo) or in the presence of 10 μM Na2MoO4 (+Mo). N-sufficient or N-limiting conditions were achieved by addition of either 15 mM (NH4)2SO4 (+N) or 9.5 mM serine (−N), respectively. β-Galactosidase activity is given in Miller units (19). Results represent the means and standard deviations of three independent measurements.
FIG. 3.
Transcriptional analysis of the R. capsulatus mop gene region. (A) Physical and genetic maps of the mop gene region. The physical map is given for EcoRI (E). Black bars below the genetic map indicate DNA fragments 1 to 3 emerging from RT-PCR (see Materials and Methods and panel B). The corresponding primer pairs used for RT-PCR are listed in Table 2. Hybrid plasmid pJW59, carrying a transcriptional mop-lacZ fusion, is based on the mobilizable broad-host-range plasmid pML5. (B) Transcriptional analysis of the mop gene region by RT-PCR. Total RNA was isolated from R. capsulatus cells grown in the presence of 10 μM Na2MoO4. Either RNA samples were treated with reverse transcriptase to synthesize cDNA (+) or, as a negative control, reverse transcriptase was omitted (−). Amplification products corresponding to DNA fragments 1 and 2 are marked by arrows. A 50-bp DNA ladder (Fermentas, St. Leon-Rot, Germany) was used as a length standard.
Regulation of mod, mor, mop, and anf transcription by molybdenum.
To analyze Mo regulation of selected genes, R. capsulatus reporter strains carrying fusions between these genes and the promoterless E. coli lacZ gene were used (Table 1). In detail, we examined expression of lac fusions with anfA (pKS131A), mopA-modA (pSL21I), mopB (pSL21II), morA (pMOT15), morC (pMOT16), and mop (pJW59). The lac fusions were introduced into wild-type R. capsulatus (B10S) and mutant strains defective for either MopA (R423AI), MopB (R423BI) or both (R423CI). These mutant strains contain a gentamicin resistance (Gm) cassette (Fig. 1A), which drives expression of downstream genes reading in the same direction as the Gm gene (29). Therefore, although mopA is the first gene of the mopA-modABCD operon, expression of the Mo transport system is not abolished in mutant strains R423AI (mopA) and R423CI (mopA mopB). In agreement with previous studies (13), transcription of modA and anfA was not only repressed by molybdenum but also inhibited by ammonium. To analyze whether other Mo-regulated genes were also controlled by the N source, R. capsulatus reporter strains were cultivated under four different growth conditions, namely, in the presence or absence of molybdenum in combination with either nitrogen-sufficient conditions (ammonium as the N source) or nitrogen-limiting conditions (serine as the N source), prior to determination of β-galactosidase activities. R. capsulatus can efficiently use serine as the sole nitrogen source, but, in contrast to ammonium, serine does not inhibit synthesis of the ModABC transport system or nitrogenase (13).
The results of expression studies on Mo-regulated genes (Table 3) may be summarized as follows. (i) In the wild-type background significant expression of both anfA and mopA-modA occurred only under Mo- and N-limiting conditions (13; this study). (ii) Mo repression was mediated by either MopA or MopB, which are able to replace each other in repression of mopA-modA and anfA. In other words, MopA autoregulates its own expression. (iii) Like the anfA and mopA-modA genes, morA and morC were repressed by MopA or MopB in the presence of Mo. However, Mo repression of morC was less pronounced compared to that of anfA, mopA-modA, and morA. Significant expression of MorC in the presence of Mo might suggest that the protein has, in addition to its energizer function of the putative ABC transporter in the absence of Mo, another yet-unknown function. (iv) While mopA-modA (like anfA) was strongly inhibited by ammonium, the N source had only a minor influence on expression of morA and morC. (v) In contrast to that of mopA, expression of mopB was not regulated by molybdenum or ammonium. As a consequence, the cellular MopA/MopB ratio should strongly differ in response to Mo and N availability, if expression data reflect the actual amounts of MopA and MopB protein. (vi) In contrast to the Mod and Mor systems and the Fe-nitrogenase, which were Mo repressed, expression of the putative Mo homeostasis protein Mop was activated by molybdenum. (vii) Interestingly, Mo activation specifically required the MopA protein, whereas MopB had little influence on mop transcription. Like R. capsulatus MopA, E. coli ModE can act as both a repressor and an activator (2). It represses the modABC operon and activates transcription of genes involved in Mo-co biosynthesis. It is worth noting, however, that expression of R. capsulatus Mo-co biosynthesis genes moeA and moeB is not Mo regulated (15) (data not shown). (viii) In contrast to that of mopA-modA and anfA, expression of mop was almost unaffected by ammonium. This finding might be explained by a role of the postulated Mo homeostasis protein as an Mo donor not only for Mo-nitrogenase, which is expressed exclusively under nitrogen-limiting conditions, but also for other Mo-containing enzymes such as DMSO reductase and xanthine dehydrogenase (16).
The morABC genes are not required for Mo-nitrogenase activity.
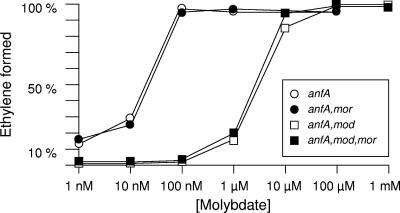
R. capsulatus mutant strains defective for the modABC genes are impaired in high-affinity Mo uptake as estimated from the Mo-nitrogenase activity (29). While the parental strain exhibited full Mo-nitrogenase activity at Mo concentrations of as low as 100 nM, a modABC mutant strain required at least 100-fold-higher Mo concentrations for maximum Mo-nitrogenase activity (Fig. 4) (29). A second, yet-uncharacterized low-affinity transport system has been discussed as being responsible for Mo uptake at concentrations of above 10 μM (29). Since the morAB and morC genes were shown to be repressed by molybdenum, we asked whether the putative MorABC transporter was involved in low-affinity Mo uptake. For this purpose, R. capsulatus mutant strains defective for either ModABC (R438II) (Fig. 1), MorABC (AW12) (Fig. 2), or both (R438II-AW12) were assayed for their Mo-nitrogenase activities at different Mo concentrations (Fig. 4). To rule out any interference with Fe-nitrogenase, which does not require molybdenum for activity, Mo-nitrogenase activity was measured in an anfA mutant background (KS94A), thus preventing transcription of Fe-nitrogenase genes. Based on Mo-nitrogenase activity, the morABC mutant strains were indistinguishable from their parental strains (Fig. 4), strongly suggesting that MorABC is not the previously postulated low-affinity Mo uptake system.
FIG. 4.
Effect of increasing molybdate concentrations on the activity of Mo-nitrogenase. R. capsulatus strains were grown in AK-NL minimal medium containing the indicated Mo concentrations and 9.5 mM serine as the sole nitrogen source (nitrogenase-derepressing conditions). The activity of Mo-nitrogenase was determined by the reduction of acetylene to ethylene, as assayed by gas chromatography, and is expressed as a percentage of the maximal value obtained in Mo-sufficient medium (100% corresponds to 662 nmol ethylene produced × h−1 × mg protein−1). R. capsulatus strains KS94A (anfA), KS94A-AW12 (anfA morABC), KS94A-R438II (anfA modABC), and KS94A-R438II-AW12 (anfA morABC modABC) were used.
Transcription start sites of Mo-regulated genes.
Typically, repressor binding sites either overlap or are located downstream of the transcription start site of the respective target genes. This has previously been demonstrated for the Mo boxes implicated in binding of MopA and MopB upstream of anfA and the mopA-modABCD operon (13). The situation is more complex for the divergently transcribed morAB and morC operons, which are expected to share a single Mo box located in the intergenic region between the two operons (Fig. 2). The transcription start site of the morC gene was determined by primer extension analysis (Fig. 5B). For this purpose, total RNAs isolated from R. capsulatus wild-type cultures grown in either the presence or absence of Mo were used as templates for reverse transcription with primer PJW18, complementary to the 5′ ends of morC mRNA (see Materials and Methods) (Table 2). Reverse transcripts based on RNA isolated from cultures grown in either the presence or absence of Mo were identical in length and of comparable intensity. This finding was in line with morC-lacZ expression studies showing significant expression under both conditions (Table 3). The transcription start site of morC mapped upstream of the putative Mo box (Fig. 5A), suggesting that binding of MopA and MopB to this Mo box interferes with transcription. Despite several attempts using three different primers complementary to the 5′ ends of morA mRNA, no transcription start site could be determined for morA.
In parallel, we determined the transcription start site of the mop gene (Fig. 5C). Reverse transcripts based on RNA isolated from cultures grown in either the presence or absence of Mo were identical in length but clearly differed in intensity, which was in line with mop-lacZ expression studies showing that maximal mop expression occurred in the presence of Mo (Table 3). The mop transcription start site was mapped downstream of the putative Mo box (Fig. 5A). As activator binding sites are typically located upstream of the transcription start site, this finding is consistent with MopA-dependent mop gene activation.
Binding of MopA and MopB to target promoters.
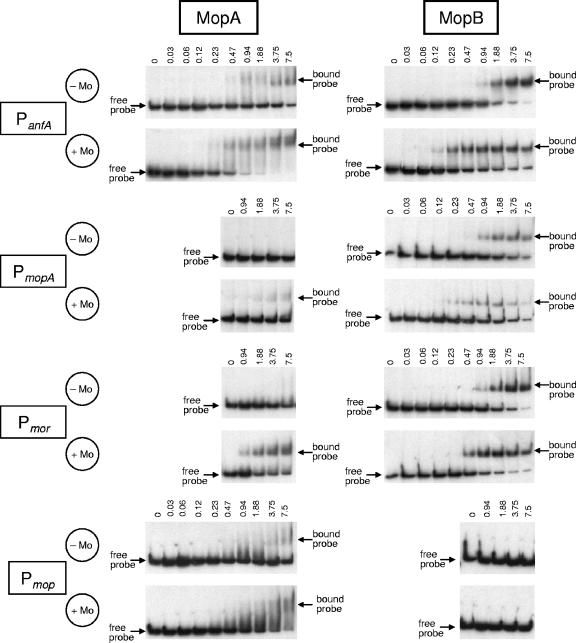
As shown above by expression studies, MopA and MopB regulate transcription of their target genes in response to Mo availability. The presence of conserved Mo boxes in the promoter regions of all target genes suggested that MopA and MopB specifically bind to these promoters. DNA mobility shift assays were carried out to verify this assumption. For this purpose, MopA and MopB were overexpressed and purified as C-terminally His-tagged recombinant proteins from E. coli (see Materials and Methods). DNA fragments, ranging from 222 to 272 bp, encompassing selected promoters were PCR amplified using appropriate primer pairs (Table 2) and radioactively labeled at their 5′ ends (see Materials and Methods). All binding assays were performed in the presence of poly(dI-dC) as competitor DNA.
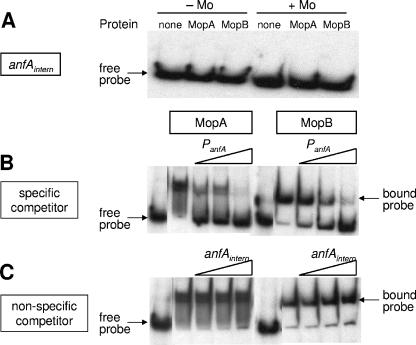
The results of DNA mobility shift assays with increasing amounts of either MopAHis or MopBHis are shown in Fig. 6. Both MopA and MopB bound to the anfA promoter (PanfA). Binding of both regulators occurred in the absence of Mo but was clearly improved in the presence of Mo. In contrast to binding to the anfA promoter, neither MopA nor MopB bound to a control DNA fragment derived from an internal region of anfA (Fig. 7A), thus corroborating binding specificity. Binding of MopA and MopB to radioactively labeled anfA promoter fragments could be reversed by addition of increasing amounts of nonlabeled anfA promoter DNA (Fig. 7B) but not by the internal anfA fragment (Fig. 7C). The importance of the Mo box upstream of anfA as a cis-regulatory element has been demonstrated by analysis of mutant promoters carrying small deletions within this element (13). Taken together, these findings strongly suggest that MopA and MopB control anfA expression by binding to this Mo box overlapping the transcription start site (Fig. 5A). Although in vitro binding of MopA and MopB to the anfA promoter implies that no additional proteins are required for Mo repression of anfA, fine-tuning by other protein factors in vivo cannot be excluded.
FIG. 6.
DNA mobility shift assays with Mo-regulated promoter fragments and purified recombinant MopA and MopB proteins. DNA fragments encompassing the promoters of anfA (PanfA), mopA-modABCD (PmopA), and mop (Pmop) and the intergenic region between the divergently transcribed mor operons (Pmor) were generated by PCR amplification using appropriate primer pairs (Table 2) prior to 32P labeling (see Materials and Methods). Incubation of increasing amounts of MopA and MopB (0, 0.03, 0.06, 0.12, 0.23, 0.47, 0.94, 1.88, 3.75, and 7.5 μM) with labeled DNA fragments was carried out either in the absence (−Mo) or presence (+Mo) of molybdenum. All reactions were performed with 5 fmol 32P-labeled DNA fragment probes.
FIG. 7.
Specificity controls for binding of MopA and MopB to anfA promoter DNA. (A) Use of an internal region of anfA (anfAintern) as negative control for DNA mobility shift assays. The control DNA fragment (anfAintern) (Table 2) was PCR amplified, 32P labeled, and incubated with either 7.5 μM MopA or MopB in the absence (−Mo) or presence (+Mo) of molybdenum. (B) Use of unlabeled anfA promoter fragments as specific competitor DNA. MopA or MopB (2.5 μM) and 32P-labeled anfA promoter fragments were mixed with a 400-, 800-, or 1,600-fold excess of unlabeled competitor DNA (compared to the labeled probe). (C) Use of anfAintern in competition assays. MopA or MopB (2.5 μM) and 32P-labeled anfA promoter fragments were mixed with a 550-, 1,100-, or 2,200-fold excess of unlabeled anfA internal fragments (compared to the labeled probe). All reactions were performed with 5 fmol 32P-labeled PanfA probes.
Under comparable conditions, binding of MopA to the mopA-modABCD promoter (PmopA) and the intergenic region between the divergently transcribed mor operons (Pmor) was much weaker than binding of MopB, and binding of MopA to PmopA and Pmor was not detectable at all in the absence of Mo (Fig. 6). Most interestingly, only MopA (and not MopB) bound to the promoter of the mop gene (Pmop), coding for a putative Mo homeostasis protein (Fig. 6). Binding of MopA in the absence of Mo was barely detectable. More efficient binding occurred in the presence of Mo. As one would expect for a cis-regulatory element serving exclusively as a binding site for MopA, the mop-specific Mo box differs at three positions from all the other Mo boxes, which serve as binding sites for both MopA and MopB (Fig. 5A).
Compared to binding to the anfA, mopA, and mor promoters, binding of MopA to the mop promoter was fairly weak (even in the presence of Mo). Generally, binding of activator proteins to their target promoters is believed to be much weaker than binding of repressor proteins to operator sequences (5). The suggested role of MopA acting either as a repressor (for anfA, mopA-modABCD, morAB, and morC expression) or as an activator (for mop regulation) perfectly agrees with this general observation.
Conclusions.
MopA and MopB have overlapping functions, as they can substitute for each other in Mo repression of anfA, mopA-modABCD, morAB, and morC (13; this study). In addition to its role as a repressor, MopA serves as an activator of transcription of the mop gene (this study), which is the first example of a specialized function of MopA in Mo regulation.
It is worth noting that MopB has been described to be essential for activity of DMSO reductase (dor encoded) in R. capsulatus strain 37b4 (28). A putative Mo box has been identified upstream of the dorX gene, consistent with the view that the dorX gene is the target for MopB-dependent Mo regulation in strain 37b4 (18). DorX, in turn, has been suggested to activate transcription of an operon whose products are required for Mo-co biosynthesis and, hence, DMSO reductase activity. R. capsulatus strain B10S, which was used in this study, also has the capacity to synthesize DMSO reductase. However, in contrast to strain 37b4, strain B10S does not contain a dorX-like gene at the equivalent position relative to the other dor genes (data not shown) or elsewhere in the chromosome (www.ergo-light.com), suggesting that regulation of DMSO reductase activity differs in the two R. capsulatus strains.
The current model for Mo regulation in R. capsulatus suggests that the Mo-dependent regulators, MopA and MopB, are involved in regulation of the internal Mo concentration by repressing transcription of the modABCD transport operon (13; this study) and thus limiting the amount of the transporter at high Mo concentrations. Mutant strains defective for ModABC express Fe-nitrogenase at Mo concentrations of up to 1 μM, while synthesis of Fe-nitrogenase is repressed at much lower concentrations in the parental strain (29), suggesting that the putative MorABC transporter does not substitute for the ModABC system. Although expression of the morAB and morC genes is controlled by molybdenum, at present it remains unknown whether the gene products are involved in Mo uptake at all. The presence of the high-affinity Mo transporter ModABC, which provides sufficient Mo for the Mo-nitrogenase at low Mo concentrations, is physiologically favorable, as Mo-nitrogenase is more efficient than Fe-nitrogenase with respect to N2 reduction rates (24).
MopA-dependent mop gene activation occurred only in the presence of Mo, thus ensuring that the putative Mo homeostasis protein is not expressed under Mo-limiting conditions. However, since expression of mopA (as part of the mopA-modABCD operon) is down-regulated in response to increasing Mo concentrations, MopA-dependent synthesis of the Mop protein is expected to decrease when Mo is abundant.
Acknowledgments
We thank Thomas Drepper for helpful discussions, Corinna Hasecke and Britta Schubert for analysis of the DMSO reductase gene region from R. capsulatus strain B10S, Silke Leimkühler for construction of plasmids pSL21I and pSL21II, and Andrea Kreuz for construction of plasmids pMOT15 and pMOT16.
This work was supported by a financial grant from Deutsche Forschungsgemeinschaft (Ma 1814/3-1).
Footnotes
Published ahead of print on 6 October 2006.
REFERENCES
- 1.Alexeyev, M. F. 1995. Three kanamycin resistance gene cassettes with different polylinkers. BioTechniques 18:52-56. [PubMed] [Google Scholar]
- 2.Anderson, L. A., E. McNairn, T. Leubke, R. N. Pau, and D. H. Boxer. 2000. ModE-dependent molybdate regulation of the molybdenum cofactor operon moa in Escherichia coli. J. Bacteriol. 182:7035-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, L. A., T. Palmer, N. C. Price, S. Bornemann, D. H. Boxer, and R. N. Pau. 1997. Characterization of the molybdenum-responsive ModE regulatory protein and its binding to the promoter region of the modABCD (molybdenum transport) operon of Escherichia coli. Eur. J. Biochem. 246:119-126. [DOI] [PubMed] [Google Scholar]
- 4.Babst, M., H. Hennecke, and H. M. Fischer. 1996. Two different mechanisms are involved in the heat-shock regulation of chaperonin gene expression in Bradyrhizobium japonicum. Mol. Microbiol. 19:827-839. [DOI] [PubMed] [Google Scholar]
- 5.Collado-Vides, J. 1993. A linguistic representation of the regulation of transcription intiation. II. Distinctive features of sigma 70 promoters and their regulatory binding sites. BioSystems 29:105-128. [DOI] [PubMed] [Google Scholar]
- 6.Delgado, M. J., A. Tresierra-Ayala, C. Talbi, and E. J. Bednar. 2006. Functional characterization of the Bradyrhizobium japonicum modA and modB genes involved in molybdenum transport. Microbiology 152:199-207. [DOI] [PubMed] [Google Scholar]
- 7.Drepper, T., K. Raabe, D. Giaourakis, M. Gendrullis, B. Masepohl, and W. Klipp. 2002. The Hfq-like protein NrfA of the phototrophic purple bacterium Rhodobacter capsulatus controls nitrogen fixation via regulation of nifA and anfA expression. FEMS Microbiol. Lett. 215:221-227. [DOI] [PubMed] [Google Scholar]
- 8.Grunden, A. M., R. M. Ray, J. K. Rosentel, F. G. Healy, and K. T. Shanmugam. 1996. Repression of the Escherichia coli modABCD (molybdate transport) operon by ModE. J. Bacteriol. 178:735-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall, D. R., D. G. Gourley, G. A. Leonard, E. M. H. Duke, L. A. Anderson, D. H. Boxer, and W. N. Hunter. 1999. The high-resolution crystal structure of the molybdate-dependent transcriptional regulator (ModE) from Escherichia coli: a novel combination of domain folds. EMBO J. 18:1435-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 11.Hübner, P., J. C. Willison, P. M. Vignais, and T. A. Bickle. 1991. Expression of regulatory nif genes in Rhodobacter capsulatus. J. Bacteriol. 173:2993-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klipp, W., B. Masepohl, and A. Pühler. 1988. Identification and mapping of nitrogen fixation genes in Rhodobacter capsulatus: duplication of a nifA-nifB region. J. Bacteriol. 170:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutsche, M., S. Leimkühler, S. Angermüller, and W. Klipp. 1996. Promoters controlling expression of the alternative nitrogenase and the molybdenum uptake system in Rhodobacter capsulatus are activated by NtrC, independent of σ54, and repressed by molybdenum. J. Bacteriol. 178:2010-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labes, M., A. Pühler, and R. Simon. 1990. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for Gram-negative bacteria. Gene 89:37-46. [DOI] [PubMed] [Google Scholar]
- 15.Leimkühler, S., S. Angermüller, G. Schwarz, R. R. Mendel, and W. Klipp. 1999. Activity of the molybdopterin-containing xanthine dehydrogenase of Rhodobacter capsulatus can be restored by high molybdenum concentrations in a moeA mutant defective in molybdenum cofactor biosynthesis. J. Bacteriol. 181:5930-5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leimkühler, S., M. Kern, P. S. Solomon, A. G. McEwan, G. Schwarz, R. R. Mendel, and W. Klipp. 1998. Xanthine dehydrogenase from the phototrophic purple bacterium Rhodobacter capsulatus is more similar to its eukaryotic counterparts than to prokaryotic molybdenum enzymens. Mol. Microbiol. 27:853-869. [DOI] [PubMed] [Google Scholar]
- 17.Masepohl, B., T. Drepper, and W. Klipp. 2004. Nitrogen fixation in the photosynthetic purple bacterium Rhodobacter capsulatus, p. 141-173. In W. Klipp, B. Masepohl, J. R. Gallon, and W. E. Newton (ed.), Genetics and regulation of nitrogen fixation in free-living bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 18.McCrindle, S. L., U. Kappler, and A. G. McEwan. 2005. Microbial dimethylsulfoxide and trimethylamine-N-oxide respiration. Adv. Microb. Physiol. 50:147-201. [DOI] [PubMed] [Google Scholar]
- 19.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 20.Mouncey, N. J., L. A. Mitchenall, and R. N. Pau. 1996. The modE gene product mediates molybdenum-dependent expression of genes for the high-affinity molybdate transporter and modG in Azotobacter vinelandii. Microbiology 142:1997-2004. [DOI] [PubMed] [Google Scholar]
- 21.Neubauer, H., I. Pantel, P. E. Lindgren, and F. Götz. 1999. Characterization of the molybdate transport system ModABC of Staphylococcus carnosus. Arch. Microbiol. 172:109-115. [DOI] [PubMed] [Google Scholar]
- 22.Pau, R. N. 2004. Molybdenum uptake and homeostasis, p. 225-256. In W. Klipp, B. Masepohl, J. R. Gallon, and W. E. Newton (ed.), Genetics and regulation of nitrogen fixation in free-living bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 23.Raabe, K., T. Drepper, K.-U. Riedel, B. Masepohl, and W. Klipp. 2002. The H-NS-like protein HvrA modulates expression of nitrogen fixation genes in the phototrophic purple bacterium Rhodobacter capsulatus by binding to selected nif promoters. FEMS Microbiol. Lett. 216:151-158. [DOI] [PubMed] [Google Scholar]
- 24.Schneider, K., U. Gollan, M. Dröttboom, S. Selsemeier-Voigt, and A. Müller. 1997. Comparative biochemical characterization of the iron-only nitrogenase and the molybdenum nitrogenase from Rhodobacter capsulatus. Eur. J. Biochem. 244:789-800. [DOI] [PubMed] [Google Scholar]
- 25.Self, W. T., A. M. Grunden, A. Hasona, and K. T. Shanmugam. 2001. Molybdate transport. Res. Microbiol. 152:311-321. [DOI] [PubMed] [Google Scholar]
- 26.Sicking, C., M. Brusch, A. Lindackers, K.-U. Riedel, B. Schubert, N. Isakovic, C. Krall, W. Klipp, T. Drepper, K. Schneider, and B. Masepohl. 2005. Identification of two new genes involved in diazotrophic growth via the alternative Fe-only nitrogenase in the phototrophic purple bacterium Rhodobacter capsulatus. J. Bacteriol. 187:92-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 28.Solomon, P. S., A. L. Shaw, M. D. Young, S. Leimkühler, G. R. Hanson, W. Klipp, and A. G. McEwan. 2000. Molybdate-dependent expression of dimethylsulfoxide reductase in Rhodobacter capsulatus. FEMS Microbiol. Lett. 190:203-208. [DOI] [PubMed] [Google Scholar]
- 29.Wang, G., S. Angermüller, and W. Klipp. 1993. Characterization of Rhodobacter capsulatus genes encoding a molybdenum transport system and putative molybdenum-pterin-binding proteins. J. Bacteriol. 175:3031-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zahalak, M., B. Pratte, K. J. Werth, and T. Thiel. 2004. Molybdate transport and its effect on nitrogen utilization in the cyanobacterium Anabaena variabilis ATCC 29413. Mol. Microbiol. 51:539-549. [DOI] [PubMed] [Google Scholar]