Abstract
Background. The adipose tissue renin-angiotensin system (RAS) contributes to regulation of fat mass and may also impact systemic functions such as blood pressure and metabolism. Methods and results. A panel of mouse models including mice lacking angiotensinogen, Agt (Agt-KO), mice expressing Agt solely in adipose tissue (aP2-Agt/Agt-KO), and mice overexpressing Agt in adipose tissue (aP2-Agt) was studied. Total body weight, epididymal fat pad weight, and circulating levels of leptin, insulin, and resistin were significantly decreased in Agt-KO mice, while plasma adiponectin levels were increased. aP2-Agt mice exhibited increased adiposity and plasma leptin and insulin levels compared to wild type (WT) controls. Angiotensinogen and type I Ang II receptor protein levels were also elevated in kidney of aP2-Agt mice. Conclusion. These findings demonstrate that alterations in adipose RAS activity significantly impact both local and systemic physiology in a way that may contribute to the detrimental health effects of obesity.
INTRODUCTION
The RAS plays a central role in blood pressure regulation and fluid-electrolyte homeostasis, and its disregulation contributes to essential hypertension [1]. Angiotensin II, the major effector of the RAS, is produced by successive enzymatic cleavage of the glycoprotein AGT by the enzymes renin and angiotensin-converting enzyme (ACE). The long-standing view that AGT, renin, and ACE were exclusive products of liver, kidney, and the vasculature, respectively, has been modified in light of discovery that local RAS exist in numerous tissues [2, 3].
The adipose RAS has been implicated in modulation of adiposity and overt obesity through its ability to stimulate both preadipocyte differentiation and lipogenesis in mature adipocytes [4, 5]. Angiotensin II enhances lipogenesis by directly increasing the activity and expression of key lipogenic enzymes, leading to increased triglyceride synthesis and storage and ultimately “fatter” fat cells. We first demonstrated this response to Ang II in vitro in both murine cell lines and primary human adipocytes [4]. Subsequently it has been confirmed in vivo by the finding that transgenic mice overexpressing Agt in adipose tissue displayed increased adiposity and total fat mass, without changes in leanness or fat free mass [6]. Adipose-derived Agt is also released into the circulation where it serves as substrate for conversion to bioactive Ang II, contributes to plasma Ang II levels and increases blood pressure [6]. Therefore detrimental consequences of excess Ang II, such as volume expansion and hypertension, may be exacerbated by conditions that lead to elevated production of Agt in adipose tissue [7–10]. Many rodent and human models indicate that Agt expression in adipose tissue is positively correlated with adipose mass, leading to the possibility that the adipose RAS contributes to conditions comorbid with obesity. Angiotensinogen protein is significantly increased in adipose tissue of numerous rodent models and human subjects that become obese through varied mechanisms [11–14]. At the other extreme, loss of Ang II production by targeted deletion of the Agt gene dramatically reduces adipose mass through adipocyte hypotrophy [6, 15, 16]. Data from an assortment of human studies also support a role for the RAS in regulation of adiposity, but the precise relationships vary according to adipose depot sampled, hypertensive status and population variables [17–20].
In this study, we determined the effects of both ubiquitous and adipose tissue-specific manipulation of Agt levels on adipose tissue metabolism and systemic indices of insulin sensitivity. Given the potential role of the adipocyte RAS in blood pressure regulation, we also examined the feedback between the adipocyte RAS and that in kidney to determine if adipose changes in Ang II production impacted the RAS in a distal location known to be important in blood pressure control.
MATERIALS AND METHODS
Experimental animals
The Agt gene was inactivated in the mouse as previously described [21]. Transgenic mice (aP2-Agt) overexpressing Agt in adipose tissue were generated by using a transgenic construct containing an adipocyte-specific promoter (adipocyte protein aP2), the mouse Agt cDNA, and a polyadenylation site [6]. Mice expressing Agt only in adipose tissue (aP2-Agt/Agt-KO) were generated by crossing transgenic mice (aP2-Agt) with Agt-deficient mice (Agt-KO) [6]. Wild-type (WT; ICR-CD1 strain, Harlen, Gannat, France), Agt-KO, aP2-Agt/Agt-KO, and aP2-Agt male mice were bred at the CNRS 6543, Centre de Biochimie at Nice, France, and shipped to the Department of Nutrition at the University of Tennessee, Knoxville. Mice were housed 4–6 per cage and fed a chow (cat # 12450B, Research Diets, New Brunswick, NJ) and water ad libitum in a 12 : 12 h light : dark cycle at constant temperature (22°C). Body weight was measured and mice were sacrificed at 28 weeks. The epididymal fat pad was removed and weighed as an index of adiposity. All experiments described were conducted in compliance with the Institutional Animal Care and Use Committee (IACUC) at the University of Tennessee.
Plasma measurements
Following an overnight fast, blood was collected by cardiac puncture for glucose, insulin, leptin, adiponectin, and resistin assays. Plasma leptin and insulin levels were measured by RIA using mouse leptin and rat insulin RIA kits, respectively, obtained from Linco Research (St Charles, MO). Blood glucose concentrations were measured using a glucose analyzer (LIFESCAN Co, MI). Plasma adiponectin and resistin levels were analyzed by ELISA kits obtained from B-Bridge International, Inc (San Jose, CA) and Phoenix Pharmaceuticals, Inc (Belmont, CA), respectively.
Western blot analysis
Proteins were extracted from epididymal fat depots and kidneys of WT, Agt-KO, aP2-Agt/Agt-KO, and aP2-Agt mice after homogenization with a protease inhibitor cocktail and quantitated by the method of Bradford [22]. Total kidney protein extracts (∼ 50 μg) and epididymal fat pad protein extracts (∼ 25 μg) were electrophoretically separated by SDS/PAGE on 8–10% running gel and transferred to nitrocellulose membranes in transfer buffer (20% methanol, 12 mM Tri base, 96 mM glycine, at pH 8.3), blocked overnight and incubated with primary polyclonal antibodies detecting AGT (52 kDa), type-I Ang II receptor (AGTR1; 47 kDa), and type-II Ang II receptor (AGTR2; 46 kDa) (Santa Cruz Biotechnology Inc, Santa Cruz, CA) at 1 : 100, 1 : 200, 1 : 200 dilution, respectively, for 3 hours at room temperature. Signals were detected using enhanced chemiluminescence (ICN Biomedicals, Inc, Costa Mesa, CA). Duplicate gels were prepared and stained with 0.1% Coomassie blue R250 and then destained in 7% acetic acid, 5% methanol to visualize protein bands for total protein quantification and confirmation of equal protein loading. The bands obtained in the Western blots were scanned using Digital Imaging and Analysis Systems (Zero-Dscan software, Scanalytics Inc, Fairfax, VA).
Statistical analysis
All data are expressed as the mean ± SEM. ANOVA was used to determine between-group differences in measured parameters. A significant overall F-test was followed by post hoc comparisons to identify significantly different groups using the Bonferroni test for multiple comparisons (SAS, Carry, NC). Statistical significance for all tests was defined as P < .05.
RESULTS
Effect of adipose Agt on fat pad weight and metabolic parameters
The effects of genetic manipulation of adipose Agt expression on body weight and fat pad mass are shown in Table 1. Consistent with previous results [6, 15, 16], total body weight and epididymal fat pad mass of Agt-KO mice were significantly lower than those of WT controls. Re-expressing Agt solely in adipose tissue through the use of an adipocyte-specific promoter increased both body and fat pad weights relative to those of Agt-KO mice (8.5% and 20%, resp), but neither change was statistically significant. It is worth noting that mice re-expressing Agt only in adipose tissue still exhibited Agt levels that were significantly lower than in WT due to lack of contribution from the endogenous gene, a point reflected in their intermediate levels of both adipose mass (Table 1) and blood pressure [6]. Although mice overexpressing Agt in adipose tissue (aP2-Agt) exhibited only a modest difference in body weight (50 g, WT vs 52 g, aP2-Agt), adiposity was significantly increased (∼ 50% greater than WT) as evidenced by the weight of epididymal fat pads (P < .05).
Table 1.
Body weight, fat pad weight, and blood parameters in 28-wk-old WT, Agt-KO, aP2-Agt/Agt-KO, and aP2-Agt mice.
| WT | Agt-KO | aP2-Agt/Agt-KO | aP2-Agt | |
| (n = 10) | (n = 8) | (n = 9) | (n = 9) | |
| Body weight (g) | 50.0 ± 2.4b | 35.0 ± 0.9a | 38.0 ± 1.1a | 52.0 ± 2.8b |
| Epididymal fat weight (g) | 1.1 ± 0.18b | 0.4 ± 0.02a | 0.5 ± 0.18a | 1.6 ± 0.15c |
| Leptin (ng/mL) | 5.7 ± 0.1b | 4.0 ± 0.05a | 5.5 ± 0.06b | 8.3 ± 0.1c |
| Insulin (ng/mL) | 1.2 ± 0.03b | 0.6 ± 0.03a | 0.8 ± 0.03a | 2.3 ± 0.1c |
| Resistin (ng/mL) | 43.4 ± 0.7b | 8.7 ± 1.3a | 42.0 ± 1.6b | 44.3 ± 0.1b |
| Adiponectin (μg/mL) | 11.0 ± 1.7a | 19.1 ± 1.1b | 11.2 ± 2.1a | 11.0 ± 2.1a |
Values with different letters are significantly different (P < .05); mean ± SEM; n: number of animals.
We next evaluated the effects of manipulating adipose Agt production on plasma leptin [23] and insulin levels, two hormones known to be involved in regulation of energy homeostasis [23]. As shown in Table 1, the plasma levels of both leptin and insulin were significantly decreased in Agt-KO mice compared to those in WT mice. Re-expression of Agt solely in adipose tissue (aP2-Agt/Agt-KO) restored leptin to WT levels, despite the lack of a marked increase in adiposity (Table 1). Insulin levels were modestly (25%) but not significantly increased by Agt re-expression in adipose tissue. Increasing adipose tissue Agt expression beyond wild-type levels by driving its expression with an adipocyte-specific promoter (aP2-Agt) elicited an approximately 50% increase in plasma leptin levels and almost doubled circulating insulin levels, both of which were statistically significant and which occurred despite similar glucose levels in these (and all) groups of mice (data not shown).
To further define potential effects of the adipose RAS on insulin sensitivity we measured levels of two plasma adipokines, adiponectin, and resistin, that are widely used as markers of insulin sensitivity and resistance, respectively [24–26]. Circulating adiponectin levels increased by 73% with targeted deletion of Agt, while plasma resistin concentration fell by almost 80% (Table 1). When Agt expression was restored specifically to adipose tissue, the levels of both hormones returned to levels not significantly different from WT. However, neither adiponectin nor resistin concentrations were affected by overexpression of Agt in adipose tissue of WT mice, despite considerable hyperinsulinemia and hyperleptinemia.
Effects of adipose Agt on protein levels of RAS components in adipose tissue and kidney
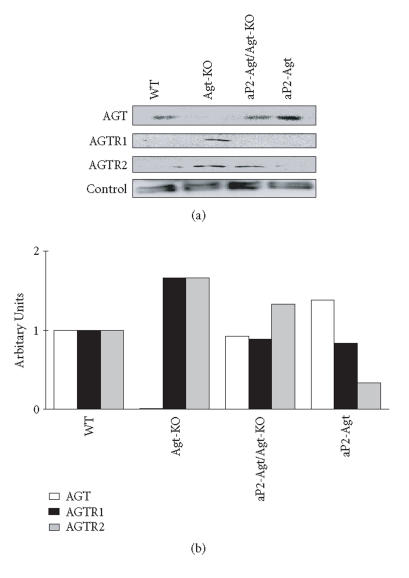
We previously established that blood pressure increases with adipose expression of Agt and that aP2-Agt mice are hypertensive [6]. Because activation of the renal RAS has been linked to hypertension [27], and because Ang II can exert both negative and positive feedback regulation of RAS components [27–29], we determined if altering adipose Ang II activated the intrarenal RAS, which could highlight a possible mechanism for hypertension displayed in aP2-Agt mice. We also evaluated the differential expression and regulation of Ang II receptors within adipose tissue in response to paracrine changes in Ang II levels. Levels of both AGTR1 and AGTR2 receptors are nearly doubled in adipose tissue of Agt-KO mice. With re-expression of Agt in adipose tissue, AGTR1 levels completely returned to WT levels while AGTR2 content was only partially restored, suggesting a compensatory response to the loss of Ang II (Figure 1). Elevating adipose Agt expression in WT mice is associated with reduced AGTR2 but normal AGTR1 levels, indicating a potential feedback mechanism to control AGTR2 receptor expression in accordance with local Ang II production. Consistent with data in Figure 1, we previously reported that overexpressing Agt in adipose tissue increased AGT content by ∼ 50% over WT [6].
Figure 1.
Expression of RAS proteins in adipose tissue of WT, Agt-KO, aP2-Agt/Agt-KO, and aP2-Agt mice. Representative immunoblots of AGT, AGTR1, and AGTR2 proteins are shown. A 95 kD protein is included as an internal loading control. The bar graph represents average expression of RAS proteins from two Western blots for two different experiments (n = 2).
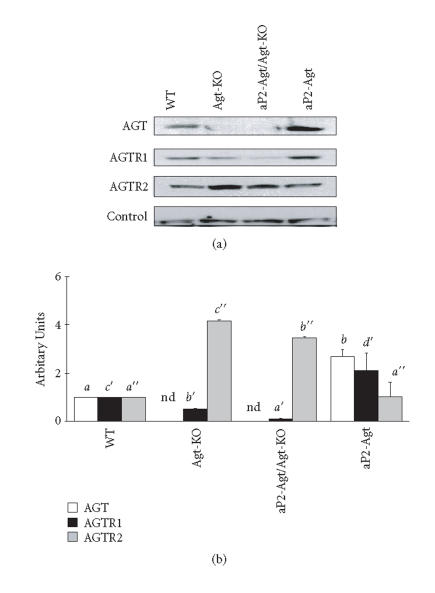
Expression and regulation of these RAS components revealed a distinct profile in kidney (Figure 2). Renal AGT levels were significantly elevated when the Agt gene was overexpressed in adipose tissue, more than doubling the level of AGT protein found in WT kidneys. This upregulation was tissue-specific, as hepatic AGT did not differ between WT and aP2-Agt animals (data not shown). AGTR1 protein was reduced in both Agt-KO and aP2-Agt/Agt-KO mice, while AGTR2 was markedly elevated in both KO models by approximately 3.5-fold. Overexpression of Agt in adipose tissue increased renal AGTR1 levels but had no effect on AGTR2. Therefore AGTR1 protein in kidney appeared to correlate inversely with circulating levels of AGT. On the other hand, AGTR2 in kidney responded only to absence or very low levels of circulating AGT, where its upregulation may be important in correcting the renal morphological defects seen in Agt-KO animals. Activation of the intrarenal RAS in aP2-Agt mice, as manifested by upregulation of both AGT and AGTR1 protein levels, confirms the ability of the adipose RAS to regulate not only adipocyte physiology but also that of distal tissues. These effects may cause at least part of the hypertension observed in this model.
Figure 2.
Expression of RAS proteins in the kidney of WT, Agt-KO, aP2-Agt/Agt-KO, and aP2-Agt mice. Representative immunoblots of AGT, AGTR1, and AGTR2 proteins are shown. A 75 kD protein is included as an internal loading control. Bars depict mean ± SEM of the quantitated AGT, AGTR1, and AGTR2 protein bands, independently obtained from 3 different experiments. Values with different letters are significantly different (P < .05); nd: not detected.
DISCUSSION
We used genetically manipulated mice to determine if altering activity of the adipose RAS in turn impacts physiological parameters relevant to obesity and its co-morbidities hypertension and diabetes. This work was driven by our previous finding that adipose Agt mRNA levels varied considerably between human patients [30], and by the potential for these changes to alter sensitivity to both diabetes and hypertension and thus susceptibility to disease. We first examined changes in adiposity and systemic markers of insulin sensitivity. Mice lacking Ang II, via targeted deletion of Agt, displayed significantly lower plasma levels of insulin, resistin, and leptin and increased levels of adiponectin, all of which suggest increased insulin sensitivity. Therefore the consequences of systemic loss of Ang II are consistent with clinical and laboratory findings showing that RAS antagonism, either by receptor blockade or ACE inhibition, improves insulin sensitivity [31–33] and increases plasma adiponectin levels [33]. These findings differ from those previously reported for the same mice at 6 weeks of age [6], and suggest that the changes in insulin and leptin status in response to Agt deletion are indirect effects that develop over time.
Re-expressing Agt exclusively in adipose tissue produced a modest increase in plasma AGT concentration (∼ 25% of WT) that was sufficient (as reported here) to revert plasma leptin, adiponectin, and resistin to wild-type levels. These changes occurred in the absence of significant increases in either insulin levels or fat mass, although a trend for increased adiposity was evident. Restoration of plasma leptin could be attributable to a subtle gain of adipose mass [23], but it could also be due to direct paracrine effects of Ang II on transcription of the leptin gene in adipocytes, as we previously reported for murine 3T3-L1 [4] and primary human adipocytes [34]. Whether Ang II also directly regulates the expression of other adipokines, such as adiponectin and resistin, is unknown. If so, subtle changes in adipose Agt expression could therefore alter insulin sensitivity in a paracrine or autocrine manner by modifying the production of adipokines that act on distal insulin-sensitive target tissues.
Elevating the production of Agt in adipose tissue of WT mice caused both hyperinsulinemia and hyperleptinemia, a metabolic phenotype that was in many ways the opposite of Agt deletion. Adiposity increased by ∼ 50% in these transgenic mice, consistent with the stimulation of lipogenesis by Ang II [4] and by Agt overexpression [6]. This change in adiposity was not reflected in body weight, perhaps due to differential, depot-specific actions of Ang II on adipocytes [35]. It is worth noting that the measure of adiposity employed here was based solely on the weight of the epididymal adipose depot; it is plausible that other fat depots responded differently. In the light of an almost 2-fold increase in plasma insulin levels, it was surprising that adiponectin and resistin did not differ between WT and aP2-Agt mice. This may indicate differential sensitivity of different metabolic markers to Agt manipulation. Further studies will be necessary to determine the basis for this unexpected response.
Despite differences in insulin levels, plasma glucose was not statistically impacted by genotype (not shown). One potential explanation is that the hyperinsulinemia of aP2-WT mice is sufficient to maintain euglycemia. Conversely, Agt-KO mice maintain euglycemia because of increased insulin sensitivity and a concomitant reduction in insulin secretion. These findings need to be further explored using thorough in vivo measures of insulin sensitivity and glucose tolerance.
Our second goal was to determine how altering adipose Agt production impacted RAS regulation distally through endocrine actions, as Ang II exerts various effects on the expression of major component genes of the RAS by feedback mechanisms [28, 29, 35–37]. We focused on kidney due to its primary role in blood pressure regulation and the fact that aP2-Agt mice are hypertensive. We found that AGTR1 protein was lower in Agt-KO mice than in wild type. This contrasts with a previous report showing upregulation of renal Agtr1 mRNA and protein expression in Agt-KO mice, raising the possibility that differences in RAS regulation in Agt null mice may also be associated with genetic background [37, 38]. We also found that renal levels of AGTR1 were significantly increased in aP2-Agt mice compared with WT mice, a result that is somewhat surprising in light of the fact that chronic Ang II infusion in rats did not alter whole kidney AGTR1 content [39]. Although we did not specifically measure plasma Ang II concentrations in the current study, we predict that they would be elevated due to the increase in circulating AGT levels and the fact that aP2-Agt mice are hypertensive [6]. In contrast, the observation that renal AGTR2 receptor protein expression was not altered in response to elevated Agt was expected [39].
Multiple studies have demonstrated a somewhat paradoxical regulation of Agt by Ang II, in which hormone substrate levels are actually increased at both the mRNA and protein levels by bioactive hormone. We demonstrate here that elevating adipose Agt expression activates AGT synthesis in kidney, presumably due to an increase in plasma Ang II [40]. Although not explicitly measured, we expect that the increase in renal Agt expression translates into elevated intrarenal Ang II content, which, coupled with increased renal AGTR1, may contribute to the hypertension documented in aP2-Agt mice [41–43]. Brain-specific overexpression of Agt also has been shown to increase blood pressure [38]. Therefore it is plausible that hypertension of aP2-Agt mice is due to one or a combination of elevated circulating Ang II, activation of the intrarenal RAS Ang II, and/or activation of other local RAS such as that in the brain. Further studies will be necessary to determine the contribution(s) of each of these tissues and the mechanisms mediating this regulation.
In conclusion, we have demonstrated that the adipose RAS extends its physiological actions well beyond the local environment of the adipocyte, impacting maintenance of blood pressure and insulin sensitivity in the whole animal. Given that RAS activity in adipose tissue varies with adiposity, these findings highlight the potential role played by the adipose RAS in the metabolic syndrome and underscore the importance of continued investigation into this possibility.
ACKNOWLEDGMENTS
This work was supported in part by the American Heart Association, National Center (NMM), the TN Agricultural Experiment Station (NMM), The Physicians Medical Education and Research Foundation at Knoxville, TN (NMM and MSP), The University of Tennessee Center of Excellence for Genomics and Bioinformatics (JHK), and by the Office of Biological and Environmental Research, US Department of Energy, Under contract DE-AC05-00OR22725 with UT-Battelle LLC, the managing organization of ORNL for the US DOE (BHV).
References
- 1.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacological Reviews. 2000;52(1):11–34. [PubMed] [Google Scholar]
- 2.Leung PS. The peptide hormone angiotensin II: its new functions in tissues and organs. Current Protein and Peptide Science. 2004;5(4):267–273. doi: 10.2174/1389203043379693. [DOI] [PubMed] [Google Scholar]
- 3.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system—an endocrine and paracrine system. Endocrinology. 2003;144(6):2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 4.Jones BH, Standridge MK, Moustaid N. Angiotensin II increases lipogenesis in 3T3-L1 and human adipose cells. Endocrinology. 1997;138(4):1512–1519. doi: 10.1210/endo.138.4.5038. [DOI] [PubMed] [Google Scholar]
- 5.Darimont C, Vassaux G, Ailhaud G, Negrel R. Differentiation of preadipose cells: paracrine role of prostacyclin upon stimulation of adipose cells by angiotensin-II. Endocrinology. 1994;135(5):2030–2036. doi: 10.1210/endo.135.5.7956925. [DOI] [PubMed] [Google Scholar]
- 6.Massiera F, Bloch-Faure M, Ceiler D, et al. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. The FASEB Journal. 2001;15(14):2727–2729. doi: 10.1096/fj.01-0457fje. [DOI] [PubMed] [Google Scholar]
- 7.Engeli S, Schling P, Gorzelniak K, et al. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? International Journal of Biochemistry and Cell Biology. 2003;35(6):807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 8.Engeli S, Sharma AM. Emerging concepts in the pathophysiology and treatment of obesity-associated hypertension. Current Opinion in Cardiology. 2002;17(4):355–359. doi: 10.1097/00001573-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Gorzelniak K, Engeli S, Janke J, Luft FC, Sharma AM. Hormonal regulation of the human adipose-tissue renin-angiotensin system: relationship to obesity and hypertension. Journal of Hypertension. 2002;20(5):965–973. doi: 10.1097/00004872-200205000-00032. [DOI] [PubMed] [Google Scholar]
- 10.Voy BH, Kim S, Urs S, Joshi R, Moustaid-Moussa N. The adipose renin angiotensin system: genetics, regulation and physiological function. In: Moustaid-Moussa N, Berdanier CD, editors. Genomics and Proteomics in Nutrition. New York, NY: Marcel Dekker; 2004. [Google Scholar]
- 11.Hainault I, Nebout G, Turban S, Ardouin B, Ferre P, Quignard-Boulange A. Adipose tissue-specific increase in angiotensinogen expression and secretion in the obese (fa/fa) Zucker rat. American Journal of Physiology. Endocrinology and Metabolism. 2002;282(1):E59–E66. doi: 10.1152/ajpendo.2002.282.1.E59. [DOI] [PubMed] [Google Scholar]
- 12.Tsai Y-S, Kim H-J, Takahashi N, et al. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ. Journal of Clinical Investigation. 2004;114(2):240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahmouni K, Mark AL, Haynes WG, Sigmund CD. Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. American Journal of Physiology. Endocrinology and Metabolism. 2004;286(6):E891–E895. doi: 10.1152/ajpendo.00551.2003. [DOI] [PubMed] [Google Scholar]
- 14.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. American Journal of Physiology. Regulatory Integrative and Comparative Physiology. 2004;287(4):R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Urs S, Massiera F, et al. Effects of high-fat diet, angiotensinogen (agt) gene inactivation, and targeted expression to adipose tissue on lipid metabolism and renal gene expression. Hormone and Metabolic Research. 2002;34(11-12):721–725. doi: 10.1055/s-2002-38263. [DOI] [PubMed] [Google Scholar]
- 16.Massiera F, Seydoux J, Geloen A, et al. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology. 2001;142(12):5220–5225. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- 17.van Harmelen V, Elizalde M, Ariapart P, et al. The association of human adipose angiotensinogen gene expression with abdominal fat distribution in obesity. International Journal of Obesity and Related Metabolic Disorders. 2000;24(6):673–678. doi: 10.1038/sj.ijo.0801217. [DOI] [PubMed] [Google Scholar]
- 18.Giacchetti G, Faloia E, Sardu C, et al. Gene expression of angiotensinogen in adipose tissue of obese patients. International Journal of Obesity and Related Metabolic Disorders. 2000;24(suppl 2):S142–S143. doi: 10.1038/sj.ijo.0801305. [DOI] [PubMed] [Google Scholar]
- 19.Van Harmelen V, Ariapart P, Hoffstedt J, Lundkvist I, Bringman S, Arner P. Increased adipose angiotensinogen gene expression in human obesity. Obesity Research. 2000;8(4):337–341. doi: 10.1038/oby.2000.40. [DOI] [PubMed] [Google Scholar]
- 20.Faloia E, Gatti C, Camilloni MA, et al. Comparison of circulating and local adipose tissue renin-angiotensin system in normotensive and hypertensive obese subjects. Journal of Endocrinological Investigation. 2002;25(4):309–314. doi: 10.1007/BF03344010. [DOI] [PubMed] [Google Scholar]
- 21.Tanimoto K, Sugiyama F, Goto Y, et al. Angiotensinogen-deficient mice with hypotension. Journal of Biological Chemistry. 1994;269(50):31334–31337. [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Ahima RS, Flier JS. Leptin. Annual Review of Physiology. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. Journal of Biological Chemistry. 2003;278(4):2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 25.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Medicine. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 26.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 27.Navar LG. The intrarenal renin-angiotensin system in hypertension. Kidney International. 2004;65(4):1522–1532. doi: 10.1111/j.1523-1755.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 28.Ingelfinger JR, Jung F, Diamant D, et al. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. American Journal of Physiology. 1999;276(2 pt 2):F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 29.Schunkert H, Ingelfinger JR, Hirsch AT, et al. Evidence for tissue-specific activation of renal angiotensinogen mRNA expression in chronic stable experimental heart failure. Journal of Clinical Investigation. 1992;90(4):1523–1529. doi: 10.1172/JCI116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones BH, Standridge MK, Taylor JW, Moustaid-Moussa N. Angiotensinogen gene expression in adipose tissue: analysis of obese models and hormonal and nutritional control. American Journal of Physiology. 1997;273(1 pt 2):R236–R242. doi: 10.1152/ajpregu.1997.273.1.R236. [DOI] [PubMed] [Google Scholar]
- 31.McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. American Journal of Cardiology. 2003;91(12 suppl 1):30H–37H. doi: 10.1016/s0002-9149(03)00432-6. [DOI] [PubMed] [Google Scholar]
- 32.Umeda M, Kanda T, Murakami M. Effects of angiotensin II receptor antagonists on insulin resistance syndrome and leptin in sucrose-fed spontaneously hypertensive rats. Hypertension Research. 2003;26(6):485–492. doi: 10.1291/hypres.26.485. [DOI] [PubMed] [Google Scholar]
- 33.Furuhashi M, Ura N, Higashiura K, et al. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42(1):76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- 34.Kim S, Whelan J, Claycombe K, Reath DB, Moustaid-Moussa N. Angiotensin II increases leptin secretion by 3T3-L1 and human adipocytes via a prostaglandin-independent mechanism. Journal of Nutrition. 2002;132(6):1135–1140. doi: 10.1093/jn/132.6.1135. [DOI] [PubMed] [Google Scholar]
- 35.Giacchetti G, Sechi LA, Griffin CA, Don BR, Mantero F, Schambelan M. The tissue renin-angiotensin system in rats with fructose-induced hypertension: overexpression of type 1 angiotensin II receptor in adipose tissue. Journal of Hypertension. 2000;18(6):695–702. doi: 10.1097/00004872-200018060-00006. [DOI] [PubMed] [Google Scholar]
- 36.Nyui N, Tamura K, Mizuno K, et al. Stretch-induced map kinase activation in cardiomyocytes of angiotensinogen-deficient mice. Biochemical and Biophysical Research Communications. 1997;235(1):36–41. doi: 10.1006/bbrc.1997.6706. [DOI] [PubMed] [Google Scholar]
- 37.Tamura K, Umemura S, Sumida Y, et al. Effect of genetic deficiency of angiotensinogen on the renin-angiotensin system. Hypertension. 1998;32(2):223–227. doi: 10.1161/01.hyp.32.2.223. [DOI] [PubMed] [Google Scholar]
- 38.Lochard N, Silversides DW, Van Kats JP, Mercure C, Reudelhuber TL. Brain-specific restoration of angiotensin II corrects renal defects seen in angiotensinogen-deficient mice. Journal of Biological Chemistry. 2003;278(4):2184–2189. doi: 10.1074/jbc.M209933200. [DOI] [PubMed] [Google Scholar]
- 39.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in ANG II-induced hypertensive rats. American Journal of Physiology. Renal Physiology. 2002;282(1):F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. Journal of the American Society of Nephrology. 2001;12(3):431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. American Journal of Physiology. Renal Physiology. 2004;286(5):F965–F971. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 42.Navar LG, Harrison-Bernard LM. Intrarenal angiotensin II augmentation in angiotensin II dependent hypertension. Hypertension Research. 2000;23(4):291–301. doi: 10.1291/hypres.23.291. [DOI] [PubMed] [Google Scholar]
- 43.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43(5):1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]