Abstract
The medial geniculate nucleus of the thalamus responds to auditory information and is a critical part of the neural circuitry underlying aversive conditioning with auditory signals for shock. Prior work has shown that lesions of this brain area selectively disrupt conditioning with auditory stimuli and that neurons in the medial geniculate demonstrate plastic changes during fear conditioning. However, recent evidence is less clear as to whether or not this area plays a role in the storage of auditory fear memories. In the current set of experiments rats were given infusions of protein or messenger RNA (mRNA) synthesis inhibitors into the medial geniculate nucleus of the thalamus 30 min prior to auditory fear conditioning. The next day animals were tested to the auditory cue and conditioning context. Results showed that rats infused with either inhibitor demonstrated less freezing to the auditory cue 24 h after training, while freezing to the context was normal. Autoradiography confirmed that the doses used were effective in disrupting synthesis. Taken together with prior work, these data suggest that the formation of fear memory requires the synthesis of new protein and mRNA at multiple brain sites across the neural circuit that supports fear conditioning.
Keywords: Pavlovian fear conditioning, anisomycin, medial geniculate nucleus, rat, distributed plasticity, consolidation
Abbreviations: ACSF, artificial cerebrospinal fluid; ANI, anisomycin; DMSO, dimethyl sulfoxide; DRB, 5,6-dichlorobenzimidazole 1-β-D-ribofuranoside; ERK/MAPK, extracellular-signal-related/mitogen-activated protein kinase; IEG, immediate early gene; LTP, long-term potentiation; MGm, medial division of medial geniculate thalamic nucleus; mRNA, messenger RNA; TIA, training-induced neuronal activity
A considerable amount of recent research has focused on understanding the neural circuitry underlying Pavlovian fear conditioning. Through lesion and pharmacological studies it has become evident that the amygdala is critical for the acquisition of conditioned fear (Blanchard and Blanchard, 1972; Miserendino et al., 1990; Helmstetter, 1992; Phillips and LeDoux, 1992; Helmstetter and Bellgowan, 1994). Similar studies have shown that the formation of fear memories depends on gene expression and protein synthesis in amygdala neurons (Bailey et al., 1999; Schafe and LeDoux, 2000; Maren et al., 2003). Given that the long-term synaptic changes that support memory storage are believed to require protein synthesis (Davis and Squire, 1984), these data are often taken as evidence that the amygdala is the site of storage for fear memories (Fanselow and LeDoux, 1999; Maren and Quirk, 2004).
Although it is clear that the amygdala is critically involved in the formation of fear memory, other brain regions are also known to be important in fear conditioning. For example, the dorsal hippocampus is necessary for contextual fear conditioning (Kim and Fanselow, 1992; Phillips and LeDoux, 1992) likely because it supports a representation of the conditioning context (Rudy and O’Reilly, 2001). Blockade of gene expression and protein synthesis in the dorsal hippocampus prevents contextual memory formation (Barrientos et al., 2002; Ahi et al., 2004; Gafford et al., 2004). Another set of structures important for fear conditioning is the medial geniculate thalamic nucleus (MGm), posterior intralaminar nucleus (PIN), and supra-geniculate (SG) nucleus of the thalamus, which can collectively be referred to as the auditory thalamus. Cells in this region respond to auditory information and are thought to be involved in conditioning when auditory cues are used as conditional stimuli. Previous work has shown that neurons in the auditory thalamus make reciprocal connections to the amygdala (LeDoux et al., 1990) and that high frequency stimulation of this region is capable of producing long-term potentiation (LTP) in amygdala neurons (Clugnet and LeDoux, 1990). Lesion studies have shown that neurons in the MGm are necessary for conditioning with auditory cues (e.g. LeDoux et al., 1984, 1986; Jarrell et al., 1986; McCabe et al., 1993; Campeau and Davis, 1995). However, lesion experiments cannot adequately answer whether the MGm simply acts as a relay structure for auditory information or whether it plays a more active role in forming the association.
Several lines of evidence suggest that the MGm is not simply a relay for auditory stimuli. Early work by Gabriel et al. (1975) demonstrated that cells in MGm exhibit neuronal activity specific to discriminative avoidance training (i.e. increased firing to CS+, but not CS−). Subsequently, several other experiments have observed changes in the firing properties of MGm neurons during the course of Pavlovian conditioning with auditory cues (Edeline and Weinberger, 1992; McEchron et al., 1996). Evidence indicates that these changes in MGm neurons during conditioning are not likely the result of sensitization or due to an increase in general excitability (i.e. sensory processing), but rather are specific to the conditioning process (Edeline and Weinberger, 1992; McEchron et al., 1996). Furthermore, high frequency stimulation of the major input into MGm results in LTP of these neurons (Gerren and Weinberger, 1983). LTP is the leading cellular model of long-term memory and the fact that MGm neurons show LTP suggests that these cells may play a role in memory formation and storage.
Despite the evidence demonstrating that neurons in MGm show conditioning specific changes, relatively few studies have examined the effects of acute and reversible MGm disruption in behaving animals. A series of studies by Poremba and Gabriel (1997a,b, 2001) investigated the role of the MGm, along with the amygdala and cingulate cortex, in discriminative avoidance learning in rabbits. These studies found that the MGm is critical for learning avoidance behavior and is also critical for the development of training-induced neuronal activity (TIA) in both the amygdala and cingulate cortex (Poremba and Gabriel, 1997a). Likewise, lesion or temporary inactivation of the amygdala prevents learning of the same task and TIA in both the cingulate cortex and MGm (Poremba and Gabriel 1997b, 2001; Talk et al., 2004). These data suggest that the MGm is a critical node in the neural circuit that supports avoidance conditioning in rabbits.
A handful of experiments have examined the effects of intra-MGm disruption of various intracellular processes on auditory fear conditioning. Similar to Poremba and Gabriel (1997b), work in rats with fear conditioning has demonstrated that inactivation of the amygdala with muscimol, a treatment known to disrupt fear memory (Helmstetter and Bellgowan, 1994; Muller et al., 1997) prevents training-related neuronal activity in MGm neurons (Maren et al., 2001). Another report by (Maren and colleagues 2003) showed that infusion of an N-methyl- D -aspartic acid (NMDA) receptor antagonist into the MGm before conditioning disrupted freezing to the auditory cue, but not to the context. However, the same study showed that intra-MGm infusion of a broad-spectrum protein kinase inhibitor or a protein synthesis inhibitor had no effect. The authors concluded that although synaptic transmission in MGm is critical for acquisition of auditory fear memory, local plasticity in these same synapses is not necessary. A recent study by Apergis-Schoute et al. (2005) also showed that post-training infusion of anisomycin (ANI) into the MGm had no effect on the consolidation of auditory fear memory. Interestingly, infusion of a messenger RNA (mRNA) synthesis blocker or extracellular-signal-related/mitogen-activated protein kinase (ERK/MAPK) inhibitor into MGm after training reduced freezing to the auditory cue when tested 24 h later. Based on these findings, the authors propose that transcription in MGm neurons mediates local presynaptic protein synthesis in the lateral amygdala (Apergis-Schoute et al., 2005). This could potentially explain why protein synthesis inhibitors are effective when infused into the amygdala, but not into the MGm (Schafe and LeDoux, 2000; Maren et al., 2003).
There may be several other interpretations of the lack of effect seen when ANI is infused into the MGm. It is possible that the proteins made within MGm that are critical to memory formation are made during a time period that is not covered by an immediate post-training infusion. Another possibility is that the details of translational disruption with ANI differ from other inhibitors that are effective when delivered into MGm. The current experiments were designed to test the effects of pre-training intra-MGm infusion of protein and mRNA synthesis inhibitors on the formation of fear memory. If the formation of auditory fear memory is independent of protein synthesis in the MGm, then protein synthesis inhibitors should not disrupt memory regardless of when they are infused (i.e. pre or post-training). However, if ANI is effective at the pre-training time point it would support the idea that MGm plays some role in the formation or storage of auditory fear memories.
EXPERIMENTAL PROCEDURES
Subjects
The subjects were 51 adult male Long-Evans rats (300–375 g) obtained from Harlan (Madison, WI, USA). The animals were housed individually in stainless steel cages and had free access to water and rat chow throughout the experiments. The colony room was maintained on a 14:10-h light/dark cycle. All experimental procedures were approved by the University of Wisconsin–Madison Animal Care and Use Committee in accordance with National Institutes of Health and international guidelines for the use of animals in research.
Surgery and histology
Subjects were adapted to handling and transportation for at least 2 days prior to surgery. Each rat was prepared with bilateral stainless steel 26-gauge guide cannulae (Plastics One, Roanoke, VA, USA) aimed at the medial division of the medial geniculate nucleus of the thalamus using stereotaxic coordinates (5.3 mm posterior, ±2.8 mm lateral, 5.6 mm ventral) relative to bregma (Paxinos and Watson, 1986). Cannulae were secured to the skull with the aid of stainless steel screws and epoxy. A stainless steel obdurator remained in place when the rats were not being injected to prevent the guide cannulae from becoming occluded. The rats were allowed to recover for at least 7 days before behavioral testing began.
After behavioral testing, rats were overdosed with an i.p. injection of sodium pentobarbital–ethanol solution and perfused transcardially with saline followed by 10% buffered formalin. Frozen sections (40-μm) were collected throughout the medial geniculate, mounted on slides, and stained with Cresyl Violet. Injection sites were then determined with the aid of a rat brain atlas (Paxinos and Watson, 1986).
Apparatus
Fear conditioning took place in a set of four identical observation chambers (28×20.5×21 cm) constructed of Plexiglas and stainless steel. The floor of each chamber was composed of stainless steel rods spaced 1.5 cm apart through which the footshock could be delivered. Each chamber was connected to its own shock generator-scrambler (Grason-Stadler, West Concord, MA, USA), and was illuminated by a 7.5-W white light bulb positioned directly above the chamber. Ventilation fans provided a constant background of approximately 60 dB. Testing to the auditory cue took place in a separate set of chambers. These chambers had a slanted wall on one side and rounded wall on the other. The floors were made of Plexiglas and fans provided a background noise of approximately 58dB. All chambers were housed in sound attenuating boxes. The training chambers were cleaned with a 5% ammonium hydroxide solution before each set of animals, while the boxes where auditory testing took place were cleaned with a 2% acetic acid solution.
Infusion procedure and drugs
In all cases rats received bilateral infusions into the MGm. The total volume of the infusion (0.5 μl/side) was given over 60 s and the injection cannula remained in place for an additional 90 s to ensure diffusion. The injection cannulae were cut to extend approximately 0.5 mm beyond the guide cannulae. Rats were returned to their home cages after infusions. ANI (125 μg/μl, Sigma Chemical, St. Louis, MO, USA) was dissolved in HCl and diluted with artificial cerebrospinal fluid (ACSF). A small amount of NaOH was added to neutralize the pH of the solution. 5,6-Dichlorobenzimidazole 1-β- D -Ribofuranoside (DRB; Sigma Chemical) was diluted with 100% dimethyl sulfoxide (DMSO) to a concentration of 200 ng/μl.
Behavioral procedures
Following recovery from surgery, all subjects were exposed to the restraint and injection procedure for the 3 days immediately prior to the training. During this time each rat was transported to the laboratory, wrapped in a small towel, and gently restrained by hand for several minutes. The obdurators were removed and the scalp was cleaned with Betadine. The infusion pump to be used for the intracranial injections was activated during these periods.
For conditioning, all rats were placed in the training chambers and after 6 min they were given four pairings of white noise (10 s/72 dB) and shock (1 s/1.3 mA) 90 s apart. Rats were removed from the chambers 4 min after the final shock. Thirty minutes prior to training, the animals were infused with DRB (n=7) or DMSO (n=9) in experiment 1, and ANI (n=8) or ACSF (n=7) in experiment 2. Approximately 24 h after training rats were tested for retention of the white noise cue and training context in a counterbalanced order. The testing sessions were separated by approximately 4 h for all animals. For context testing, rats were placed in training chambers for 15 min. For auditory cue testing, rats were placed in the shifted context and exposed to the white noise for 5 min after 6 min of baseline. In experiments 1 and 2 the rats were removed from the chambers 4 min after termination of the white noise and returned to their home cages. In experiment 3, rats were given ACSF (n=7) or ANI (n=7) and put through the same training procedure 30 min later. However, the rats in this experiment were tested for performance to the auditory cue 15 min, 4 h, and 72 h after training. The auditory test sessions involved a 2 min presentation of the white noise after a 6-min baseline. These tests all occurred in Context B. The rats were removed from the chambers 1 min after termination of the auditory CS. All of the behavioral testing was recorded on videotape.
Data analysis
The strength of conditional responding during the sessions was determined by the amount of time engaged in freezing behavior. For each session, an observer blind to the experimental conditions scored each subjects behavior from videotape once every 4 s for the duration of the sessions. Freezing was defined as the absence of all body movement except that required for respiration. All other behavior was scored as general activity. Freezing during training was separated into baseline, shock, and post-shock time periods. Freezing during the context test sessions was expressed as a percentage for the entire 15 min time period, while freezing during the white noise tests were separated into, baseline, white noise, and post–white noise periods. Repeated measures analysis of variance and Student’s t-test were used to test for differences between groups.
Autoradiography procedure
Eight male Long-Evans rats that served as subjects in the behavioral experiments were used for the autoradiography procedure. Three of the rats received infusions (0.5 μl) of ACSF in one hemisphere and ANI (125 μg/μl) in the opposite hemisphere (counterbalanced). Thirty minutes later these rats received infusions of 1.0 μl/side of [U-14C]-leucine (50 μCi/ml, 306 mCi/mmol specific activity, Amersham Biosciences, Piscataway, NJ, USA) into both hemispheres. The other five rats received infusions (0.5 μl/side) of 100% DMSO into one side of the MGm or DRB (20 ng/μl) into the other. These rats were then given 1 μl infusions of [U-14C]-uridine (50 μCi/ml, 474 mCi/mmol specific activity, Amersham Biosciences) into both hemispheres. One hour later, all rats were killed and perfused with saline followed by 10% buffered formalin. Brains were stored overnight in sucrose formalin and the following day the tissue was sectioned by taking 40 μm slices beginning in the area ~2 mm anterior to the medial geniculate nucleus and continuing to the area ~2 mm posterior to the same structure. The sections were then mounted on slides and exposed to autoradiographic film (Hyperfilm MP film; Amersham Biosciences) for 2 wk. Densitometry was performed on developed films using a computerized image processing system (MCID; Imaging Research, Inc., St. Catherine’s, ON, Canada).
RESULTS
Experiment 1
Out of the 51 animals in the study, 5 were removed due to misplaced cannula (see Figs. 1C and 2C for placements) and one was removed when the guide cannula became dislodged before testing was complete. Data for experiment 1 are depicted in Fig. 1. In this experiment, rats received infusion of DRB or DMSO into the MGm 30 min before training and were tested to the context and auditory cue 24 h later. We used a Student’s t-test to measure differences between to the two treatment groups. No differences were observed during the training session (Fig. 1A) indicating that the drug does not likely affect shock reactivity or performance of the freezing response (t values all <1.0). During the retention test session rats infused with DRB showed normal responding to the context (t(1,14)=.827, ns) but froze significantly less to the auditory cue, t(1,14)= 2.309, P<0.05 (Fig. 1B). The lack of an effect during the context test supports prior observations indicating that the MGm is selectively involved in conditioning with auditory signals (LeDoux et al., 1986; Campeau and Davis, 1995).
Fig. 1.
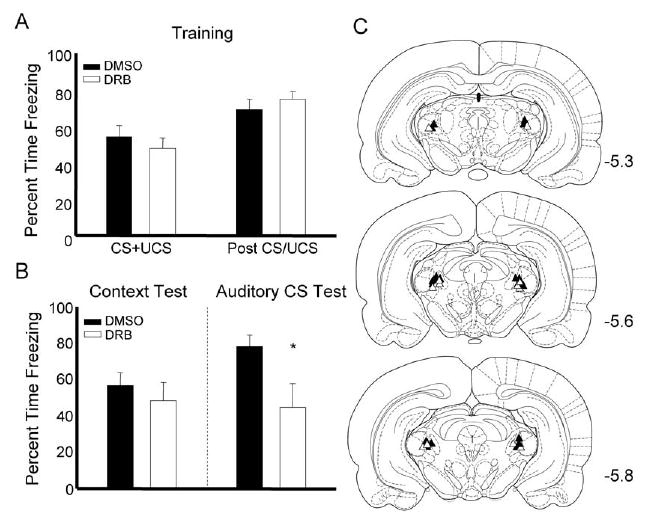
Auditory fear memory formation relies on the synthesis of mRNA in the auditory thalamus. (A) Freezing during the training session for rats infused with DMSO (black bars) or DRB (white bars). (B) Data from the test session show that animals treated with DRB freeze less during the auditory CS test. (C) Cannula placements for animals given DMSO (black triangles) or DRB (white triangles) in experiment 1; * P<0.05.
Fig. 2.
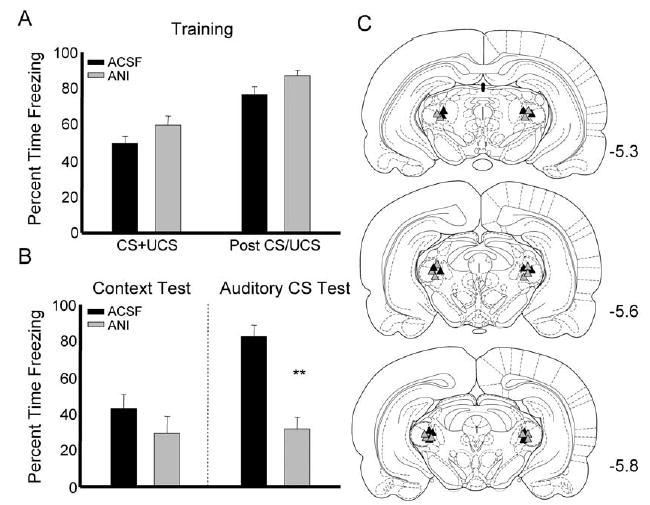
The formation of auditory fear memory requires protein synthesis in the auditory thalamus. (A) Percent time rats spent freezing during white noise-shock pairings and the period after for animals infused with ACSF (black bars) or ANI (gray bars). (B) Rats infused with ANI froze less during the auditory CS test, but showed intact freezing to the context. (C) Cannula placements for rats infused with ACSF (black triangles) or ANI (gray triangles) in experiment 2; ** P<0.01.
Experiment 2
In order to test the effect of protein synthesis inhibition on auditory fear memory, rats were infused with ANI into MGm 30 min before training. There were no significant differences between groups during the training session, although there was a non-significant trend for ANI-treated animals to freeze more during the period when white noise and shock were being paired and during the postshock period (Fig. 2A). Data from the test session showed that rats given ANI into the MGm froze significantly less than ACSF-treated rats during the auditory cue test (t(1,13)=4.136, P<0.01). Similar to the previous experiment, rats given ANI showed no difference from controls during the context test, t(1,13)=.815, ns (Fig. 2B).
Experiment 3
Experiment 3 was designed to assess the effects of ANI on short-term memory when given into the MGm. No differences were seen during the training session indicating that the drug did not affect performance during training (data not shown). Fig. 3B shows data from the test sessions that occurred at 15 min, 4 h, and 72 h after training. A repeated measure ANOVA, with group and time of testing as factors, was performed on the data during the time when the auditory CS was on and during the baseline for each testing session. This analysis showed a significant main effect of time (F(2,24)= 11.404, P<0.01), but no effect of group. Importantly, there was a significant time by group interaction for the 2 min of auditory testing (F(2,24)=14.291, P<0.01). t-Tests for the individual test sessions revealed significant decreases in performance of the ANI-treated rats only during the 72 h test, (t(1,12)= 3.743, P<0.01). Repeated measures ANOVA on the baseline freezing performance (data not shown) of the test session showed a significant effect of time (F(2,24)= 8.875, P<0.01). This effect was driven by a general decrease in baseline freezing as the testing went on. Importantly, there was no effect of group (F(1,12)= 1.024) and no significant group by time interaction (F(2,24)=.197) for the baseline data.
Fig. 3.
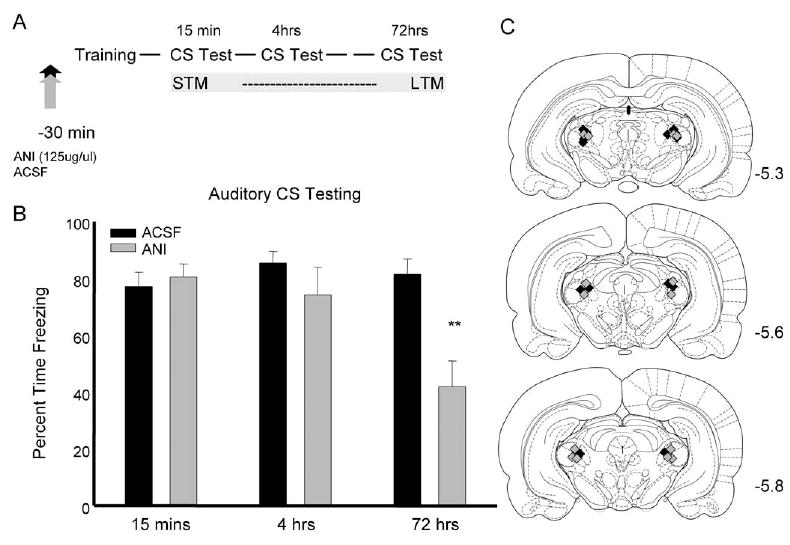
Blockade of protein synthesis prior to training blocks long-term, but not short-term, memory performance. (A) Design of the experiment indicating when the test sessions took place. (B) Percent time freezing during the three test sessions for rats infused with either ACSF (black bars) or ANI (gray bars) prior to training. Rats given ANI before training showed less freezing during the 72 h test only; ** P<0.01.
Experiment 4
Fig. 4 shows data from autoradiographic experiments designed to determine the effectiveness of the inhibitors used in the behavioral experiments. For these experiments rats were infused with ANI or DRB and ACSF or DMSO into the contralateral MGm. Thirty minutes later the ANI/ACSF-treated rats were given intra-MGm infusions of 14C-leucine, while DRB/DMSO-infused rats were administered 14C-uridine. Results showed that ANI treatment decreased rates of protein synthesis by approximately 75%, while DRB reduced mRNA synthesis rates by about 35% (Fig. 3A). Fig. 3B shows representative samples of autoradiographic images overlaid on Nissl-stained tissue from a rat given ANI and ACSF and a rat given DRB and DMSO. Dark areas on the images indicate areas where the radiolabel is being incorporated into protein and mRNA respectively (Fig. 3B).
Fig. 4.
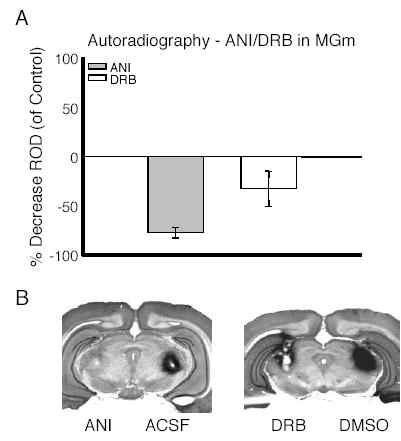
Infusion of DRB or ANI results in a decrease in rates of mRNA and protein synthesis respectively. (A) Percent decrease in relative optical density (ROD) compared with controls for rats treated with ANI or DRB. (B) Photomicrographs of two rats treated with ANI/ACSF and two rats given DRB/DMSO. Dark areas indicate sites where the radiolabel is being incorporated into protein or mRNA.
DISCUSSION
The currently accepted model of the critical neural circuit for fear conditioning has the MGm positioned as an input structure involved primarily in relaying information about auditory stimuli to the lateral nucleus of the amygdala, which has been regarded as the primary site of plasticity underlying fear conditioning (Fanselow and LeDoux, 1999; Maren and Quirk, 2004). Our data argue that the MGm may play a more active role in auditory fear memory formation, whereby the synthesis of protein and mRNA in MGm neurons is necessary for the formation of long-term associations between an auditory signal and shock. Our findings are in agreement with previous work showing that the consolidation of auditory fear memory requires the synthesis of mRNA in the medial geniculate nucleus (Apergis-Schoute et al., 2005). However, our observation that ANI delivered into the MGm also disrupted auditory fear memory is in contrast to other recent studies (Maren et al., 2003; Apergis-Schoute et al., 2005). There are a couple of factors that could explain the discrepancy in the data. Results from our autoradiography experiments showed that ANI injections resulted in a large (~75%) decrease in rates of protein synthesis in MGm. This effect is comparable to results obtained when infusing a similar dose of ANI into other brain areas (e.g. Rosenblum et al., 1993). However, Maren et al. (2003) reported a smaller net decrease in protein synthesis (~40%) after ANI administration in MGm. It is therefore possible that the lack of effectiveness of ANI in this experiment might have been due to insufficient blockade of local protein synthesis. However, this is not likely the case given that a dose of ANI twice what we used did not affect auditory fear memory formation when given into the MGm immediately post-training (Apergis-Schoute et al., 2005). Furthermore, it is also possible that similar rates of suppression were achieved in the behavioral work and our estimates of the extent of inhibition due to ANI might differ from those of Maren et al. (2003) based on procedural differences in how the assay was done (e.g. route of administration of radiolabeled compounds).
The factor that most likely explains the discrepancy between experiments is that in the present study we gave infusions of ANI 30 min before the training session, where as studies that have found no effect employed immediate post-training infusions. This finding is especially interesting when we take into consideration data showing that pre-and post-training infusions of mRNA inhibitors are effective when given into the auditory thalamus. Similar findings have been observed in the hippocampus with inhibitory avoidance training (Quevedo et al., 1999; Igaz et al., 2002) and contextual fear conditioning (Baruch et al., 2003). In these experiments, infusion of ANI shortly before training, but not immediately after, blocked memory formation. However, immediate post-training blockade of mRNA synthesis in the dorsal hippocampus disrupts memory for inhibitory avoidance (Igaz et al., 2002) and context fear conditioning (Gafford et al., 2004). There are several potential explanations for this discrepancy between protein and mRNA inhibitors.
One possible explanation of the data is that the inhibitors have different time windows of effectiveness. For example, if ANI is slower to take effect than DRB, then it would be expected that the inhibitors would need to be infused at slightly different time points to be effective. Another possibility is that some of the null results with immediate post-training infusions of ANI can be explained by the fact that ANI has some unfortunate side effects in addition to blocking de novo protein synthesis. These include activation of ERK/MAPK and certain immediate early genes (IEG) (Torocsik and Szeberenyi, 2000 ). It is possible that when given at certain time points and into certain brain areas ANI overcomes its own amnestic effects by driving kinase or IEG activity. Finally, it is also possible that ANI is effective when given prior to training due to disruption of rapid protein synthesis which might occur during acquisition and is essential to memory formation. For this to be possible, the rapid protein synthesis must occur fast enough to be unaffected by a post-training injection. It is unclear if such a mechanism exists, but there is some evidence of rapid, translation-dependent, synaptic plasticity (e.g. Bradley and Sporns, 1999).
Experiment 2 demonstrated that blockade of protein synthesis in the MGm disrupts performance to the auditory CS 24 h after training. These findings could be taken to support the notion that long-term auditory fear memory formation requires the synthesis of protein locally in MGm, alternatively it might be the case that the ANI infusions cause non-specific effects, whereby the deficit seen in experiment 2 would be likely be due to disruption of sensory processes during training, and not memory formation. If this were the case, then performance to the auditory CS would likely be disrupted at any point after training since normal learning would not have occurred. To address these two possible explanations, we tested the effects of ANI in MGm on short-term memory for the auditory cue. The data from this experiment showed intact performance in ANI-treated rats 15 min after training. This data are in agreement with several other published studies showing no disruption of short-term memory following administration of ANI (Davis and Squire, 1984; Abel et al., 1997; Schafe and LeDoux, 2000; Rudy and Matus-Amat, 2005). At 4 h after training there was still no difference between rats treated with ANI and the controls, although there appears to be a slight trend for ANI-treated rats to freeze less at this time. It would not be unexpected to see deficits at this time point given that previous work has shown that with systemic injection of ANI, effects on memory become apparent as early as 3 h after fear conditioning (Bourtchouladze et al., 1998). However, when the same set of animals were tested again at 72 h after training, rats infused with ANI showed significantly less freezing than controls. The fact that the manipulation is specific to long-term performance strongly argues that ANI is blocking memory consolidation.
The data from the current set of experiments add to a body of work describing the role of the auditory thalamus in aversive conditioning. Lesion studies have shown that the MGm is critical for conditioning with auditory cues (Jarrell et al., 1986; LeDoux et al., 1986), while electrophysiological studies have shown that neurons in MGm demonstrate conditioning specific changes during training with auditory cues (Gabriel et al., 1975; Edeline and Weinberger, 1992; McEchron et al., 1996) and that these neurons are also capable of long lasting changes in excitability (Gerren and Weinberger, 1983). The current set of experiments suggests that in addition to the electrophysiological changes, synapses in MGm may undergo long-lasting functional alterations that support memory formation and which require gene expression and protein synthesis.
Work by Gabriel and colleagues (Poremba and Gabriel, 1997b) has demonstrated that the MGm is critical for discriminative avoidance learning in rabbits and the development of TIA in the amygdala and cingulate cortex. Work from the same group showed that the amygdala is critical for both avoidance learning and the development of TIA in MGm and cingulate cortex (Poremba and Gabriel, 2001). In this experiment rabbits received intra-BLA infusion of muscimol during discriminative avoidance training. Results showed that this treatment blocked the development of TIA in MGm. When the rabbits were retrained drug free they were able to learn, but still did not shown TIA in the MGm. This could be taken as evidence that the MGm is not critical for fear memory. However, close inspection of the behavioral data from the study (Poremba and Gabriel, 1999) shows that while the performance in muscimol-infused rats does improve, they still show impairments after 3 days of retraining when TIA in the MGm is absent. The performance only becomes equivalent to controls after several more days of overtraining. Assuming that TIA in MGm is still absent at this point, this might be evidence that MGm is not critical for fear memory. However, previous work has shown that overtraining often supersedes a requirement for brain regions which are normally critical for fear memory (Maren, 1999).
A report by (Maren and colleagues 2001) came to the same basic conclusions as (Poremba and Gabriel 2001). In the Maren study rats given muscimol into the BLA failed to show CS-elicited spike firing in the MGm during testing. Given that the BLA appears to be critical for plasticity in MGm, these data support the notion that plasticity in the amygdala takes precedence over changes in other brain areas. These findings advance the idea that the amygdala is the primary site of plasticity in fear conditioning. Our results do not necessarily contradict this idea. It may be the case that the amygdala is driving plasticity in MGm; however our data would argue that plasticity in MGm is still critical for the consolidation of auditory fear memory.
Our data showing that protein and mRNA synthesis in the auditory thalamus is necessary for long-term fear memory add to data showing that the same processes are necessary in the amygdala and dorsal hippocampus (Bailey et al., 1999; Schafe and LeDoux, 2000; Barrientos et al., 2002; Gafford et al., 2004). This suggests that the critical plasticity supporting memory for conditioned fear does not rely on plasticity that resides solely in the amygdala, but suggests that memory is likely to depend on plastic changes in a distributed set of structures along the essential neural circuit for this form of learning. Across brain structures it is likely that different biochemical mechanisms are involved in the formation of memory (e.g. Rossato et al., 2004). Determining which mechanisms are involved in each brain region and how these processes interact is important to understanding how memory is consolidated at both the molecular and systems level.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Ahi J, Radulovic J, Spiess J. The role of hippocampal signaling cascades in consolidation of fear memory. Behav Brain Res. 2004;149:17–31. doi: 10.1016/s0166-4328(03)00207-9. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute AM, Debiec J, Doyere V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system. J Neurosci. 2005;25:5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behav Neurosci. 1999;113:276–282. doi: 10.1037//0735-7044.113.2.276. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, O’Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res. 2002;134:299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Baruch DE, Parsons RG, Gafford GM, Helmstetter FJ. The role of hippocampal protein synthesis in the acquisition and consolidation of contextual fear memory. Soc Neurosci Abstr. 2003:938.14. [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Sporns O. BDNF-dependent enhancement of exocytosis in cultured cortical neurons requires translation but not transcription. Brain Res. 1999;815:140–149. doi: 10.1016/s0006-8993(98)01112-3. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15:2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugnet MC, LeDoux JE. Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci. 1990;10:2818–2824. doi: 10.1523/JNEUROSCI.10-08-02818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci. 1992;106:81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Saltwick SE, Miller JD. Conditioning and reversal of short-latency multiple-unit responses in the rabbit medial geniculate nucleus. Science. 1975;189:1108–1109. doi: 10.1126/science.1162365. [DOI] [PubMed] [Google Scholar]
- Gafford GM, Parsons RG, Helmstetter FJ. Consolidation, but not reconsolidation, of contextual fear memory is disrupted by rapamycin and an inhibitor of mRNA synthesis in the dorsal hippocampus. Soc Neurosci Abstr. 2004:327.6. doi: 10.1016/j.neuroscience.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerren RA, Weinberger NM. Long term potentiation in the magnocellular medial geniculate nucleus of the anesthetized cat. Brain Res. 1983;265:138–142. doi: 10.1016/0006-8993(83)91344-6. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ. The amygdala is essential for the expression of conditional hypoalgesia. Behav Neurosci. 1992;106:518–528. doi: 10.1037//0735-7044.106.3.518. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- Igaz LM, Vianna MR, Medina JH, Izquierdo I. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J Neurosci. 2002;22:6781–6789. doi: 10.1523/JNEUROSCI.22-15-06781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell TW, Gentile CG, McCabe PM, Schneiderman N. The role of the medial geniculate region in differential Pavlovian conditioning of bradycardia in rabbits. Brain Res. 1986;374:126–136. doi: 10.1016/0006-8993(86)90401-4. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J Neurosci. 1984;4:683–698. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Pearl D, Reis DJ. Disruption of auditory but not visual learning by destruction of intrinsic neurons in the rat medial geniculate body. Brain Res. 1986;371:395–399. doi: 10.1016/0006-8993(86)90383-5. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci. 1990;10:1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci. 2001;21:RC135. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McCabe PM, McEchron MD, Green EJ, Schneiderman N. Electrolytic and ibotenic acid lesions of the medial subnucleus of the medial geniculate prevent the acquisition of classically conditioned heart rate to a single acoustic stimulus in rabbits. Brain Res. 1993;619:291–298. doi: 10.1016/0006-8993(93)91623-z. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Green EJ, Winters RW, Nolen TG, Schneiderman N, McCabe PM. Changes of synaptic efficacy in the medial geniculate nucleus as a result of auditory classical conditioning. J Neurosci. 1996;16:1273–1283. doi: 10.1523/JNEUROSCI.16-03-01273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala 117. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Amygdalar lesions block discriminative avoidance learning and cingulothalamic training-induced neuronal plasticity in rabbits. J Neurosci. 1997a;17:5237–5244. doi: 10.1523/JNEUROSCI.17-13-05237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Medial geniculate lesions block amygdalar and cingulothalamic learning-related neuronal activity. J Neurosci. 1997b;17:8645–8655. doi: 10.1523/JNEUROSCI.17-21-08645.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Amygdala neurons mediate acquisition but not maintenance of instrumental avoidance behavior in rabbits. J Neurosci. 1999;19:9635–9641. doi: 10.1523/JNEUROSCI.19-21-09635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A, Gabriel M. Amygdalar efferents initiate auditory thalamic discriminative training-induced neuronal activity. J Neurosci. 2001;21:270–278. doi: 10.1523/JNEUROSCI.21-01-00270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Vianna MR, Roesler R, de Paris F, Izquierdo I, Rose SP. Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learn Mem. 1999;6:600–607. doi: 10.1101/lm.6.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behav Neural Biol. 1993;59:49–56. doi: 10.1016/0163-1047(93)91145-d. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Bonini JS, Coitinho AS, Vianna MR, Medina JH, Cammarota M, Izquierdo I. Retrograde amnesia induced by drugs acting on different molecular systems. Behav Neurosci. 2004;118:563–568. doi: 10.1037/0735-7044.118.3.563. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behav Neurosci. 2005;119:154–163. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talk A, Kashef A, Gabriel M. Effects of conditioning during amygdalar inactivation on training-induced neuronal plasticity in the medial geniculate nucleus and cingulate cortex in rabbits (Oryctolagus cuniculus) Behav Neurosci. 2004;118:944–955. doi: 10.1037/0735-7044.118.5.944. [DOI] [PubMed] [Google Scholar]
- Torocsik B, Szeberenyi J. Anisomycin uses multiple mechanisms to stimulate mitogen-activated protein kinases and gene expression and to inhibit neuronal differentiation in PC12 phaeochromocytoma cells. Eur J Neurosci. 2000;12:527–532. doi: 10.1046/j.1460-9568.2000.00933.x. [DOI] [PubMed] [Google Scholar]


