Abstract
Purpose
p42/p44 MAPK (MAPK) negatively modulates protein secretion stimulated by cholinergic and α1D-adrenergic agonists but does not play a role in EGF-stimulated protein secretion. Therefore, the purpose of this study was to determine the roles of protein kinase C (PKC), intracellular [Ca2+] ([Ca2+]i) and the non-receptor tyrosine kinases Pyk2 and Src play in the activation of agonist- and EGF-stimulated MAPK activation.
Methods
Lacrimal gland acini were isolated by collagenase digestion and incubated with phorbol 12-myristate 13-acetate (PMA) to activate PKC or ionomycin, a Ca2+ ionophore. Acini were preincubated with the PKC inhibitors calphostin C or Ro-31-8220, EGTA to chelate Ca2+, or the c-Src inhibitor PP1 prior to stimulation with the cholinergic agonist carbachol, the α1D-adrenergic agonist phenylephrine, or EGF. The amount of activated MAPK, Pyk2 and c-Src was measured by western blot analysis.
Results
PMA and ionomycin significantly increased activation of MAPK in a time- and concentration-dependent manner. Inhibition of PKC partially inhibited carbachol-stimulated MAPK activation while completely inhibiting phenylephrine- and EGF-stimulated MAPK activation. Chelation of Ca2+ also partially inhibited carbachol -stimulated MAPK with no effect on phenylephrine- and EGF-stimulated MAPK activation. Carbachol increased the phosphorylation of Pyk2 on tyrosine 402 and c-src on tyrosine 416 in a time-dependent manner. The c-src inhibitor PP1 inhibited carbachol-stimulated phosphorylation of Pyk2.
Conclusions
We conclude that cholinergic agonists use Ca2+ and PKC to phosphorylate Pyk2 and c-Src that subsequently stimulate MAPK activity. In contrast, α1D-adrenergic agonists and EGF do not use Pyk2 and Src but do use PKC to activate MAPK.
The lacrimal gland is the major contributor to the aqueous layer of the tear film secreting water, electrolytes, and protein onto the ocular surface. Regulation of secretion is tightly controlled and any disruption in either quantity or quality can have deleterious effects on the ocular surface. This control is dependent upon stimulation of nerves in the cornea activating an afferent pathway to stimulate efferent pathways in the lacrimal gland causing the gland to secrete 1.
Acinar cells are the main cell type in the lacrimal gland comprising approximately 80% of the gland and are the main cell type involved in secretion 2. These cells are highly polarized with tight junctions at the luminal membranes creating distinct basolateral and apical membranes. Neurotransmitters released from nerves bind to the appropriate receptor on the basolateral membrane of the acinar cell initiating the signal that leads to secretion of water, electrolytes, and protein across the apical membrane.
Norepinephrine released from sympathetic nerves and acetylcholine released from parasympathetic nerves are major stimuli of lacrimal gland protein secretion. Norepinephrine binds to α1D-adrenergic receptors to activate endothelial nitric oxide synthase to generate nitric oxide (NO) 3. NO increases cGMP through activation of guanylate cyclase leading to protein secretion. This agonist also activates protein kinase C (PKC)-ɛ 4 to stimulate secretion. Acetylcholine binds to M3 muscarinic receptors to stimulate protein secretion by activating Gαq/11 and phospholipase C (PLC)β5, 6. PLCβ hydrolyzes phosphatidylinositol bisphosphate into diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG activates PKCα, -δ, and –ɛ in the lacrimal gland while IP3 releases Ca2+ from intracellular stores 4, 7. Ca2+, either alone or in conjunction with calmodulin, leads to protein secretion.
Growth factors of the epidermial growth factor (EGF) family are also known to stimulate lacrimal gland protein secretion 8. While it is known that many growth factors in this family increase intracellular [Ca2+], only the signal transduction pathway used by EGF has been studied extensively 8, 9. EGF stimulates secretion through the recruitment of PLCγ that in turn activates PKC and increases intracellular [Ca2+] 9.
In addition to the pathways described, both G protein-linked agonists and EGF also activate p42/p44 mitogen-activated protein kinase (MAPK) albeit by different mechanisms. Similar to other G-protein coupled receptors, the α1D-adrenergic receptors in the lacrimal gland transactivate the EGF receptor (EGFR) via activation of a matrix metalloproteinase and ectodomain shedding of EGF (unpublished observations). The activated EGFR phosphorylates Shc leading to the recruitment of Grb2. This activates a cascade of protein kinases namely Ras, Raf, MEK and ultimately MAPK.
In contrast to α1D-adrenergic receptors, muscarinic receptors in the lacrimal gland do not transactivate the EGFR to activate MAPK. Rather, these receptors activate the non-receptor tyrosine kinase proline-rich tyrosine kinase 2 (Pyk2) and c-Src. Pyk2, also known as RAFTK, is a Ca2+-dependent member of the Focal Adhesion Kinase (FAK) family 10, 11. Cholinergic agonist-stimulated Pyk2 and c-Src leads to activation of MAPK in lacrimal gland as inhibition of c-Src with a specific inhibitor decreases cholinergic agonist-stimulated MAPK activation while increasing protein secretion 12.
Long term activation of MAPK leads to cell growth and proliferation 13. Short term activation has been shown to be involved secretion 12, 14. In the lacrimal gland, activation of MAPK negatively modulates cholinergic- and α1D-adrenergic agonist-stimulated protein secretion and thus may control agonist-stimulated protein secretion. Interestingly, EGF-stimulated MAPK does not affect EGF-stimulated protein secretion 9.
In the present study, we investigated the role of PKC and Ca2+ on agonist- and growth factor-stimulated MAPK and the role of Pyk2 and c-Src in the activation of MAPK in the lacrimal gland. We demonstrate that MAPK is activated by stimulation of PKC and is dependent on intracellular Ca2+and that cholinergic agonists use Ca2+, PKC, Pyk2, and c-Src to activate MAPK. In contrast, α1D-adrenergic agonists and EGF use PKC to activate MAPK.
MATERIALS AND METHODS
Antibodies against phosphorylated (active) p42/p44 MAPK (Tyr204), total MAPK, EGFR and total Src were from purchased Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phosphorylated Pyk2 (Tyr402), total Pyk2 and phosphorylated Src (Tyr416) were from Cell Signaling (Beverly, MA). Antibodies against phosphorylated Pyk2 on Tyr580 and Tyr881 were purchased from Biosource International (Camarillo, CA). EGF was from Peprotech Inc (Rocky Hill, NJ). Anti-tyrosine antibody (4G10) was obtained from Upstate Biotechnology (Chicago, IL). Ionomycin was purchased from Alexis Biochemicals (San Diego, CA). Collagenase Type III was purchased from Worthington Biochemicals (Freehold, NJ). All other reagents were purchased from Sigma Chemical Company (St. Louis, MO) and were the highest quality available.
Preparation of Rat Lacrimal Gland Acini
All animals were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Acini were prepared using a modification of a method developed by Herzog et al 15. In brief, lacrimal glands were trimmed, fragmented, and incubated with collagenase CLSIII (100 U/ml) in Krebs-Ringer Bicarbonate (KRB) buffer (119 mM NaCl, 4.8 mM KCl, 1 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 25 mM NaHCO3) supplemented with 10 mM HEPES, 5.5 mM glucose (KRB-HEPES), and 0.5% BSA, pH 7.4 at 37 °C. Lobules were subjected to gentle pipetting through tips of decreasing diameter, filtered through nylon mesh (150 μm pore size), and centrifuged briefly. The pellet was washed twice by centrifugation (50 x g, 2 minutes) through a 4% BSA solution made in KRB-HEPES buffer. The dispersed acini were allowed to recover for 30 min in fresh KRB-HEPES buffer containing 0.5% BSA.
Measurement of MAPK, Pyk2, and c-Src activity
The activation of p42/44 MAPK, Pyk2, and c-Src were examined using western blot techniques. Acini were incubated with the cholinergic, muscarinic agonist carbachol (10−4 M), the α1D-adrenergic agonist phenylephrine (10−4 M), or EGF (10−7 M) for 5 min. Inhibitors were added 10 minutes prior to addition of carbachol. To terminate incubation, ice-cold KRB buffer was added and centrifuged briefly. The pellet was homogenized in RIPA buffer (10 mM Tris-HCl, pH7.4, 150 mM NaCl, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 100 μg/ml phenylmethylsulfonyl fluoride, 30 μl/ml aprotinin, 1 mM Na3VO3), sonicated, and centrifuged at 20,000 x g for 30 minutes. Proteins in the supernatant were separated by SDS-PAGE on an 10% gel, transferred onto nitrocellulose membrane, which were blocked overnight at 4°C in 5% non-fat dried milk in buffer containing 10 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.05% Tween-20 (TBST). The blots were then probed with antibodies directed against the non-phosphorylated form of the enzyme (total) or the phosphorylated form of the enzyme (activated) followed by HRP-conjugated secondary antibody. Immunoreactive bands were digitally scanned and analysed using ImageJ (National Institutes of Health). The amount of phosphorylated enzyme in each sample was standardized to either the amount of total enzyme or total MAPK.
Immunoprecipitation Experiments
Lacrimal gland acini were incubated for 5 min with either PMA (10−6 M) or EGF (10−7 M) as a positive control. To terminate incubation, the acini were centrifuged, the supernatant discarded, and ice-cold RIPA buffer was added. The homogenate was centrifuged at 3,000 rpm for 30 min, and the supernatant was incubated overnight at 4 °C on a rocker platform in the presence of an antibody against EGFR. Protein A-agarose was added for 2 h and incubated for 4 °C. The immunoprecipitate was collected by brief centrifugation, washed three times with RIPA buffer, and resuspended in Laemmli sample buffer. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Western blot analysis was performed using an antibody against phosphorylated tyrosine or the immunoprecipiating antibody, the EGFR.
Data presentation and statistical analysis
Data are expressed as fold increase above basal value, which was standardized to 1.0. Results are expressed as mean ± SEM. Data were analysed by Student’s t-test. p<0.05 was considered statistically significant.
RESULTS
Effect of Time and Concentration of Phorbol Esters on p42/p44 MAPK Activation
We previously showed that the cholinergic agonist carbachol activates PKCα, -δ, and –ɛ, the α1D-adrenergic agonist phenylephrine activates PKCɛ, and EGF activates PKCα and –δ to stimulate lacrimal gland protein secretion. As PKC is known to activate MAPK 16–18, we determined if PKC plays a role in the activation of p42/p44 MAPK in the lacrimal gland. Lacrimal gland acini were incubated with the phorbol ester, phorbol 12-myristate 13-acetate (PMA), which activates classical and novel isoforms of PKC, for varying times and concentrations. The amount of phosphorylated MAPK was then determined via western blot analysis. PMA (10−6 M) significantly increased the activation of MAPK in a time-dependent manner with a maximum stimulation at 5 minutes of 3.5 ± 0.8 fold above basal (Figure 1A). This increase persisted for at least 30 minutes.
Figure 1. Effect of PMA on p42/p44 MAPK Activation.
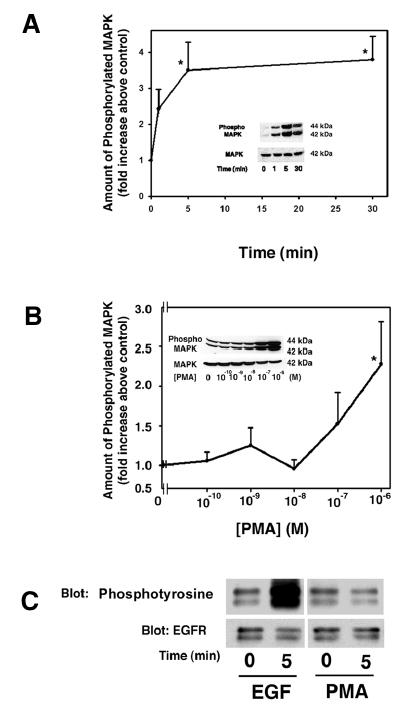
Lacrimal gland acini were incubated with PMA (10−6 M) for 0–30 min (A) or with PMA (10−10–10−6 M) for 5 min (B) and the amount of activated p42/p44 MAPK/total p42 MAPK was measured. Insert blots are representative of 3–7 independent experiments. Data are mean ± SEM. * indicates statistical significance from t=0 or basal. (C) Acini were also incubated with EGF (10−7 M) or PMA (10−6 M) for 5 min, EGFR was immunoprecipitated and samples blotted for phosphotyrosine or EGFR.
Lacrimal gland acini were also incubated with increasing concentrations of PMA for 5 minutes and the amount of phosphorylated MAPK was determined. As shown in Figure 1B, PMA (10−6 M) significantly increased MAPK activation 2.3 ± 0.5 fold increase above basal.
To determine if PMA activates MAPK through activation of the EGFR, lacrimal gland acini were incubated with PMA (10−6 M) or EGF (10−7 M) as a positive control for 5 min. Proteins were subjected to immunoprecipitation experiments using an anti-EGFR antibody. The immunoprecipitated proteins were analyzed by western blot analysis with either an anti-phosphotyrosine antibody or the same anti-EGFR antibody used for immunoprecipitation to control for the amount of proteins in each sample. As shown in Figure 1C, EGF caused a substantial increase in the amount of tyrosine phosphorylation of the EGFR while PMA did not increase the amount of tyrosine phosphorylation of the EGFR.
These data indicate that activation of PKC stimulates MAPK in rat lacrimal gland acini but not by activating the EGFR.
Effect of Inhibition of PKC on Agonist-stimulated MAPK Activation
As PKC can activate p42/p44 MAPK, we determined the role of PKC in cholinergic-, α1D-adrenergic- and EGF-stimulated activated MAPK. Acini were preincubated for 10 min with either calphostin C (10−10 – 10−6 M) or Ro-31-8220 (10−9 – 10−5 M), two PKC inhibitors, prior to incubation for 5 min with carbachol (Cch, 10−4 M), phenylephrine (Ph, 10−4 M), or EGF (10−7 M). Cch (10−4 M) significantly increased activation of MAPK 4.55 ± 0.77 fold over basal (data not shown). Calphostin C inhibited this activation in a concentration dependent manner with a maximum decrease, which was significant, of 73.5 ± 10.4% at 10−6 M. In another set of experiments, Ro-31-8220 also significantly decreased carbachol-stimulated MAPK activation in a biphasic manner with a maximum of 77.8 ± 20.3% at 10−8 M and 67.8 ± 23.3% at 10−5 M, which were significantly decreased from carbachol alone (Figure 2). In these experiments, Cch significantly increased activation of MAPK 2.00 ± 1.23 fold over basal (data not shown).
Figure 2. Effect of Inhibition of PKC on Agonist-stimulated p42/p44 MAPK Activation.
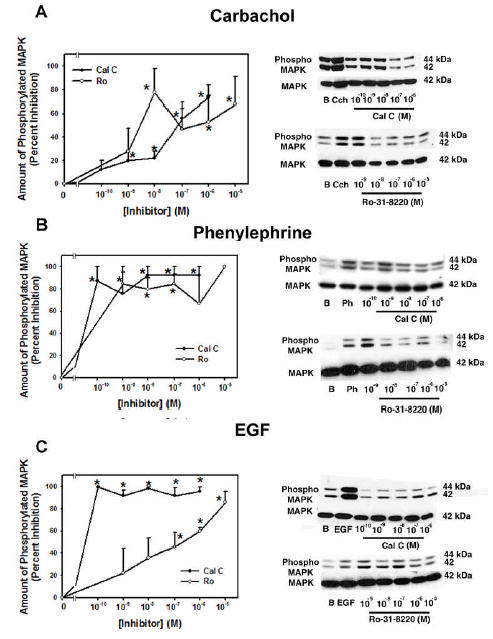
Acini were preincubated for 10 min with either calphostin C (10−10–10−6 M) or Ro-31-8220 (10−9–10−5 M). Acini were then stimulated with (A) carbachol (10−4 M), (B) phenylephrine (10−4 M), and (C) EGF (10−7 M) for 5 min and activated p42/p44 MAPK/total p42 MAPK was measured. Data are mean ± SEM from 3 independent experiments. * indicates statistical significance from basal. Blots are representative experiments.
Ph (10−4 M), an α1D-adrenergic receptor agonist, significantly increased MAPK 2.27 ± 0.91 fold increase above basal (data not shown). This activation was significantly inhibited by calphostin C a maximum of 92.6 ± 7.3% at 10−7 M. In another set of experiments, Ro-31-8220 also significantly inhibited phenylephrine-stimulated p42/p44 MAPK activation a maximum of 84.7 ± 10.3 at 10−9 M (Figure 2). In these experiments, Ph significantly increased activation of MAPK 1.82 ± 0.20 fold over basal (data not shown).
The growth factor EGF (10−7 M) increased MAPK activation by 2.32 ± 0.21 fold above basal (data not shown). Calphostin C significantly decreased EGF-stimulated MAPK activation in a concentration dependent manner with a maximum inhibition of 99.7 ± 0.3% at 10−6 M. In separate experiments, Ro-31-8220 also significantly decreased EGF-stimulated MAPK a maximum of 85.7 ± 9.8% at 10−5 M (Figure 2). In these experiments, EGF significantly increased activation of MAPK 3.01 ± 0.57 fold over basal (data not shown).
These results suggest that PKC is necessary for cholinergic-, α1D-adrenergic -, and EGF-stimulated activation of MAPK though calphostin C inhibited α1D-adrenergic agonist-and EGF-stimulated MAPK at lower concentrations than it inhibited cholinergic agonists.
Effect of Ca2+ on MAPK activation
As cholinergic and α1D-adrenergic agonists and EGF increase intracellular Ca2+7,9,19, we determined the role that Ca2+ plays in the activation of p42/p44 MAPK. Acini were incubated with the calcium ionophore, ionomycin (Figure 3). Lacrimal gland acini were incubated with ionomycin 10−9 – 10−5 M for 5 min followed by western blot analysis for activated MAPK. Ionomycin increased the amount of activated MAPK by 2.83 ± 0.33, 3.84 ± 1.08, 3.66 ± 0.86, 4.03 ± 0.99 and 2.55 ± 0.55 fold increase above basal at 10−9, 10−8, 10−7, 10−6, and 10−5 M, respectively. These values were significantly increased above basal activation.
Figure 3. Effect of Ionomycin on p42/p44 MAPK Activation.
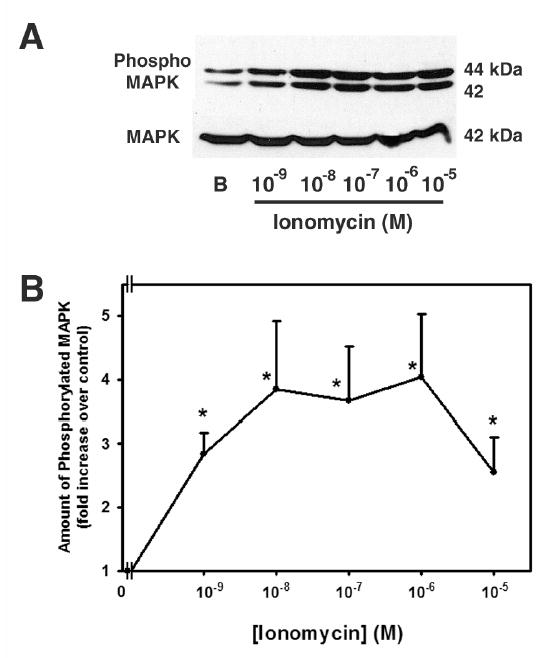
Acini were incubated for 10 min with increasing concentrations of ionomycin (10−9 – 10−5 M). Activated p42/p44 MAPK/total p42 MAPK was analyzed via western blot. Representative experiment is shown in (A). The results of 5 independent experiments are shown in (B). Data are mean ± SEM. * indicates statistical significance from basal.
To explore the role of Ca2+in agonist-stimulated MAPK activation, Ca2+-free buffer was made by omitting Ca2+ and adding EGTA (2 mM) to the KRB-BSA. Acini were resuspended in the Ca2+-free buffer prior to stimulation with Cch, Ph, or EGF (Figure 4). Activated MAPK was significantly increased by 2.79 ± 0.57, 1.78 ± 0.26, and 2.24 ± 0.47 fold above basal by Cch (10-4 M), Ph (10−4 M), and EGF (10−7 M), respectively (data not shown). Cch-stimulated MAPK activation was significantly decreased by 54.0 ± 19.1% by chelation of Ca2+. In contrast, chelation of Ca2+ did not significantly decrease Ph- and EGF-stimulated MAPK activation by which were decreased 17.7 ± 13.6% and 27.8 ± 13.8%, respectively.
Figure 4. Effect of Chelation of Ca2+ on p42/p44 MAPK Activation.
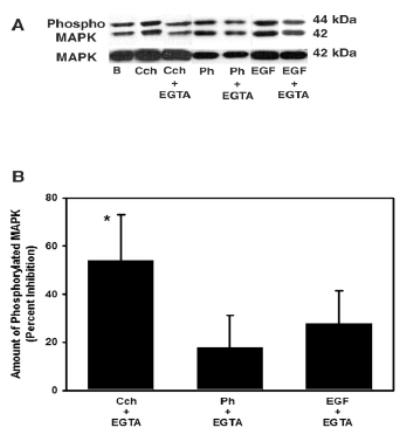
Acini were incubated for 10 min with either carbachol (Cch, 10−4), phenylephrine (10−4 M), or EGF (10-7 M) for 10 min in the presence of extracellular Ca2+ or in the absence of Ca2+ plus 2 mM EGTA and the amount of phosphorylated p42/p44 MAPK/total p42 MAPK was measured. Representative blot in shown in (A). The results of 3–5 independent experiments were analyzed and are shown in (B). Data are mean ± SEM. * indicates statistical significance from basal.
These results indicate that Ca2+ alone can activate MAPK. In addition, Ca2+ plays a pivotal role in cholinergic-, but not α1D-adrenergic agonist- or EGF-stimulated MAPK activation.
Effect of Time on Agonist Activation of Pyk2
We previously showed that Cch activated Pyk2 on Tyr402 after 5 min stimulation, but Ph and EGF did not 12. Therefore, we examined the time-dependency of this activation and determined if Ph or EGF activated Pyk2 at a wide range times. Lacrimal gland acini were incubated with Cch (10−4 M), Ph (10−4 M), or EGF (10−7 M) for 0-10 min. Acini were homogenized and the amount of phosphorylation at Tyr402 (active) Pyk2 and total Pyk2 were determined by western blot analysis. Cch increased the amount of phospho-Pyk2 with a maximum response after 1 min while Ph and EGF did not increase phospho-Pyk2 at any time tested (Figure 5A). When the results from three independent experiments were analyzed, Cch increased phospho-Pyk2 to 1.52 ± 0.01 fold above basal after 30s, 1.75 ± 0.19 fold after 1 min, 1.31 ± 0.04 fold after 5 min, and 1.13 ± 0.04 after 10 min (Figure 5B). The increase was significantly different from basal at 30s and 5 min. Neither Ph nor EGF increased phosphorylated Pyk2 (Figure 5B).
Figure 5. Effect of Time on Agonist-induced Pyk2 Activation.
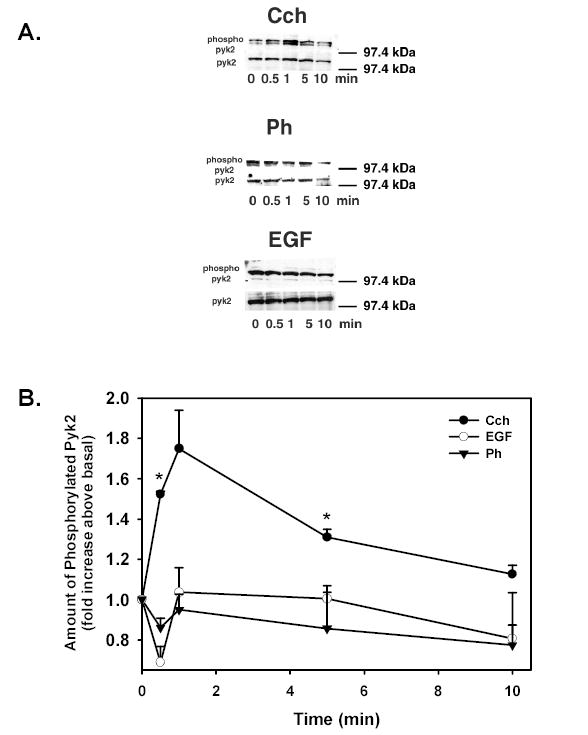
Acini were stimulated with Cch (10−4 M), phenylephrine (Ph 10−4 M), or EGF (10−7 M) for 0–10 min and phosphoPyk2 (Tyr402)/total Pyk2 was measured. Representative blots are shown in (A). The results from 3 independent experiments were analyzed and are shown in (B). Data are mean ± SEM * indicates statistical significance from basal.
In addition to phosphorylation on Tyr402, it is known that Pyk2 can be phosphorylated on Tyr580 and Tyr881 20. The samples stimulated with Cch were also analyzed using antibodies to Pyk2 phosphorylated on these tyrosine residues. Cch did not increase the amount of phosphorylation on either Tyr580 or Tyr881 indicating that phosphorylation of Tyr402 is responsible for activation of Pyk2 in the lacrimal gland (data not shown).
These results indicate that cholinergic agonists, but not α1D-adrenergic agonists or EGF, activate Pyk2 in a time-dependent manner. As Pyk2 stimulation is Ca2+ dependent, it is consistent that the Ca2+ dependent cholinergic agonist is the only agonist to activate Pyk2.
Effect of Time on Agonist Activation of c-Src
We previously showed that Cch activates c-Src and inhibition of c-Src decreased Cch-stimulated MAPK while increasing Cch-stimulated protein secretion 12. In addition, as Pyk2 and c-Src interact to be active 20, we examined the time-dependency of c-Src activation and if Ph or EGF activated c-Src. Acini were incubated with Cch (10−4 M), Ph (10−4 M) or EGF (10−7 M) for 0-10 min and the amount of phosphorylated (activated) c-Src was measured by western blot analysis using an antibody against phosphorylated c-Src (Tyr416). Each condition was standardized for the number of cells using total c-Src. As shown in Figure 6A, Cch stimulated the phosphorylation of c-Src by 30s and 1 min while Ph and EGF did not. When 4-5 independent experiments were analyzed, Cch significantly increased the amount of phosphorylated c-Src to 1.73 ± 0.19 fold above basal after 30s (Figure 6B). Cch further increased the amount of phosphorylated c-Src, though the increase was not significant, to 2.51 ± 0.59 fold above basal after 1 min, 2.61 ± 0.64 fold after 5 min, and 1.68 ± 0.32 fold after 10 min. Neither Ph nor EGF increased the amount of phosphorylated c-Src at any time tested (Figure 6B).
Figure 6. Effect of Time on Agonist-induced c-Src Activation.
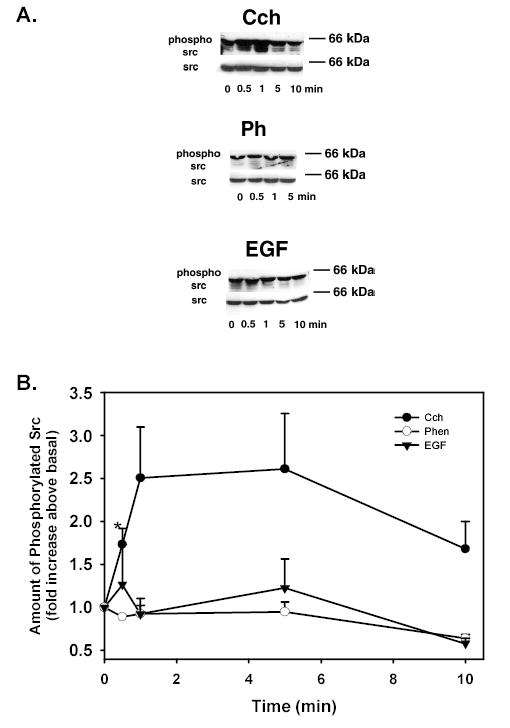
Acini were stimulated with Cch (10−4 M), phenylephrine (Ph 10−4 M), or EGF (10−7 M) for 0–10 min and phospho-c-Src(Tyr416)/total c-Src was measured. Representative blots are shown in (A). The results from 3 independent experiments were analyzed and are shown in (B). Data are mean ± SEM. * indicates statistical significance from basal.
These results indicate that cholinergic agonists increase the amount of phosphorylated c-Src in a time-dependent manner while α1D-adrenergic agonists and EGF did not.
Effect of PKC and Ca2+ on activation of Pyk2
As Cch activates Pyk2 and c-Src and activation of MAPK is dependent upon PKC, we determined the role of PKC activation with PMA and Ca2+ in Cch-stimulated Pyk2 and c-Src activation. Lacrimal gland acini were incubated with Cch (10−4 M), the PKC activator PMA (10−6 M), and acini were homogenized and the amount of phosphorylated Pyk2 on Tyr402 and c-Src and Tyr416 was determined by western blot analysis. Each sample was normalized to the amount of total p42/p44 MAPK to correct for the number of cells in each condition. Cch and PMA significantly increased the amount of phosphorylated Pyk2 2.25 ± 0.24 and 1.88 ± 0.20 fold above basal, respectively (Figure 7A).
Figure 7. Effect of PKC and Ca2+and c-Src on Pyk2 Activation.
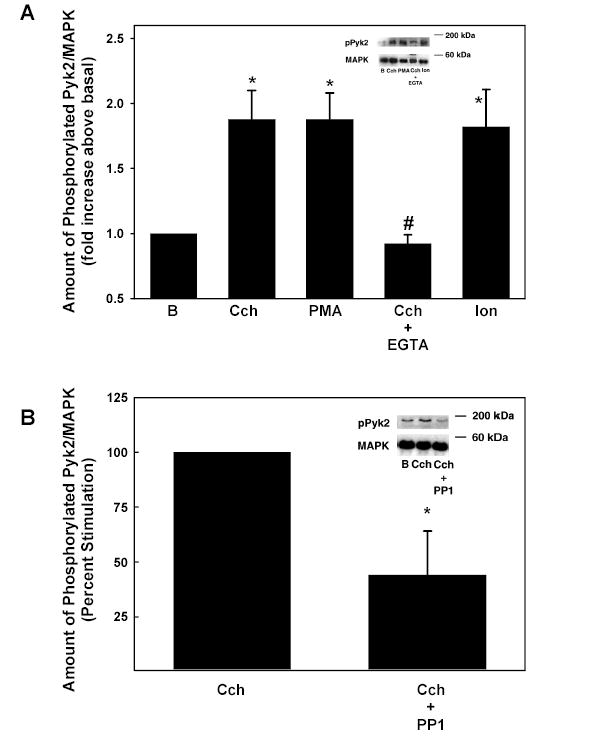
Acini were stimulated for 5 min with Cch (10−4 M), PMA (10−6 M), Cch and 2 mM EGTA, and ionomycin (10−7 M). The amount of phosphorylated Pyk2 (Tyr402)/total Pyk2 is shown in A. Data are mean ± SEM from 3–6 independent experiments. Acini were also preincubated with PP1 for 15 min prior to stimulation with Cch. The amount of phosphorylated Pyk (Tyr402) is shown in B. Data are mean ± SEM from 4 independent experiments. * indicates statistical significance from basal. # denotes statistical significance from Cch alone.
To determine the effects of Ca2+ on Cch-stimulated Pyk2, acini were incubated with ionomycin (10−7 M) for 5 min or in KRB buffer containing 2 mM EGTA and stimulated with Cch (10−4 M) for 5 min. Cch significantly increased the phosphorylation of Pyk2 1.88 ± 0.22 fold above basal. This increase was completely inhibited with chelation of Ca2+. Ionomycin also significantly increased the amount of phosphorylated Pyk2 1.82 ± 0.29 fold above basal (Figure 7A).
These results indicate that PKC and Ca2+ can play a role in the activation of Pyk2. Furthermore, Ca2+ plays a major role in cholinergic agonist activation of Pyk2.
Effect of c-Src on Cholinergic Agonist-stimulated Pyk2 activation
To determine if Pyk2 activates with c-Src during cholinergic agonist stimulation, acini were preincubated with the c-Src inhibitor PP1 (10−5 M) 21 for 15 minutes prior to stimulation with Cch (10−4 M) for 5 min. The amount of phosphorylated Pyk2 was determined by western blot analysis. PP1 significantly inhibited phosphorylation of Pyk2 on Tyr402 56.12 ± 19.48% from 1.41 ± 0.16 fold increase above basal with Cch alone to 1.08 ± 0.18 fold increase above basal in the presence of Cch plus PP1 (Figure 7B).
These results indicate that Pyk2 and c-Src are both necessary for Cch to activate MAPK in the lacrimal gland.
DISCUSSION
Activation of MAPK by EGF is central to the control of both short and long term processes in multiple cell types 13. In addition G protein-linked agonists can activate MAPK by transactivating the EGFR using the triple membrane passing mechanism that releases EGF family of growth factors by ectodomain shedding 22. G protein linked agonists can also stimulate MAPK activity by other mechanisms depending upon the cell type and the stimulus. In the lacrimal gland there are three different known agonists (EGF, cholinergic agonists, and α1D-adrenergic agonists) that activate MAPK and each uses a distinct cellular pathway (Figure 8) 2. EGF activates MAPK by the classical pathway using phosphorylation of the EGFR, recruitment of Shc and Grb2, stimulation of Sos that induces Ras to activate Raf (MAPKKK), MEK (MAPKK), and ERK (MAPK) 12. In the present manuscript we found that EGF does not phosphorylate Pyk2 or Src. EGF activation of MAPK does not stimulate protein secretion, although EGF can stimulate protein secretion using PLCγ, intracellular Ca2+, and PKC 9. Cholinergic agonists stimulate PLCβ using Gαq/11 to increase the intracellular [Ca2+] and activate the PKC isoforms α, ɛ, and δ (in rank order of importance) 4. Cholinergic agonists also activate MAPK, but do not transactivate the EGFR 12. In the present manuscript we demonstrated that these agonists activate MAPK by the increase in intracellular [Ca2+] and activation of PKC that phosphorylates Pyk2 and Src. In contrast to cholinergic agonists, α1-adrenergic agonists cause a small increase in intracellular [Ca2+] and activate PKCɛ to stimulate secretion and PKCα and -δ to inhibit secretion 4. α1D-Adrenergic agonists transactivate the EGFR via a matrix metalloproteinase. In the present manuscript we found that α1-adrenergic agonists do not activate Pyk2 and Src. Activation of MAPK by cholinergic and α1-adrenergic agonists attenuates stimulated protein secretion. Thus EGF, cholinergic agonists, and α1D-adrenergic agonists each activate MAPK in the lacrimal gland causing the same functional result but utilize distinct signaling components.
Figure 8.
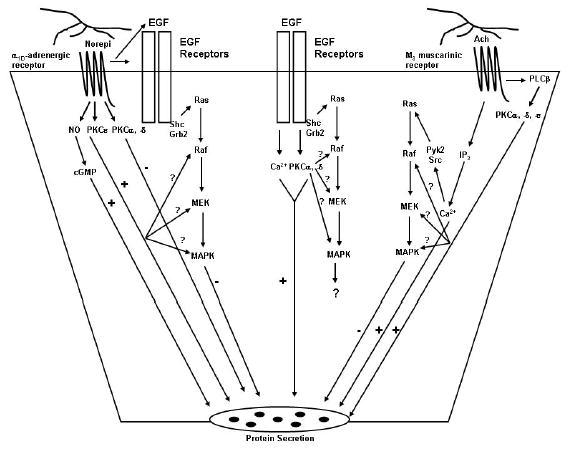
Schematic diagram of the signal transduction pathways used by cholinergic and α1-adrenergic agonists and EGF to activate MAPK and stimulate protein secretion. Norepi, norepinephrine; PKC, protein kinase C; NO, nitric oxide; Ach, acetylcholine; PLCβ, phospholipase Cβ.
In spite of the fact that different agonists activate MAPK in the lacrimal gland, this enzyme can be stimulated in an agonist-independent fashion. Increasing the intracellular [Ca2+] and activation of PKC causes phosphorylation of MAPK. Thus the calcium ionophore ionomycin and phorbol esters each activate lacrimal gland MAPK in time and concentration dependent manner. However, the three agonists that activate MAPK in the lacrimal gland have differential dependency on Ca2+ and PKC. Cholinergic agonist phosphorylation of MAPK was blocked 54% by chelating extracellular Ca2+ with EGTA. In contrast, α1D-adrenergic agonist- and EGF-stimulation was not significantly inhibited by chelating extracellular Ca2+. Thus only cholinergic agonist activation of MAPK was Ca2+ dependent which is consistent with our previous findings that cholinergic agonists cause a substantial increase in the intracellular [Ca2+], but α1D-adrenergic agonists and EGF cause only small, although significant, increases.
Activation of PKC is a second mechanism by which MAPK is activated in the lacrimal gland. We used two different PKC inhibitors, RO 31-8220 and calphostin C. We found that cholinergic activation of MAPK was only partially blocked 59% and 68% by RO 31-8220 and calphostin C, respectively. This is consistent with our finding that cholinergic activation of MAPK is also Ca2+-dependent. In contrast to cholinergic agonists, the effect of α1-adrenergic agonists and EGF on MAPK was completely blocked by both PKC inhibitors. Thus cholinergic agonists activate MAPK by both increasing the intracellular [Ca2+] and activating PKC, whereas α1-adrenergic agonists and EGF only use PKC.
We found that cholinergic agonists, but neither α1-adrenergic agonists nor EGF, use Pyk2 and Src to increase MAPK activity. Pyk2 and Src are well known to activate MAPK although the mechanism of action differs between tissues. In some tissues Pyk2 and Src play a role in the transactivation of the EGFR 23, 24. In other tissues Pyk2 and Src bypass the EGFR and interact with MAPK directly or with other components of the signaling pathway 11. In the lacrimal gland cholinergic agonists do not transactivate the EGFR, so Pyk2 and Src must use an alternative mechanism. Pyk2 has an SH1 domain that allows it to interact with a variety of proteins including Src family kinases, Grb2/Sos complex, p130Cas, paxillin, Hic-5, and Graf 25. In PC12 cells, H2O2 stimulation causes Pyk2 and Src to interact and be co-immunoprecipitated 26. An increase in intracellular Ca2+ causes Pyk2 to be autophosphorylated on Tyr402 11,20,25. The phosphorylated Pyk2 binds to and phosphorylates Src, which then phosphorylates Pyk2 on Tyr580 and Tyr881 and fully activates it. We studied cholinergic activation of Pyk2 and c-Src in the lacrimal gland. We found that Pyk2 could be activated by increasing the intracellular [Ca2+] with ionomycin and by activating PKC with phorbol esters. Furthermore, chelating extracellular Ca2+ with EGTA blocked activation of Pyk2 by cholinergic agonists. We also found that inhibition of c-Src with PP1 blocked cholinergic agonist activation of Pyk2. This suggests that Pyk2 and c-Src are acting together to mediate the effects of cholinergic agonists on MAPK in the lacrimal gland.
Interestingly, in rat lacrimal gland, cholinergic agonists caused phosphorylation on only Tyr402 of Pyk2 and not Tyr580 or Tyr881. This is in contrast to H2O2 stimulation of PC12 cells, where H2O2 caused an increase in the phosphorylation of Pyk2 on Tyr580 and Tyr881 as well as Tyr402 26 or C6 glioma cells in which activation of the P2Y12 receptors and noradrenaline stimulation of mesenteric small arteries caused phosphorylation on Tyr402 and Tyr597 (phosphorylation of Tyr881 was not measured in these studies) 27, 28. In PC12 cells, phosphorylation of Tyr580 and Tyr881 requires phospholipase D2 (PLD2) activity 26 whereas phosphorylation of Tyr881 in astrocytes assists with the binding of Grb2 29. It is known that cholinergic agonists do not require Grb2 stimulation for MAPK activation in the lacrimal gland 12, which is consistent with the lack of phosphorylation of Tyr881. However, the role of PLD2 in Pyk2 and MAPK activation in the rat lacrimal gland is not known.
Although we know that Pyk2/c-Src do not interact with the EGFR upon cholinergic stimulation 12, we do not know the signaling proteins that these non-receptor tyrosine kinases use to activate MAPK. We previously showed that cholinergic agonists do not activate Grb2 or Shc, so potential target proteins are Sos, Ras, Raf, MEK, MAPK, or proteins that interact with these components.
In conclusion our study demonstrates that although activation of MAPK in the lacrimal gland is Ca2+ and PKC dependent, three stimuli of lacrimal gland secretion differentially activate MAPK. Cholinergic agonists use Ca2+ and PKC to phosphorylate Pyk2 and c-Src that activate a target protein distal to the EGFR, Shc and Grb2 that subsequently stimulate MAPK activity. In contrast α1D-adrenergic agonists and EGF do not use Pyk2 and Src but do activate PKC. α1D-Adrenergic agonists activate PKC to transactivate the EGFR that in turn activates Shc, Grb2, Sos, Ras, Raf, MEK, and finally MAPK. Activation of MAPK attenuates stimulated protein secretion.
Footnotes
Supported by NIH EY0677
References
- 1.Botelho SY. Tears and the Lacrimal Gland. Sci Am. 1964;211:78–86. doi: 10.1038/scientificamerican1064-78. [DOI] [PubMed] [Google Scholar]
- 2.Hodges RR, Dartt DA. Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol. 2003;231:129–96. doi: 10.1016/s0074-7696(03)31004-6. [DOI] [PubMed] [Google Scholar]
- 3.Hodges RR, Shatos MA, Tarko RS, Vrouvlianis J, Gu J, Dartt DA. Nitric oxide and cGMP mediate α1D-adrenergic receptor-stimulated protein secretion and p42/p44 MAPK activation in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2005;46(8):2781–2789. doi: 10.1167/iovs.05-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoukhri D, Hodges RR, Sergheraert C, Toker A, Dartt DA. Lacrimal gland PKC isoforms are differentially involved in agonist-induced protein secretion. Am J Physiol. 1997;272(1 Pt 1):C263–9. doi: 10.1152/ajpcell.1997.272.1.C263. [DOI] [PubMed] [Google Scholar]
- 5.Mauduit P, Jammes H, Rossignol B. M3 Muscarinic Acetykcholine Receptor Coupling to PLC in Rat Exorbital Lacrimal Acinar Cells. Am J Physiol. 1993;264(6):C1550–C1560. doi: 10.1152/ajpcell.1993.264.6.C1550. [DOI] [PubMed] [Google Scholar]
- 6.Meneray MA, Fields TY. Adrenergic stimulation of lacrimal protein secretion is mediated by G(q/11)alpha and G(s)alpha. Curr Eye Res. 2000;21(2):602–7. [PubMed] [Google Scholar]
- 7.Dartt DA, Dicker DM, Ronco LV, Kjeldsen IM, Hodges RR, Murphy SA. Lacrimal Gland inositol trisphosphate isomer and inositol tetrakisphopshate production. . Am J Physiol. 1990;259(2):G274–G281. doi: 10.1152/ajpgi.1990.259.2.G274. [DOI] [PubMed] [Google Scholar]
- 8.Chen LL, Johansson JK, Hodges RR, Zoukhri D, Ghinelli E, Rios JD, Dartt DA. Differential effects of the EGF family of growth factors on protein secretion, MAPK activation, and intracellular calcium concentration in rat lacrimal gland. Exp Eye Res. 2005;80(3):379–89. doi: 10.1016/j.exer.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Tepavcevic V, Hodges RR, Zoukhri D, Dartt DA. Signal transduction pathways used by EGF to stimulate protein secretion in rat lacrimal gland. Invest Ophthalmol Vis Sci. 2003;44(3):1075–81. doi: 10.1167/iovs.02-0794. [DOI] [PubMed] [Google Scholar]
- 10.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J, Plowman G, Rudy B, Schlessinger J. Protein tyrosine kinase Pyk2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 11.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12(3):123–33. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 12.Ota I, Zoukhri D, Hodges RR, Rios JD, Tepavcevic V, Raddassi I, Chen LL, Dartt DA. Alpha 1-adrenergic and cholinergic agonists activate MAPK by separate mechanisms to inhibit secretion in lacrimal gland. Am J Physiol Cell Physiol. 2003;284(1):C168–78. doi: 10.1152/ajpcell.00151.2002. [DOI] [PubMed] [Google Scholar]
- 13.Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. . Nat Rev Mol Cell Biol. 2005;6(11):827–37. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 14.Bertelsen LS, Barrett KE, Keely SJ. Gs protein-coupled receptor agonists induce transactivation of the epidermal growth factor receptor in T84 cells: implications for epithelial secretory responses. J Biol Chem. 2004;279(8):6271–9. doi: 10.1074/jbc.M311612200. [DOI] [PubMed] [Google Scholar]
- 15.Herzog V, Sies H, Miller F. Exocytosis in secretory cells of rat lacrimal gland. Peroxidase release from lobules and isolated cells upon cholinergic stimulation. J Cell Biol. 1976;70(3):692–706. doi: 10.1083/jcb.70.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70(11):1537–47. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364(6434):249–52. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 18.Luttrell LM. Activation and targeting of mitogen-activated protein kinases by G-protein-coupled receptors. Can J Physiol Pharmacol. 2002;80(5):375–82. doi: 10.1139/y02-045. [DOI] [PubMed] [Google Scholar]
- 19.Hodges RR, Dicker DM, Rose PE, Dartt DA. Alpha 1-adrenergic and cholinergic agonists use separate signal transduction pathways in lacrimal gland. Am J Physiol. 1992;262(6 ):p–x02013;96. doi: 10.1152/ajpgi.1992.262.6.G1087. [DOI] [PubMed] [Google Scholar]
- 20.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383(6600):547–50. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 21.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271(2):695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 22.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 23.Kanno H, Horikawa Y, Hodges RR, Zoukhri D, Shatos MA, Rios JD, Dartt DA. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol. 2003;284(4):C988–98. doi: 10.1152/ajpcell.00582.2001. [DOI] [PubMed] [Google Scholar]
- 24.Shah BH, Baukal AJ, Shah FB, Catt KJ. Mechanisms of Extracellularly Regulated Kinases 1/2 Activation in Adrenal Glomerulosa Cells by Lysophosphatidic Acid and Epidermal Growth Factor 10.1210/me.2005–0082. Mol Endocrinol. 2005;19(10):2535–2548. doi: 10.1210/me.2005-0082. [DOI] [PubMed] [Google Scholar]
- 25.Blaukat A, Ivankovic-Dikic I, Gronroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem. 1999;274(21):14893–901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- 26.Banno Y, Ohguchi K, Matsumoto N, Koda M, Ueda M, Hara A, Dikic I, Nozawa Y. Implication of phospholipase D2 in oxidant-induced phosphoinositide 3-kinase signaling via Pyk2 activation in PC12 cells. J Biol Chem. 2005;280(16):16319–24. doi: 10.1074/jbc.M410903200. [DOI] [PubMed] [Google Scholar]
- 27.Van Kolen, K, K Gilany, L Moens, EL Esmans, and H Slegers. P2Y(12) receptor signalling towards PKB proceeds through IGF-I receptor cross-talk and requires activation of Src, Pyk2 and Rap1. Cell Signal 2005. [DOI] [PubMed]
- 28.Ohanian V, Gatfield K, Ohanian J. Role of the actin cytoskeleton in G-protein-coupled receptor activation of PYK2 and paxillin in vascular smooth muscle. Hypertension. 2005;46(1):93–9. doi: 10.1161/01.HYP.0000167990.82235.3c. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Reiser G. The role of the Ca2+-sensitive tyrosine kinase Pyk2 and Src in thrombin signalling in rat astrocytes. J Neurochem. 2003;84(6):1349–57. doi: 10.1046/j.1471-4159.2003.01637.x. [DOI] [PubMed] [Google Scholar]


