Abstract
Carbapenem-resistant Acinetobacter baumannii isolates were obtained from 17 patients between September 2004 and August 2005 at the Academisch Ziekenhuis Vrije Universiteit Brussel, Brussels, Belgium. These multidrug-resistant isolates, which belonged to a single clone, remained susceptible to colistin and tigecycline only and produced the carbapenem-hydrolyzing oxacillinase OXA-58. This study highlights the importance of the intercountry spread of this β-lactamase-mediated resistance mechanism and its epidemic evolution.
Imipenem and meropenem are among the drugs of choice for the treatment of nosocomial infections due to multidrug-resistant (MDR) Acinetobacter baumannii (2). However, their efficacies are increasingly being compromised and carbapenem-resistant isolates are becoming widespread in several regions of the world (7, 16). A large variety of molecular mechanisms for resistance to carbapenems have been reported in A. baumannii, i.e., not only the acquisition of carbapenem-hydrolyzing β-lactamases of molecular Ambler class B (metalloenzymes) and the acquisition of mostly class D enzymes (oxacillinases) (19, 24) but also rare mutations in genes coding for penicillin-binding proteins (9) and decreased outer membrane permeability (5). Whereas the class B enzymes found in A. baumannii are of the IMP and VIM types (24), the acquired carbapenem-hydrolyzing class D β-lactamases (CHDLs) are members of three subgroups of enzymes: the OXA-23, OXA-24, and OXA-58 enzymes (16, 18). OXA-58 shares less than 50% amino acid identity with the members of two other CHDL groups, which share 60% amino acid sequence identity. Recently, a fourth subgroup of OXA β-lactamases sharing less than 63% amino acid identity with subgroups 1 and 2 has been identified (4, 11). These enzymes (OXA-69-like enzymes) correspond to naturally occurring oxacillinases of A. baumannii; and it is likely that their overexpression, associated with a degree of an outer membrane permeability defect, may partially explain carbapenem resistance in A. baumannii (12).
Outbreaks of CHDL-producing A. baumannii isolates have increasingly been reported worldwide: OXA-24 in Spain; OXA-40 in Portugal; OXA-23 in Brazil, China, Singapore, and the United Kingdom (3); and OXA-58 in several countries worldwide (7, 10, 20).
The aim of this study was to analyze the molecular mechanisms of carbapenem resistance in A. baumannii isolates identified at the university hospital of the Academisch Ziekenhuis Vrije Universiteit Brussel (AZ-VUB). On 20 August 2004, a couple living in Belgium was involved in a severe motor vehicle traffic accident in Greece that resulted in severe injuries. They were initially hospitalized in the same general district hospital and were subsequently referred to two different hospitals in the north of Greece. The 17-year-old woman was transferred to the intensive care unit (ICU) of AZ-VUB on 29 August 2004. Her 20-year-old husband was repatriated to Belgium a few days later and was also admitted to AZ-VUB on 8 September 2004. Bacteriological screening of samples of the wounds obtained on the day of their admission revealed the presence of MDR A. baumannii strains susceptible only to colistin.
Despite the increased awareness of the MDR A. baumannii strains and the implementation of strict barrier precautions, MDR A. baumannii strains with the same antibiotic resistance phenotype were subsequently isolated from 17 patients over a 6-month period. In all patients, the MDR A. baumannii isolates were detected from various clinical samples. Twelve of the 17 patients (71%) were hospitalized in the ICU at the time of isolation of these organisms. This was very unusual, since during the 2 years before the outbreak, only three carbapenem-nonsusceptible strains (resistant isolates or isolates with intermediate susceptibility) of Acinetobacter spp. (1.2%) had been detected in the laboratory of the AZ-VUB hospital.
The A. baumanni isolates recovered during this outbreak and the clinical data for all 17 patients were analyzed (Table 1). The patients' ages ranged from 17 to 77 years (mean age, 58 years). Twelve patients (70.6%) were infected and five patients (29.4%) were colonized, according to national Belgian criteria (NSIH surveillance data, 2000-2003 report [http://www.nsih.be/about/rap_fr.asp]) and to those of the Centers for Disease Control and Prevention (13). Most A. baumannii isolates were isolated from the respiratory tracts (13 patients) of ICU patients, but transmission also occurred in wards other than the ICU (general medical ward, general surgery).
TABLE 1.
Case histories of patients infected with OXA-58-producing A. baumannii strains
| Patient | Straina | PFGE no. | Date of hospitalization (mo/day/yr) | Age (yr) | Sexb | Ward | Date of isolation (mo/day/yr) | Underlying disease | Site of isolation | Treatmentc | Outcomed | PFGE typee |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 04/0574 | 1 | 09/08/04 | 20 | M | ICU | 09/08/04 | Multiple injuries | Blood | MEM, GM | A′ | |
| B | DIV5521 | 2 | 08/10/04 | 72 | M | General medical | 09/09/04 | Coronary surgery, COPDf | Bronchial aspirate | CS, GM | A′ | |
| C | DIV5523 | 3 | 09/15/04 | 69 | M | ICU | 10/05/04 | Stroke | Bronchial aspirate | Deceased | A′ | |
| D | DIV5525 | 4 | 09/05/04 | 17 | M | ICU | 10/02/04 | Multiple injuries | Bronchial aspirate | CS, GM | A′ | |
| E | DIV5531 | 5 | 09/07/04 | 21 | M | ICU | 10/04/04 | Multiple injuries | Wound | A′ | ||
| F | DIV5547 | 6 | 11/17/04 | 66 | M | General medical | 12/08/04 | COPD | Sputum | CS, GM | A′ | |
| F | DIV5548 | 7 | 12/12/04 | Sputum | CS, GM | A′ | ||||||
| G | 05/0045 | 8 | 12/06/04 | 77 | M | General medical | 01/04/05 | COPD | Sputum | MEM, GM | A′ | |
| H | 05/0046 | 9 | 10/25/04 | 55 | F | Abdominal surgery | 01/10/05 | Necrotizing fasciitis | Wound | Deceased | A′ | |
| I | 05/0076 | 10 | 12/24/04 | 58 | M | ICU | 01/13/05 | COPD | Sputum | A′ | ||
| J | 05/0292 | 11 | 01/28/05 | 74 | F | ICU | 02/20/05 | Stroke | Sputum | CS, GM | Deceased | A" |
| K | 05/0324 | 12 | 02/20/05 | 67 | M | ICU | 02/27/05 | Stroke | Blood | CS, GM | Deceased | A" |
| L | 05/0372 | 13 | 03/04/05 | 48 | F | ICU | 03/09/05 | Multiple injuries | Bronchial aspirate | CS, GM | Deceased | A" |
| M | 05/0382 | 14 | 02/27/05 | 65 | M | ICU | 03/13/05 | Coronary surgery | Bronchial aspirate | CS, GM | A" | |
| N | 05/0403 | 15 | 02/17/05 | 75 | F | ICU | 03/17/05 | Coronary surgery | Sputum | CS, GM | A" | |
| O | 05/0404 | 16 | 03/13/05 | 60 | M | ICU | 03/18/05 | Stroke | Sputum | CS, GM | A" | |
| P | 05/0405 | 17 | 03/13/05 | 71 | M | ICU | 03/17/05 | Diabetic ketoacidosis, stroke | Sputum | Deceased | A" | |
| Q | 05/406 | 18 | 03/15/05 | 70 | M | Neurology | 03/16/05 | Pulmonary neoplasm | Bronchial aspirate | A" |
All isolates were imipenem resistant and blaOXA-58 positive by PCR except strain DIV5548, which was imipenem sensitive and blaOXA-58 negative by PCR.
M, male; F, female.
Only treatments for patients who received antibiotic treatment for their A. baumannii infections are shown. The treatments consisted of meropenem (MEM), gentamicin (GM), and colistin (CS). Colistin was administered three times a day for a total dose of 6 × 106 U/day; the dose was reduced in the case of renal insufficiency; gentamicin was administered at 6 mg/kg of body weight once a day, with further adjustment to levels in serum.
Patient L died after 35 days; and patients C, H, J, K, and P died within 28 days.
Pulsotypes are those according to Tenover et al. (23).
COPD, chronic obstructive pulmonary disease.
The A. baumannii isolates were identified by means of a commercial identification gallery (API 32GN system; BioMérieux, Marcy l'Etoile, France). Antimicrobial susceptibility testing was performed by the disk diffusion method and by using the interpretative criteria of the Clinical and Laboratory Standards Institute (6). Overall, 18 MDR Acinetobacter baumannii isolates recovered from 17 patients (Table 1) were sent to a central reference laboratory for further analysis. The MICs of the different antibiotics were determined by Etest (AB Biodisk, Solna, Sweden), according to the manufacturer's instructions. Pulsed-field gel electrophoresis (PFGE) was performed with ApaI-restricted whole-cell DNAs embedded in 1% agarose plugs and separated in a 1% pulsed-field-certified agarose gel by using a contour-clamped homogeneous electric field DRII system (Bio-Rad, Marnes-La-Coquette, France), as described previously (10). DNA (genomic and plasmid) extractions and analysis, isoelectric focusing, and conjugation assays with rifampin-resistant A. baumannii strain CIP 7010 (MIC > 256 μg/ml) and strain 04/0574 (MIC = 4 μg/ml) were performed as described previously (10).
Genes coding for Ambler class B and D carbapenemases were sought by PCR with primers specific for the blaIMP (22), blaVIM (22), blaOXA-23-like (8), blaOXA-26-like (1), and blaOXA-58 and blaOXA-69-like (20) genes. Similarly, the class A β-lactamase blaTEM gene (10), the blaOXA-20 gene (forward primer, 5′-GATGGGACGGCGCTAAAAGA-3′; reverse primer, 5′-TACCCAACCGACCCACCAAC-3′), the chromosomal class C β-lactamase blaAmpC gene, and the presence of ISAba1 inserted upstream of a blaAmpC β-lactamase gene were sought by PCR (21). Both strands of the PCR products were sequenced with an automated sequencer (ABI 3100; Applied Biosystems, Foster City, CA). The nucleotide and deduced amino acid sequences were analyzed with software available over the Internet (http://www.ncbi.nlm.nih.gov/).
Seventeen of the 18 A. baumannii isolates were resistant to all β-lactams, including imipenem (MICs, ≥32 μg/ml) and meropenem (MICs, 4 to >32 μg/ml); ciprofloxacin (MICs, >32 μg/ml); and amikacin (MICs, >256 μg/ml). Various susceptibilities were found for rifampin (MICs, 4 to >256 μg/ml). The isolates remained susceptible only to tigecycline (MICs, 2 μg/ml) and were borderline susceptible to colistin (MICs, 3 μg/ml). Routine susceptibility tests revealed the heterogeneous expression of resistance to some β-lactam agents, as shown by the growth of colonies inside the zones of inhibition of the imipenem, meropenem, and aztreonam disks. Following subcultures, the antibiotic susceptibility patterns yielded resistance patterns similar to those for the original isolates. The MICs of sulbactam (32 μg/ml), meropenem (>32 μg/ml), and imipenem (>32 μg/ml) for strain 04/0574, isolated from patient A (the index patient) on the day of admission, were higher than those for strain DIV5547 (sulbactam MIC, 8 μg/ml; meropenem MIC, 4 μg/ml; imipenem MIC, 32 μg/ml) from patient F, suggesting that even though the resistant strains are clonally related, they might exhibit different patterns of resistance. While this phenomenon is not yet fully understood, it has already been reported in Acinetobacter baumannii (20). It is tempting to suggest that rearrangements in plasmids and/or modification in outer membrane proteins could account for the different MICs, as recently shown for a carbapenem-resistant Klebsiella pneumoniae strain with blaVIM-1 (14). By contrast, strain DIV5548, which was also isolated from patient F 4 days after the recovery of the first isolate, was found to be susceptible both to imipenem (MIC, 0.5 μg/ml) and to meropenem (MIC, 3 μg/ml).
Seventeen of the A. baumannii isolates were positive for blaOXA-58-like, blaOXA-20, blaOXA-69-like, and natural and chromosomally located blaAmpC genes. Carbapenem-susceptible strain DIV5548 was positive only for the blaOXA20, blaOXA-69-like, and blaAmpC genes. Isoelectric focusing confirmed the expression of OXA-58 (pI 7.0), OXA-20 (pI 7.5), and AmpC (pI > 8.5). An unidentified band (pI 6.2) was also observed by isoelectric focusing and could not be associated with the OXA-69-like enzymes since their expected pIs are about 8.5 (data not shown) (4, 11). Sequencing of the amplified fragments confirmed the presence of these genes and showed that the sequence of the blaOXA-58 gene was identical to that of the prototype OXA-58 β-lactamase described in Toulouse, France (10), and other southern European regions (7, 15), thus underscoring the wide geographical spread of this gene. The presence of a blaOXA-69-like gene was confirmed by PCR, and sequencing revealed the presence of the blaOXA-76 alleles previously described in A. baumannii isolates from different regions (Spain, Turkey, Hong Kong, and Singapore) (4, 11).
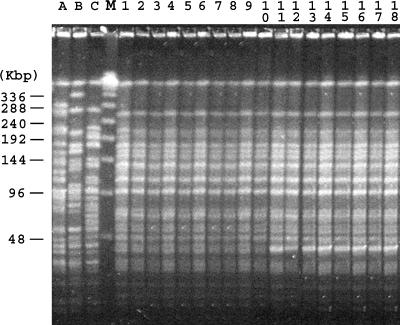
In line with previous findings (10, 20), the blaOXA-58 gene was plasmid mediated. In all carbapenem-resistant isolates, a 50-kb plasmid was present but it was not self-transferable to a rifampin-resistant A. baumannii strain. Electroporation of an isolated plasmid into A. baumannii CIP7010 identified the blaOXA-58 gene in the electroporants. Héritier et al. (11) demonstrated that OXA-58 has a weak carbapenemase activity and plays a role in carbapenem resistance in A. baumannii, particularly when it is highly expressed. The isolates from the different wards of the hospital gave similar PFGE patterns (Fig. 1), with the patterns differing by only one band for some isolates. Imipenem-susceptible isolate DIV5548 from patient F showed the same PFGE pattern as the carbapenem-resistant strains (Fig. 1) but lacked blaOXA-58. This observation confirms, as recently suggested by Poirel and Nordmann (17), that OXA-58-producing A. baumannii isolates may occasionally lose the blaOXA-58 gene and its surrounding genetic structure in the absence of carbapenem selection pressure. The blaOXA-76 gene was, however, detected by PCR in this strain, further suggesting that these β-lactamase genes may be present in carbapenem-susceptible strains, as shown by Héritier et al. (12).
FIG. 1.
PFGE patterns of A. baumannii isolates. The assigned numbers of A. baumannii isolates are shown over the lanes of the gel and correspond to those in Table 1. The positions of the molecular size markers (lane M; in kilobases) are shown on the left side of the gel. Lanes A, B, and C, OXA-58-producing isolates MAD (18), MAC (15), and SUR (15), respectively, isolated in France.
From March to September 2005, 11 novel carbapenem-resistant A. baumannii isolates were identified from patients hospitalized in the AZ-VUB hospital. All strains were analyzed by arbitrarily primed PCR and were clonally related to the previously described strains (data not shown). Since September 2005, only one carbapenem-resistant A. baumannii isolate was identified, and this occurred after the admission to the AZ-VUB hospital of a patient transferred from South Africa. This isolate expressed an OXA-23 carbapenemase and was unrelated to the OXA-58 epidemic clone, as determined by arbitrarily primed PCR. No new carbapenem-resistant cases related to the OXA-58 outbreak were detected.
OXA-58-producing A. baumannii isolates have been reported in several countries, suggesting that they have a wide distribution (7, 15, 20). Hospital outbreaks have been so far documented in France and Greece. This is the first description of OXA-58 carbapenem-resistant A. baumannii isolates in Belgium and the first documented intercountry transfer, from Greece to Belgium, of an OXA-58-producing A. baumannii strain. Importantly, the fact that a large outbreak arose in the hospital, despite the early implementation of appropriate barrier precautions, underlines the epidemic potential of such resistant strains.
Our study also highlights the importance of international travel in the spread of antimicrobial resistance. The global emergence of carbapenemase genes is worrying; and consideration should be given to screening all patients admitted to hospital from a foreign country, especially countries that are known to have a high incidence of MDR strains, for multidrug-resistant pathogens and to isolating all patients infected or colonized with such strains.
Acknowledgments
This work was funded in part by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, Paris, France, and mostly by the European Community (6th PCRD, LSHM-CT-2003-503-335).
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Afzal-Shah, M., N. Woodford, and D. M. Livermore. 2001. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D beta-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 45:583-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, S., and S. Amyes. 2006. OXA β-lactamases in Acinetobacter: the story so far. J. Antimicrob. Chemother. 57:1-3. [DOI] [PubMed] [Google Scholar]
- 4.Brown, S., H. K. Young, and S. G. B. Amyes. 2005. Characterisation of OXA-51, a novel class D carbapenemase found in genetically unrelated clinical strains of Acinetobacter baumannii from Argentina. Clin. Microbiol. Infect. 11:11-15. [DOI] [PubMed] [Google Scholar]
- 5.Clark, R. B. 1996. Imipenem resistance among Acinetobacter baumannii: association with reduced expression of a 33-36 kDa outer membrane protein. J. Antimicrob. Chemother. 38:245-251. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 7.Coelho, J., N. Woodford, M. Afzal-Shah, and D. Livermore. 2006. Occurrence of OXA-58-like carbapenemases in Acinetobacter spp. collected over 10 years in three continents. Antimicrob. Agents Chemother. 50:756-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donald, H. M., W. Scaife, S. G. Amyes, and H. K. Young. 2000. Sequence analysis of ARI-1, a novel OXA β-lactamase, responsible for imipenem resistance in Acinetobacter baumannii 6B92. Antimicrob. Agents Chemother. 44:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gehrlein, M., H. Leying, W. Cullmann, S. Wendt, and W. Opferkuch. 1991. Imipenem resistance in Acinetobacter baumannii is due to altered penicillin-binding proteins. Chemotherapy 37:405-412. [DOI] [PubMed] [Google Scholar]
- 10.Héritier, C., A. Dubouix, L. Poirel, N. Marty, and P. Nordmann. 2005. A nosocomial outbreak of Acinetobacter baumannii isolates expressing the carbapenem-hydrolysing oxacillinase OXA-58. J. Antimicrob. Chemother. 55:115-118. [DOI] [PubMed] [Google Scholar]
- 11.Héritier, C., L. Poirel, T. Lambert, and P. Nordmann. 2005. Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:3198-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Héritier, C., L. Poirel, P. E. Fournier, J. M. Claverie, D. Raoult, and P. Nordmann. 2005. Characterization of the naturally occurring oxacillinase of Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:4174-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horan, T. C., and R. P. Gaynes. 2004. Surveillance of nosocomial infections, p. 1653-1702. In C. G. Mayhall (ed.), Hospital epidemiology and infection control, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 14.Loli, A., L. S. Tzouvelekis, E. Tzelepi, A. Carattoli, A. C. Vatopoulos, P. T. Tassios, and V. Miriagou. 2006. Sources of diversity of carbapenem resistance levels in Klebsiella pneumoniae carrying blaVIM-1. J. Antimicrob. Chemother. 58:669-672. [DOI] [PubMed] [Google Scholar]
- 15.Marqué, S., L. Poirel, C. Héritier, S. Brisse, M. D. Blasco, R. Filip, G. Coman, T. Naas, and P. Nordmann. 2005. Regional occurrence of plasmid-mediated carbapenem-hydrolyzing oxacillinase OXA-58 in Acinetobacter spp. in Europe. J. Clin. Microbiol. 43:4885-4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordmann, P., and L. Poirel. 2002. Emerging carbapenemases in gram-negative aerobes. Clin. Microbiol. Infect. 8:321-331. [DOI] [PubMed] [Google Scholar]
- 17.Poirel, L., and P. Nordmann. 2006. Genetic structures at the origin of the acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-58 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:1442-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poirel, L., S. Marque, C. Héritier, C. Segonds, G. Chabanon, and P. Nordmann. 2005. OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:202-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirel, L., and P. Nordmann. 2002. Acquired carbapenem-hydrolyzing beta-lactamases and their genetic support. Curr. Pharm. Biotechnol. 3:117-127. [DOI] [PubMed] [Google Scholar]
- 20.Pournaras, S., A. Markogiannakis, A. Ikonomidis, L. Kondyli, K. Bethimouti, A. N. Maniatis, N. J. Legakis, and A. Tsakris. 2006. Outbreak of multiple clones of imipenem-resistant Acinetobacter baumannii isolates expressing OXA-58 carbapenemase in an intensive care unit. J. Antimicrob. Chemother. 57:557-561. [DOI] [PubMed] [Google Scholar]
- 21.Segal, H., E. C. Nelson, and B. G. Elisha. 2004. Genetic environment and transcription of ampC in an Acinetobacter baumannii clinical isolate. Antimicrob. Agents Chemother. 48:612-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senda, K., Y. Arakawa, S. Ichiyama, K. Nakashima, H. Ito, S. Ohsuka, K. Shimokata, N. Kato, and M. Ohta. 1996. PCR detection of metallo-β-lactamase gene (blaIMP) in gram-negative rods resistant to broad-spectrum beta-lactams. J. Clin. Microbiol. 34:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenover, F. C., R. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]