Abstract
Trichomonas vaginalis infection in men is an important cause of nongonococcal urethritis. Effective detection of the parasite in men using culture requires examination of multiple specimens. We compared culture and PCR-enzyme-linked immunosorbent assay in urethral swabs, urine, and semen for T. vaginalis detection in male sexual partners of women with trichomoniasis identified by wet mount and culture. Trichomonads were detected by at least one positive test in 205/280 men (73.2%) who submitted at least one specimen for culture and PCR. Whereas InPouch TV culture detected only 46/205 cases (22.5%), PCR detected 201/205 (98.0%). Urethral swab cultures from men with urethritis were more likely to be positive with shorter incubation than specimens from men without urethritis. T. vaginalis was detected more often in men with wet-mount-positive partners. Even with a sensitive PCR assay, reliable detection of T. vaginalis in male partners required multiple specimens. The majority of male sexual partners in this study were infected, emphasizing the importance of partner evaluation and treatment.
Infection with the protozoan parasite Trichomonas vaginalis is the most common nonviral sexually transmitted infection (STI), with prevalence estimates frequently surpassing those for gonorrhea and chlamydia (36). Infection of the female genital tract can result in vaginitis, cervicitis, and urethritis, and trichomoniasis has been associated with adverse pregnancy outcomes (4, 23, 26). Though it was once virtually ignored, T. vaginalis infection in men is now recognized as an important cause of nongonococcal urethritis (9, 27, 29) and is associated with prostatitis (17, 25, 31) and male factor infertility (6, 21). In addition, trichomoniasis is a risk factor for sexual transmission of human immunodeficiency virus (HIV) (1, 3, 5, 19). T. vaginalis disrupts the urogenital epithelia and enhances HIV replication in vitro (7). Increased vaginal and endocervical inflammation in women and urethral inflammation in men with trichomoniasis likely contribute to enhanced HIV transmission. Because trichomoniasis is so widespread, substantial proportions of HIV infections might be attributable to T. vaginalis infection in populations where both are prevalent (2, 32).
Few studies have examined concordant trichomoniasis in sexual partners. In studies conducted in the early 1990s using culture for T. vaginalis detection, infection was identified in 22 to 48% of male partners of women with trichomoniasis (14, 18). Since that time, more-sensitive nucleic acid amplification assays have been developed for detection of the parasite (8, 10, 11, 13, 20, 22, 30). T. vaginalis detection in men is also improved when multiple urogenital specimens are tested (12, 15, 16). The current study was designed to examine the concordance of T. vaginalis infection in the male sexual partners of women with trichomoniasis attending 3 sexually transmitted diseases (STD) clinics in the United States. In this report, we focus on the performance of culture and PCR from urethral swabs, urine, and semen and culture from vaginal swabs for T. vaginalis detection in women and their male partners.
MATERIALS AND METHODS
Study design and sample.
Women with trichomoniasis and their male sexual partners were enrolled in the public health department STD clinics located in Durham and Raleigh, North Carolina, and in Birmingham, Alabama, in a clinical study designed to examine concordance of T. vaginalis infection among sexual partners. The study design is described in detail elsewhere (30a). Briefly, women with trichomoniasis were identified on the basis of a vaginal swab positive by wet mount or culture and were asked to identify their most frequent and most recent sexual partner. The “most frequent” sexual partner was defined as the partner with whom the woman had the greatest number of sexual encounters in the preceding 60 days; the “most recent” partner was defined as the partner with whom the subject last had sex prior to enrollment. Male partners aged 18 years and older who presented to one of the STD clinics following standard referral or conditional partner notification efforts were eligible for the study. Human experimentation guidelines of the U.S. Department of Health and Human Services were followed in the conduct of this research. All enrolled subjects provided written informed consent. The study was approved by the Institutional Review Boards of the University of North Carolina at Chapel Hill and the University of Alabama at Birmingham.
Clinical data and specimen collection.
Study subjects received standard care and treatment in the clinics and then responded to a questionnaire including questions on demographics, STD signs and symptoms, STD history, and sexual behaviors. Study specimens were obtained after routine clinic specimens; for the men, this included a urethral swab for T. vaginalis culture and first-catch urine for gonorrhea and chlamydia testing (Amplicor CT/NG PCR; Roche Diagnostics, Indianapolis, IN), T. vaginalis culture, and PCR. To minimize discomfort for study subjects, only a single urethral swab was collected. This swab was used for culture but not PCR, since the former is an FDA-cleared test for detection of T. vaginalis. Men were also asked to provide a semen specimen for T. vaginalis culture and PCR. After specimen collection, all partners were given metronidazole (2 g orally in a single dose).
All specimens were initially processed and culture pouches were inoculated at the local health departments. Urine and semen specimens were transported to research laboratories at University of North Carolina at Chapel Hill or University of Alabama at Birmingham for further processing and PCR testing as described below.
T. vaginalis culture.
Vaginal swabs from women and urethral swabs from men were immediately used to inoculate the InPouch TV culture system (Biomed, White City, OR). For urine sediment cultures, 10 ml of first-catch urine was centrifuged for 10 min at 1,500 rpm at room temperature. The supernatant was aspirated, 0.25 ml of Diamond's modified medium (Remel, Lenexa, KS) was added to the pellet, and 0.05 ml of resuspended sediment was used to inoculate the culture. The remainder of the resuspended urine sediment was stored at 4°C for up to 4 days before processing for PCR. Semen was allowed to liquefy and was centrifuged for 10 min at 1,500 rpm at room temperature. The sediment from 0.5 to 1 ml of semen was inoculated into an InPouch TV system, and the remaining sample was centrifuged, resuspended in 0.1 ml of Diamond's medium, and stored at 4°C for up to 4 days before preparation for PCR. When the semen volume was ≤1 ml, culture but not PCR was performed. All cultures were incubated in a humidified atmosphere with 5% CO2 at 37°C and examined for at least 1 min per sample by a trained microscopist daily for 5 days or until a positive result was obtained. A positive culture was defined as visualization of parasites with morphology and motility characteristic of T. vaginalis. No motile parasites were observed at any reading in negative cultures.
T. vaginalis PCR-ELISA.
For amplification of T. vaginalis DNA, we used the previously described TV PCR-enzyme-linked immunosorbent assay (ELISA) (11) with a slight modification of the specimen processing procedure. Urine or semen sediment that had been resuspended in Diamond's medium (0.5 ml) was added to an equal volume of CT/NG urine wash solution from the Amplicor CT/NG urine specimen prep kit (Roche Applied Science, Indianapolis, IN) and further processed according to the manufacturer's instructions. Specimens with less than 0.5 ml were adjusted to 0.5 ml with sterile phosphate-buffered saline. Fifty microliters of prepared sample was used as a template in the PCR as previously described (11). Briefly, primers TVK3 and TVK7 (digoxigenin labeled) (13) were used to amplify T. vaginalis DNA. Positive and negative controls were purified T. vaginalis DNA and sterile water, respectively. PCR products were detected using the PCR DIG ELISA detection kit (Roche Diagnostic Systems) with the biotinylated TVK probe and ELISA controls as described previously (10). Standard measures were taken to minimize contamination. Separate workspaces were maintained for specimen processing, PCR, and post-PCR work, and sterile, disposable laboratory supplies were used.
PCR primers TVK3 and TVK7 were previously tested against human DNA, a variety of sexually transmitted pathogens, and other Trichomonas species and were shown to amplify only T. vaginalis DNA (13). Additional analytical specificity was provided by the requirement for hybridization of the internal TVK probe; any DNA spuriously amplified by the primers would not produce a positive PCR-ELISA result. In first-catch urine (≤30 ml) from men, the assay has a sensitivity of 92.7% and an adjusted specificity (corrected to account for the imperfect sensitivity of T. vaginalis culture) of 95.2% compared to culture of urethral swabs or urine sediment (11).
Data analysis.
Laboratory data were double entered by two different members of the study staff into a database created on EpiInfo 6.02 (CDC, Atlanta, GA). Data were analyzed, without identifiers, using SAS version 8 (SAS institute, Cary, NC), STATA version 7 (Stata Corp., College Station, TX), and SigmaStat version 3.11 (Systat Software, Inc., Point Richmond, CA). For analyses of specimens from the male partners, results from all men were included, whether they were identified as the most frequent or the most recent partner of a woman in the study. For women with 2 partners enrolled, results from only the most frequent partner were included in analyses of associations involving characteristics of the women.
RESULTS
Study population and specimens.
A total of 540 women with trichomoniasis were enrolled in the study from November 2001 through July 2003; 287 male partners of 261 women were contacted and agreed to participate. Table 1 summarizes the demographic and clinical characteristics of the participants. While the majority of women (74%) in the study complained of symptoms including vaginal discharge, vaginal itching, dysuria, or lower abdominal pain, most of the men (75%) were asymptomatic.
TABLE 1.
Characteristics of study participants
| Characteristic | No. (%) of:
|
|
|---|---|---|
| Women with trichomoniasis (n = 540) | Male partners (n = 287) | |
| Age range (yr) | ||
| <20 | 54 (10.0) | 15 (5.2) |
| 20-24 | 143 (26.5) | 70 (24.4) |
| 25-29 | 94 (17.4) | 56 (19.5) |
| 30-39 | 145 (26.9) | 71 (24.7) |
| ≥40 | 104 (19.3) | 75 (26.1) |
| Race/ethnicity | ||
| Black, non-Hispanic | 503 (93.2) | 278 (96.9) |
| White, non-Hispanic | 32 (5.9) | 3 (1.1) |
| Black, Hispanic | 1 (0.2) | 1 (0.4) |
| White, Hispanic | 1 (0.2) | 0 (0.0) |
| Other | 3 (0.6) | 5 (1.7) |
| Clinical observations | ||
| Asymptomatica | 140 (26.0) | 215 (74.9) |
| Abnormal vaginal or penile dischargeb | 449 (83.2) | 81 (28.2) |
| Elevated WBCs on cervical or urethral Gram stainc | 249/290 (85.9) | 95 (33.1) |
| STId | ||
| T. vaginalis | 540 (100.0) | 206/287 (71.8) |
| N. gonorrhoeae | 49 (9.9) | 27/281 (9.6) |
| C. trachomatis | 52 (11.9) | 31/277 (11.2) |
| Syphilis | 12 (2.4) | 7/253 (2.8) |
| HIV | 4 (1.5) | 0/113 (0.0) |
Patients denied symptoms, including vaginal or penile discharge or itching, dysuria, or lower abdominal pain.
Observed by clinician during physical exam.
Elevated WBC is defined as >5 or >4 WBC/hpf for cervical or urethral Gram stain, respectively.
T. vaginaliswas diagnosed by vaginal swab wet mount or culture for women and urethral, urine, or semen culture or urine or semen PCR for men. N. gonorrhoeae and C. trachomatiswere diagnosed by Amplicor CT/NG PCR from first-void urine for men and according to routine clinic protocols for women. Syphilis was diagnosed by positive rapid plasma reagin and confirmed by positive Treponema pallidum hemagglutination assay. HIV was diagnosed by ELISA and confirmed by Western blotting.
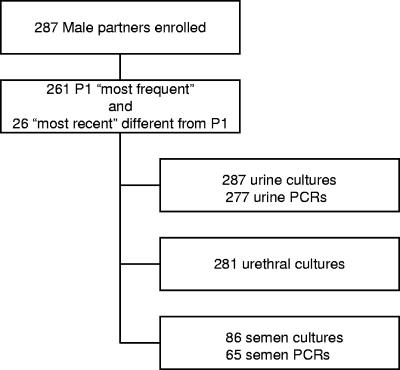
All 287 men provided first-catch urine for T. vaginalis culture; 10 urine specimens could not be processed for PCR because of transport or laboratory problems, and 6 men declined to provide a urethral swab for T. vaginalis culture (Fig. 1). Eighty-six men provided semen for T. vaginalis culture; 65 of these specimens contained a sufficient volume for PCR.
FIG. 1.
Specimens obtained from male partners of women with trichomoniasis. From 540 women in the study, 287 male partners were enrolled. For analyses of specimens from the male partners, results from all men were included, whether they were identified as a woman's most frequent partner (the partner with whom the woman had the greatest number of sexual encounters in the preceding 60 days) or most recent partner (the partner with whom the subject last had sex prior to enrollment). For women with 2 partners enrolled, results from only the most frequent partner were included in analyses of associations involving characteristics of the women.
T. vaginalis detection by culture.
InPouch TV cultures were inoculated with vaginal swabs from women or with urethral swabs, urine sediment, or semen sediment from men and vaginal swabs from women, and cultures were examined daily for up to 5 days as described in Materials and Methods. We compared the time to positive culture for 67 positive specimens from men and 79 positive vaginal swabs from women with negative wet mount results. Seventeen percent of positive cultures did not become positive until 4 or 5 days after inoculation. Results were similar for specimens from men and women (data not shown). The day on which male cultures became positive was not influenced by whether or not cultures from the female partner had a wet-mount-positive result. Urethral swab cultures from men with trichomoniasis and urethritis (urethral discharge or >4 white blood cells [WBC]/high-power field [hpf] on urethral gram stain) in the absence of gonorrhea or chlamydial infection were slightly more likely to be positive on the first day after inoculation than urethral swab cultures from men with asymptomatic trichomoniasis (P = 0.076, Fisher's exact test).
Significantly more trichomoniasis cases were identified by PCR than by culture.
Positive specimens and Trichomonas detection test combinations are shown in Table 2. Among 287 male sexual partners of women with trichomoniasis who submitted at least one specimen for culture, T. vaginalis was detected in 47 (16.4%) men. PCR identified 4 times more infections (P < 0.001, chi-square test); T. vaginalis was detected in 201 (71.8%) of 280 partners who submitted at least one specimen for PCR (Table 2). Overall, T. vaginalis was detected by at least one positive test in 205/280 (73.2%) men who submitted at least one specimen for culture and at least one specimen for PCR. InPouch TV culture detected 47/205 cases (22.9%), and PCR detected 201/205 cases (98.0%).
TABLE 2.
T. vaginalis detection in urogenital specimens from male sexual partners of women with trichomoniasis
| Test | Specimen | No. positive/no. tested | % Positive | 95% CIc |
|---|---|---|---|---|
| Culturea | Urethral swab | 35/281 | 12.5 | 8.8-16.9 |
| Urine | 23/287 | 8.0 | 5.2-11.8 | |
| Semen | 9/86 | 10.5 | 4.9-18.9 | |
| Any | 47/287 | 16.4 | 12.3-21.2 | |
| PCRb | Urine | 192/277 | 69.3 | 63.5-74.7 |
| Semen | 35/65 | 53.8 | 41.0-66.3 | |
| Any | 201/280 | 71.8 | 66.1-77.0 |
InPouch TV cultures read daily for up to 5 days after inoculation.
PCR-ELISA with primers TVK3/7.
Exact 95% CI.
Testing multiple urogenital specimens from men increased detection of T. vaginalis.
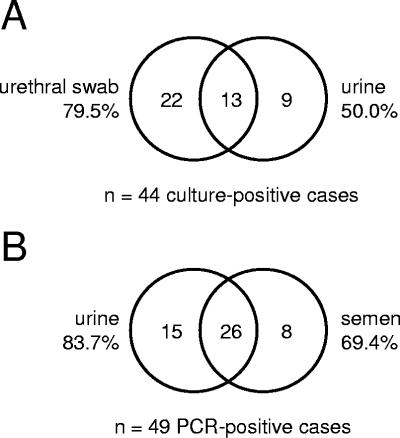
Among 281 sets of urine specimens and urethral swabs from male sexual partners of women with trichomoniasis, T. vaginalis was detected by culture from at least one specimen in 44 (15.7%) cases. Urethral swab cultures were more sensitive than urine cultures with 79.5% of cases diagnosed from swabs and 50.0% from urine (Fig. 2A) (P = 0.007, chi-square test). In a subset of 84 men who provided urine specimens, urethral swabs, and semen for culture, T. vaginalis was detected in at least 1 specimen in 12 (14.3%) cases. Five urethral swabs, 8 urine specimens, and 9 semen specimens were positive; all 3 specimens were positive in only 3 (25%) cases. In this small subset of samples, the sensitivities of the 3 specimens were not significantly different. Comparisons of PCR detection in specimens from men in this study are limited to urine and semen. From 62 men who provided both urine and semen for PCR, T. vaginalis was detected in at least one specimen in 49 (79.0%) cases. Urine was slightly, but not significantly, more sensitive than semen (Fig. 2B) (P = 0.144, McNemar's chi-square test).
FIG. 2.
T. vaginalis detection in multiple urogenital specimens from men. (A) Cases detected by use of urethral swab culture and urine specimen culture. A total of 44 infections were identified from 281 men. (B) Cases detected by use of urine or semen PCR. A total of 49 infections were identified from 69 men.
Associations with T. vaginalis infection in male partners.
In this study, there were no significant associations between T. vaginalis infection in men and urethritis (urethral discharge or >4 WBC/hpf on urethral gram stain) or chlamydial infection. The prevalence of gonorrhea was higher in male partners with trichomoniasis than in men in whom T. vaginalis was not detected. This association was not statistically significant for men in whom T. vaginalis was detected using urine (prevalence ratio, 1.4; 95% confidence interval [CI], 0.6 to 3.4) or urethral swab samples (prevalence ratio, 1.6; 95% CI, 0.6 to 3.8). However, among 63 men who provided semen for T. vaginalis detection by PCR, the 2 infections were significantly associated (P = 0.027, Fisher's exact test). In this subset, gonorrhea was detected only in partners in whom T. vaginalis was detected in the semen.
Trichomoniasis in men in the study, defined as T. vaginalis detection in any specimen by culture or PCR, was influenced by the female partner's wet mount result (P = 0.044, chi-square test). Nearly 75% (169/226) of men with wet-mount-positive partners were infected with T. vaginalis, compared to 60.4% (29/48) of men with wet-mount-negative partners (odds ratio, 1.94; 95% CI, 1.01 to 3.73).
DISCUSSION
We detected T. vaginalis in 72% of the male sexual partners of women with trichomoniasis, using culture and PCR from urine, urethral swabs, and semen. Not surprisingly, substantially more T. vaginalis infections in men were detected by PCR than by culture. Others have reported similar results in recent studies of T. vaginalis in men attending STD clinics (30, 35). The study was designed to examine concordant infection in the partners of women with trichomoniasis, and positive index cases were defined using wet mount microscopy and culture but not PCR. Because of the case ascertainment bias inherent in using less-sensitive detection methods, the female study population consisted of women with generally high organism burdens of T. vaginalis, which is reflected in the large proportion of symptomatic women. Consequently, the sexual partners of women in the study comprised a population of men with high exposure to T. vaginalis.
The T. vaginalis PCR assay used in this study was previously validated with men's urine (12); this is the first report of its use with semen. There were too few culture-positive cases in men identified in the current study to provide a valid reference standard for conventional sensitivity and specificity calculations for the assay with semen, but we expect that the test performs similarly with urine and semen. A limitation of the assay is the lack of an internal amplification control. If the specimen preparation procedure did not eliminate PCR inhibitors, false-negative results could have occurred. Thus, the prevalence of T. vaginalis infection in the male partners of infected women may be even higher than reported here.
Whether PCR or culture was used to detect T. vaginalis in this population, testing multiple specimens from men substantially increased the number of cases identified. If only one specimen had been used for culture, 20 to 50% of cases would have been missed. These results are consistent with previous studies (12, 15, 16, 34) documenting recovery of T. vaginalis by culture of multiple genitourinary specimens in men. Using PCR to detect T. vaginalis, fewer missed cases would have resulted from the use of a single specimen; nevertheless, PCR detection from both urine and semen increased the number of cases by 16% compared to urine alone. However, the study design did not permit PCR testing from urethral swabs, and this was the most sensitive sample type for culture. Thus, it is possible that urethral swab PCR may have eliminated the observed benefits of testing multiple specimens.
The incubation time required for positive InPouch TV cultures was similar for specimens from men and vaginal swabs from wet-mount-negative women. Approximately 17% of positive cultures required incubation for more than 3 days. We observed cultures daily for up to 5 days after inoculation, in accordance with the manufacturer's instructions in an early version of the product insert dated August 1999. The current InPouch TV product insert instructs users to read cultures only up to 3 days after inoculation. Following the current recommendations, 1 of every 6 culture-positive specimens would have been falsely classified as negative in this study.
Most of the male partners of women with trichomoniasis in this study were asymptomatic, and overall, urethritis was not significantly associated with trichomoniasis in this population. Schwebke and Lawing also reported that urethritis was not significantly associated with T. vaginalis infection in men attending a U.S. STD clinic, using a combination of culture and PCR (30). Using similar methods in a study conducted in an STD clinic in Malawi, Price et al. showed that, while T. vaginalis infection tended to be asymptomatic in HIV-negative men, the clinical presentation of trichomoniasis was more severe in men with HIV infection (28).
In the present study, cultures from men with urethritis were slightly more likely to be positive on the first day after inoculation than cultures from men without urethritis. Early positive cultures are plausible indicators of infections with higher parasite loads, and urethral inflammation is more likely to accompany such infections. Similarly, Wendel et al. observed an association of urethritis in men with trichomoniasis detected by culture but not by PCR (35). The superior sensitivity of nucleic acid amplification tests compared to T. vaginalis culture certainly results in detection of more asymptomatic trichomoniasis in men by PCR, and such infections may have lower organism burdens with less urethral inflammation. Whether asymptomatic T. vaginalis infections pose a lower transmission risk than symptomatic infections is not clear. Although we cannot determine the direction of transmission within couples in this study, the high rate of concordant infection among sexual partners suggests that transmission does occur in the context of asymptomatic infection in men. Using sensitive detection methods with multiple specimens, self-limiting exposures may also have been identified in some asymptomatic men. Men with positive results from multiple specimens may have had active infection, whereas those with a single positive specimen represent detection of residual DNA.
Parasite loads are probably higher in women with trichomoniasis diagnosed by wet mount microscopy than in wet-mount-negative, culture-positive women with T. vaginalis infections. Consistent with this notion, we observed a higher prevalence of trichomoniasis in the partners of wet-mount-positive women than in the partners of women who were culture positive only. This relationship was most evident when T. vaginalis infection was defined as detection by any positive culture or PCR in the male partners. However, in the related report by Seña et al., similar analyses were conducted using a more restrictive definition of infection in a subset of male partners, and the differences were not statistically significant (30a).
We examined the association of T. vaginalis infection and coinfection with Neisseria gonorrhoeae and Chlamydia trachomatis in the male partners of women with trichomoniasis. Gonorrhea, but not chlamydial infection, was consistently more frequent in men in whom T. vaginalis was detected, regardless of the specimen used for Trichomonas detection. High rates of N. gonorrhoeae and T. vaginalis coinfection in men have been previously reported (9, 24), probably reflecting the common transmission routes and risk factors for the two infections. The relationship between gonorrhea and trichomoniasis in male partners in the current study was particularly strong among men in whom T. vaginalis was detected in semen. The demographic characteristics of men who provided semen were not different from those who did not (data not shown). T. vaginalis in semen could represent more chronic or indolent infections that are not spontaneously cleared. Prolonged effects on the urogenital defense mechanisms of these men may make them more susceptible to gonococcal infection.
In the current study, we identified a higher proportion of T. vaginalis infection in the predominantly asymptomatic partners of women with trichomoniasis than in previous studies that relied on culture for parasite detection. However, even in this population of highly exposed men, reliable detection of T. vaginalis required the use of a sensitive nucleic acid amplification test with multiple urogenital specimens. When T. vaginalis is considered in the clinical evaluation of men, usually only urine or a urethral swab is collected and inadequately sensitive wet mount microscopy or culture is performed. In this and previous studies (12), semen testing has been shown to substantially increase the number of T. vaginalis infections identified in men, and detection of the pathogen in semen is clinically and epidemiologically important, since these infections can be transmitted to partners through sexual intercourse. However, collection of multiple urogenital specimens, especially semen, may not be practical outside clinical research settings. Because clinical practices may not be “best practices” with respect to T. vaginalis diagnosis in men on account of financial or logistical constraints, it is important to emphasize sexual partner notification and treatment among women with trichomoniasis.
Despite increasing recognition of the importance of T. vaginalis infection in men, parasite detection by nucleic acid amplification testing is not widely available in STD clinics. The recent adaptation of a commercially available Chlamydia trachomatis-Neisseria gonorrhoeae PCR assay for T. vaginalis detection (33) and the commercial availability of a transcription-mediated amplification research test (A. Sitay, J. Bungo, K. Dickey, W. Weisburg, T. Aguirre, D. Fuller, L. Jasper, and T. Davis, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., abstr. C-120, 2003; D. H. Martin, R. A. Lillis, M. Nasumi, B. Smith, C. Cammarata, D. Diodene and K. Dickey, Abstr. 16th Int. Soc. Sex. Transm. Dis. Res., abstr.WP-129, 2005; M. M. Hobbs, K. D. Rich, E. B. Quinlivan, R. Zeitlin, J. L. Schmitz and M. B. Miller, Abstr. 106th Gen. Meet. Am. Soc. Microbiol., abstr. C-096, 2006) should increase the use of molecular diagnostics for T. vaginalis detection.
Acknowledgments
This work was supported by the National Institutes of Health STD Clinical Trials Unit contract N01AI075329 and the NC STI/TM Cooperative Research Center grant U19AI031496.
We thank Edward W. Hook III at the University of Alabama for support of the study. Karen Lau, Kecilia Leathers, Gail Leiblang, Chris Bernart, Molly Venglarik, Desmond Wiley, and other research personnel at the Durham County Health Department, the Wake County Department of Health and Human Services, and the Jefferson County Health Department were instrumental in patient recruitment and enrollment and specimen collection. Julie Welch, Silver Wevill, and Doug Taylor at Family Health International assisted in study design and monitoring and data management.
Footnotes
Published ahead of print on 13 September 2006.
REFERENCES
- 1.Buvé, A., H. A. Weiss, M. Laga, E. Van Dyck, R. Musonda, L. Zekeng, M. Kahindo, S. Anagonou, L. Morison, N. J. Robinson, and R. J. Hayes. 2001. The epidemiology of trichomoniasis in women in four African cities. AIDS 15(Suppl. 4):S89-S96. [DOI] [PubMed] [Google Scholar]
- 2.Chesson, H. W., J. M. Blandford, and S. D. Pinkerton. 2004. Estimates of the annual number and cost of new HIV infections among women attributable to trichomoniasis in the United States. Sex. Transm. Dis. 31:547-551. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, M. S., I. F. Hoffman, R. A. Royce, P. Kazembe, J. R. Dyer, C. Costello Daly, D. Zimba, P. L. Vernazza, M. Maida, S. A. Fiscus, and J. J. Eron. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 349:1868-1875. [DOI] [PubMed] [Google Scholar]
- 4.Cotch, M. F., J. G. Pastorek II, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Regan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, G. G. Rhoads, et al. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 24:353-360. [DOI] [PubMed] [Google Scholar]
- 5.Cu-Uvin, S., H. Ko, D. J. Jamieson, J. W. Hogan, P. Schuman, J. Anderson, and R. S. Klein. 2002. Prevalence, incidence, and persistence or recurrence of trichomoniasis among human immunodeficiency virus (HIV)-positive women and among HIV-negative women at high risk for HIV infection. Clin. Infect. Dis. 34:1406-1411. [DOI] [PubMed] [Google Scholar]
- 6.Gopalkrishnan, K., I. N. Hinduja, and T. C. Kumar. 1990. Semen characteristics of asymptomatic males affected by Trichomonas vaginalis. J. In Vitro Fert. Embryo Transf. 7:165-167. [DOI] [PubMed] [Google Scholar]
- 7.Guenthner, P. C., W. E. Secor, and C. S. Dezzutti. 2005. Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1. Infect. Immun. 73:4155-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardick, J., S. Yang, S. Lin, D. Duncan, and C. Gaydos. 2003. Use of the Roche LightCycler instrument in a real-time PCR for Trichomonas vaginalis in urine samples from females and males. J. Clin. Microbiol. 41:5619-5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobbs, M. M., P. Kazembe, A. W. Reed, W. C. Miller, E. Nkata, D. Zimba, C. C. Daly, H. Chakraborty, M. S. Cohen, and I. Hoffman. 1999. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sex. Transm. Dis. 26:381-387. [DOI] [PubMed] [Google Scholar]
- 10.Kaydos, S. C., H. Swygard, S. L. Wise, A. C. Sena, P. A. Leone, W. C. Miller, M. S. Cohen, and M. M. Hobbs. 2002. Development and validation of a PCR-based enzyme-linked immunosorbent assay with urine for use in clinical research settings to detect Trichomonas vaginalis in women. J. Clin. Microbiol. 40:89-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaydos-Daniels, S. C., W. C. Miller, I. Hoffman, T. Banda, W. Dzinyemba, F. Martinson, M. S. Cohen, and M. M. Hobbs. 2003. Validation of a urine-based PCR-enzyme-linked immunosorbent assay for use in clinical research settings to detect Trichomonas vaginalis in men. J. Clin. Microbiol. 41:318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaydos-Daniels, S. C., W. C. Miller, I. Hoffman, M. A. Price, F. Martinson, D. Chilongozi, D. Namakwha, S. Gama, S. Phakati, M. S. Cohen, and M. M. Hobbs. 2004. The use of specimens from various genitourinary sites in men, to detect Trichomonas vaginalis infection. J. Infect. Dis. 189:1926-1931. [DOI] [PubMed] [Google Scholar]
- 13.Kengne, P., F. Veas, N. Vidal, J. L. Rey, and G. Cuny. 1994. Trichomonas vaginalis: repeated DNA target for highly sensitive and specific polymerase chain reaction diagnosis. Cell. Mol. Biol. 40:819-831. [PubMed] [Google Scholar]
- 14.Krieger, J. N., C. Jenny, M. Verdon, N. Siegel, R. Springwater, C. W. Critchlow, and K. K. Holmes. 1993. Clinical manifestations of trichomoniasis in men. Ann. Intern. Med. 118:844-849. [DOI] [PubMed] [Google Scholar]
- 15.Krieger, J. N., M. Verdon, N. Siegel, C. Critchlow, and K. K. Holmes. 1992. Risk assessment and laboratory diagnosis of trichomoniasis in men. J. Infect. Dis. 166:1362-1366. [DOI] [PubMed] [Google Scholar]
- 16.Krieger, J. N., M. Verdon, N. Siegel, and K. K. Holmes. 1993. Natural history of urogenital trichomoniasis in men. J. Urol. 149:1455-1458. [DOI] [PubMed] [Google Scholar]
- 17.Kuberski, T. 1980. Trichomonas vaginalis associated with nongonococcal urethritis and prostatitis. Sex. Transm. Dis. 7:135-136. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, P., N. K. Sharma, U. Sharma, R. P. Sharma, R. Idnani, and A. K. Agrawal. 1990. Trichomoniasis and candidiasis in consorts of females with vaginal discharge. Indian J. Sex. Transm. Dis. 11:54-56. [PubMed] [Google Scholar]
- 19.Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, N. Nzila, J. Goeman, F. Behets, V. Batter, and M. Alary. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95-102. [DOI] [PubMed] [Google Scholar]
- 20.Lawing, L. F., S. R. Hedges, and J. R. Schwebke. 2000. Detection of trichomonosis in vaginal and urine specimens from women by culture and PCR. J. Clin. Microbiol. 38:3585-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd, G. L., J. R. Case, D. De Frias, and R. E. Brannigan. 2003. Trichomonas vaginalis orchitis with associated severe oligoasthenoteratospermia and hypogonadism. J. Urol. 170:924. [DOI] [PubMed] [Google Scholar]
- 22.Madico, G., T. C. Quinn, A. Rompalo, K. T. McKee, Jr., and C. A. Gaydos. 1998. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J. Clin. Microbiol. 36:3205-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minkoff, H. L., D. Eisenberger-Matityahu, J. Feldman, R. Burk, and L. Clarke. 1999. Prevalence and incidence of gynecologic disorders among women infected with human immunodeficiency virus. Am. J. Obstet. Gynecol. 180:824-836. [DOI] [PubMed] [Google Scholar]
- 24.Morency, P., M. J. Dubois, G. Grésenguet, E. Frost, B. Mâsse, S. Deslandes, P. Somsé, A. Samory, F. Mberyo-Yaah, and J. Pépin. 2001. Aetiology of urethral discharge in Bangui, Central African Republic. Sex. Transm. Infect. 77:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkawa, M., K. Yamaguchi, S. Tokunaga, T. Nakashima, and S. Fujita. 1992. The incidence of Trichomonas vaginalis in chronic prostatitis patients determined by culture using a newly modified liquid medium. J. Infect. Dis. 166:1205-1206. [DOI] [PubMed] [Google Scholar]
- 26.Pastorek, J. G., II, M. F. Cotch, D. H. Martin, D. A. Eschenbach, et al. 1996. Clinical and microbiological correlates of vaginal trichomoniasis during pregnancy. Clin. Infect. Dis. 23:1075-1080. [DOI] [PubMed] [Google Scholar]
- 27.Pillay, D. G., A. A. Hoosen, B. Vezi, and C. Moodley. 1994. Diagnosis of Trichomonas vaginalis in male urethritis. Trop. Geogr. Med. 46:44-45. [PubMed] [Google Scholar]
- 28.Price, M. A., W. C. Miller, S. C. Kaydos-Daniels, I. Hoffman, D. Chilongozi, F. Martinson, D. Namakhwa, J. Malanda, and M. S. Cohen. 2004. Trichomoniasis in men and HIV infection: data from 2 outpatient clinics at Lilongwe Central Hospital, Malawi. J. Infect. Dis. 190:1448-1455. [DOI] [PubMed] [Google Scholar]
- 29.Schwebke, J. R., and E. W. Hook III. 2003. High rates of Trichomonas vaginalis among men attending a sexually transmitted diseases clinic: implications for screening and urethritis management. J. Infect. Dis. 188:465-468. [DOI] [PubMed] [Google Scholar]
- 30.Schwebke, J. R., and L. F. Lawing. 2002. Improved detection by DNA amplification of Trichomonas vaginalis in males. J. Clin. Microbiol. 40:3681-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Seña, A. C., W. C. Miller, M. M. Hobbs, J. R. Schwebke, P. A. Leone, H. Swygard, J. Atashili, and M. S. Cohen. Trichomonas vaginalis infection in male sexual partners: implications for diagnosis, treatment and prevention. Clin. Infect. Dis., in press. [DOI] [PubMed]
- 31.Skerk, V., S. Schonwald, J. Granic, I. Krhen, B. Barsic, I. Marekovic, S. Roglic, B. Desnica, and Z. Zeljko. 2002. Chronic prostatitis caused by Trichomonas vaginalis—diagnosis and treatment. J. Chemother. 14:537-538. [DOI] [PubMed] [Google Scholar]
- 32.Sorvillo, F., and P. Kerndt. 1998. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet 351:213-214. [DOI] [PubMed] [Google Scholar]
- 33.Van Der Pol, B., C. S. Kraft, and J. A. Williams. 2006. Use of an adaptation of a commercially available PCR assay aimed at diagnosis of chlamydia and gonorrhea to detect Trichomonas vaginalis in urogenital specimens. J. Clin. Microbiol. 44:366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watt, L., and R. F. Jennison. 1960. Incidence of Trichomonas vaginalis in marital partners. Br. J. Vener. Dis. 36:163-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wendel, K. A., E. J. Erbelding, C. A. Gaydos, and A. M. Rompalo. 2003. Use of urine polymerase chain reaction to define the prevalence and clinical presentation of Trichomonas vaginalis in men attending an STD clinic. Sex. Transm. Infect. 79:151-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. 2001. Global prevalence and incidence of selected curable sexually transmitted infections. World Health Organization, Geneva, Switzerland.