Abstract
Due to the lack of a rapid, simple, and inexpensive assay for detecting alphavirus infections, we combined a reverse transcription-PCR with an enzyme-linked immunosorbent assay (RT-PCR-ELISA) to identify human pathogenic alphaviruses that are endemic in the New World. By combining the sensitivity of PCR, the detection simplicity of ELISA, and the specificities of DNA probes, this method rapidly detected and differentiated closely related species and subtypes of several medically important alphaviruses. After an amplification using RT-PCR with primers targeting conserved sequences in the nonstructural protein 1 gene, sequence-specific, biotin-labeled probes targeted against Venezuelan, eastern, and western equine encephalitis or Mayaro virus genes were used for the detection of amplicons using ELISA. The assay is simple, fast, and easy to perform in an ordinary diagnostic laboratory or clinical setting. Nucleic acid derived from cell cultures infected with several alphaviruses, clinical specimens, and mosquito pools as well as frozen and paraffin-embedded animal tissues were detected and identified within 6 to 7 h in a sensitive and specific manner.
The genus Alphavirus of the family Togaviridae includes 29 virus species that are classified into seven antigenic complexes (24). Most alphaviruses are arthropod borne and transmitted among vertebrate hosts by mosquitoes. Alphaviruses have a wide geographic distribution, including all continents except Antarctica. In humans, alphaviruses cause diseases ranging from nonspecific, flu-like syndromes to arthritic and/or rash syndromes to severe encephalitis that can be fatal (9, 20). The latter outcome is common in people infected with Venezuelan equine encephalitis virus (VEEV), eastern equine encephalitis virus (EEEV), or western equine encephalitis virus (WEEV). These viruses are important emerging and reemerging human and veterinary pathogens as well as potential biological weapons (10). In addition to causing severe human disease, these viruses produce epizootics accompanied by high rates of mortality in equines and other domestic animals (9). Of the alphaviral equine encephalitides, EEEV is the most virulent for humans, with mortality rates of 30 to 70% in apparent cases and severe neurologic sequelae in many survivors (22). However, VEEV is the most important human pathogen because of its ability to infect many people during epizootics (23). The New World Mayaro virus (MAYV), along with Old World members of the Semliki Forest complex, including O'nyong-nyong, Chikungunya, Ross River, and Sindbis (SIN) viruses, causes moderate to severe polyarthralgia and a rash that can be chronically incapacitating (9, 20).
Clinical diagnosis of alphaviral infection is difficult because of the nonspecific nature of the prodromal illnesses and the presence of other viruses, such as dengue virus, that commonly produce similar diseases, especially in the tropics (20). Most infected people exhibit nonspecific clinical signs and symptoms, such as high fever, headache, dizziness, vomiting, diarrhea, and abdominal pain, before the onset of encephalitis or arthralgia. Encephalitis usually begins abruptly, and patients can become unconscious with seizures and other neurological signs of the illness. Due to the lack of a simple diagnostic method for the rapid detection of acute alphaviral infections in the early stages, assessing the epizootic/epidemic potential of the etiologic agent and implementing appropriate control measures, such as equine vaccination or mosquito control, are often delayed.
Conventional assays for the detection of alphavirus infection include virus isolation by inoculating cell cultures or mice, serological testing to detect immunoglobulin M antibodies or immunoglobulin G seroconversion, and nucleic acid amplification. All of these diagnostic methods have limitations. Virus isolation is time-consuming and expensive and requires subsequent diagnostic steps to identify the pathogen. Serological assays, including complement fixation, hemagglutination inhibition, and plaque reduction neutralization tests, require paired acute- and convalescent-phase sera to determine seroconversion, which is only retrospectively diagnostic. Immunoglobulin M antibody responses, which are the most rapid and subtype specific (3), do not usually develop until 4 to 5 days after infection (20). Most PCR-based methods, including a nucleic acid sequence-based amplification assay and real-time reverse transcription-PCR (RT-PCR), provide rapid and more sensitive means to detect alphaviruses (11, 13), but the target nucleic acids are generally present in the blood only 2 to 6 days after infection, and sequencing or other genetic analyses are often needed to identify the species or strain of alphaviral RNA amplified. Also, viral nucleic acids are generally present for only 3 to 6 days after infection.
An RT-PCR amplification technique described by Pfeffer et al. (16) provides fast, sensitive, and genus-specific detection of all human pathogenic alphaviruses tested. Two degenerate primers designed to amplify a conserved region of the nsP1 gene of all alphaviruses are used, but nucleotide sequencing or restriction enzyme digestion of the amplicon DNA is needed to identify the alphavirus amplified. A nested PCR can be used for further confirmation, but this introduces greater potential for contamination and false positives.
To overcome some of the deficiencies of the current alphavirus detection assays, we exploited the advantages of several methods by combining the sensitivity of PCR, the detection simplicity of enzyme-linked immunosorbent assay (ELISA), and the specificities of sequence-specific DNA probes to develop a rapid yet simple method for diagnosing acute alphavirus infection from samples collected when virus is present (viremia). This method is similar to that of an RT-PCR-ELISA described previously for detecting foot-and-mouth disease (1) and swine vesicular disease viruses from cell cultures (4). The detection and identification of etiologic alphaviruses were obtained within the time frame of a normal workday.
MATERIALS AND METHODS
Viruses.
Four serocomplexes of alphaviruses (VEE, EEE, WEE, and Semliki Forest) representative of many geographic locations in the New World were used (Table 1). Viruses in the VEE complex included (i) the VEEV epidemic subtype IC strain, the enzootic subtype ID and IE strains, and the subtype IAB vaccine strain; (ii) the VEE complex subtype II; and (iii) the VEE subtype III strains IIIA, IIIB, IIIC, and IIID. For the EEE complex, two North American strains and three South American strains were used. Six Mayaro virus strains from Peru, Bolivia, and Brazil were included. The WEE complex viruses used included two WEEV strains and the closely related Highland J virus. Sindbis virus (14) and the unrelated West Nile and dengue viruses, uninfected cell culture supernatants, and mosquito pool and animal tissues were included as negative controls. Viruses were quantified using a plaque assay and Vero cells (2).
TABLE 1.
Alphaviruses used for assay testing and probe design
| Antigenic complex | Species | Subtype | Strain | Origin (year) | GenBank accession no. |
|---|---|---|---|---|---|
| Venezuelan equine | VEEV | IAB | TRD | Trinidad (1943) | L01442 |
| encephalitis | VEEV | IAB | TC-83 | TRD vaccine derivative | L01443 |
| VEEV | IC | P676 | Venezuela (1963) | AF375051 | |
| VEEV | IC | 3908 | Venezuela (1995) | U55350 | |
| VEEV | IC | SH3 | Venezuela (1992) | U55360 | |
| VEEV | IC | 243937 | Venezuela (1992) | AF004459 | |
| VEEV | ID | 66637 | Venezuela (1981) | AF004458 | |
| VEEV | ID | ZPC738 | Venezuela (1997) | AF1005660 | |
| VEEV | ID | 3880 | Panama (1961) | L00930 | |
| VEEV | ID | 83U434 | Colombia (1983) | U55362 | |
| VEEV | ID | Mac87 | Venezuela (1977) | DQ138312a | |
| VEEV | ID | CoAn9004 | Colombia (1969) | DQ138313a | |
| VEEV | ID | IQT8131 | Peru (1998) | DQ390224a | |
| VEEV | IE | 63U16 | Mexico (1963) | DQ138314a | |
| VEEV | IE | 68U201 | Guatemala (1968) | U34999 | |
| VEEV | IE | Menall | Panama (1962) | AF075252 | |
| EVEV | II | Fe37c | United States (1963) | AF075251 | |
| Mucambo virus | IIIA | BeAn 8 | Brazil (1954) | AF075253 | |
| Tonate virus | IIIB | CaAn 410d | French Guiana (1973) | AF075254 | |
| Mucambo virus | IIIC | 71D-1252 | Peru (1971) | U94612 | |
| Mucambo virus | IIID | FSL190 | Peru (2000) | DQ228210a | |
| Eastern equine | EEEV | I | 82V2137 | United States (1982) | U01034 |
| encephalitis | EEEV | I | North American strain | Not reported | X63135 |
| EEEV | I | FL93-939 | United States (1993) | EF034078 | |
| EEEV | II | BeAr300851 | Brazil (1975) | EF034076 | |
| EEEV | IV | BeAr436087 | Brazil (1985) | EF034077 | |
| EEEV | III | GML903836 | Panama (1984) | EF034079 | |
| Semliki Forest | MAYV | TRVL4675 | Trinidad (1954) | U94602 | |
| MAYV | BeH504378 | Brazil (1991) | DQ138315a | ||
| MAYV | OBS2248 | Peru (1995) | DQ138316a | ||
| MAYV | BeH394881 | Brazil (1981) | DQ138317a | ||
| MAYV | Uruma | Bolivia (1955) | DQ138318a | ||
| MAYV | BeAr505411 | Brazil (1991) | DQ138319a | ||
| MAYV | BeH343148 | Brazil (1978) | DQ138320a | ||
| O'nyong-nyong virus | SG650 | Uganda (1996) | AF079456 | ||
| Ross River virus | NB-5092 | Australia (1969) | M20162 | ||
| Semliki Forest virus | A7 | Unknown | Z48163 | ||
| Western equine | WEEV | 71V-1658 | United States (1971) | AF214040 | |
| encephalitis | WEEV | Fleming | United States (1946) | AF109297 | |
| WEEV | Rio 1257 | Brazil (1961) | AY348559 | ||
| Highland J virus | B-230 | United States (1960) | U94609 | ||
| Aura virus | BeAR 10315 | Brazil (1956) | AF126284 | ||
| Fort Morgan virus | CM4-146 | United States (1973) | U94608 | ||
| Whataroa virus | M78 | New Zealand (1962) | U94606 | ||
| SIN virus | HRsp | Derived from Egypt strain (1953) | J02363 |
New sequences obtained for this study.
Samples.
Five different types of samples were used to standardize and evaluate the assays: (i) infected cell culture supernatants (baby hamster kidney or African green monkey [Vero] cells purchased from the American Type Culture Collection [Bethesda, MD]); (ii) human sera from natural VEEV infections in Panama, horse sera collected after experimental VEEV infection (8), and blood samples (1:10 dilutions in Eagle's minimal essential medium [MEM]) obtained from golden Syrian hamsters experimentally infected with VEEV and mice infected with EEEV; (iii) animal tissues (kidney from guinea pigs infected with VEEV); (iv) paraffin-embedded hamster tissues collected after infection with VEEV, EEEV, and WEEV; and (v) mosquito pools (designed to simulate pools collected during an alphaviral outbreak by mixing one mosquito collected 7 days after intrathoracic inoculation with VEEV, WEEV, EEEV, and MAYV with 49 uninfected mosquitoes).
RT-PCR and sequencing.
Viral RNA was extracted from cell culture supernatants, and animal blood was diluted 1:10 in MEM, sera, and mosquito pools using the QIAamp viral RNA mini kit (QIAGEN, Valencia, CA) according to the manufacturer's protocol. Frozen guinea pig kidneys were homogenized in MEM supplemented with 5% fetal bovine serum (FBS) and supernatant used for RNA extraction. Total RNAs were also extracted from 10-μm sections of paraffin-embedded brains using the Optimum FFPE RNA isolation kit (Ambion Diagnostics, Austin, TX) without DNase I treatment. Mosquito pools were homogenized in 1 ml of MEM containing 20% FBS and centrifuged at 13,400 × g for 2 min prior to RNA extraction. Two degenerate primers, M2W and cM3W, were used to amplify a portion of the nsP1 gene as described previously (16); cDNA was produced using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) in a total volume of 20 μl at 42°C for 20 min and then at 50°C for 10 min. Ten microliters of the cDNA reaction mixture was used for PCR amplification with 0.2 mM dATP, dCTP, and dGTP, 0.19 mM dTTP, 0.01 mM digoxigenin-11-dUTP (Roche Molecular Biochemicals, Indianapolis, IN), and 0.25 U Takara Ex Taq polymerase (Takara Biomedicals, Shiga, Japan) in a total volume of 50 μl. The PCR was performed with an initial denaturation at 94°C for 2 min, following 30 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 1 min. The final extension was incubation at 72°C for 7 min. Amplicons were purified using the QIAGEN QIAquick kit and sequenced directly or cloned into the pGEM vector (Promega, Madison, WI). In the latter case, plasmids were screened by restriction digestion with EcoRI to confirm an insert of the anticipated length. The PCR products and/or two to three clones were sequenced using BigDye ready reaction mix, version 3.1 (Applied Biosystems, Foster City, CA), and the manufacturer's protocol.
Sequence analyses.
To identify probes that bind specifically to PCR amplicons from viruses in VEE complex subtypes I and III as well as WEE, EEE, and Semliki Forest (MAYV) complexes, we sequenced the amplicons generated from five VEE complex isolates (subtypes ID-Mac87, ID-CoAn9004, ID-IQT8131, IE-63U16, and IIID-FSL190), four EEEV strains (FL93-939, BeAr300851, BeAr436087, and GML903836), and six MAYV isolates (BeH504378, OBS2248, BeH394881, Uruma, BeAr505411, and BeH343148) (Table 1). These sequences were aligned with homologous sequences from the GenBank library (nucleotide [nt] positions 188 to 569; numbering is according to VEEV strain Trinidad donkey [TRD]) (12). Four species probes were selected to test viruses in the VEE subtype I, EEE, WEE, and MAYV groups. To identify probes with species specificity, VEE probes in subtype I were selected in genome regions with minor nucleotide sequence variations. The probes for VEE subtype III, WEE, and SIN serogroups were biotinylated at the 5′ ends, and other probes were labeled at the 3′ ends (depending on the available labeling method provided by Sigma-Genosys [Woodlands, TX]; equivalent results were obtained with both labeling methods).
Hybridization and ELISA.
Four to 20 μl of digoxigenin-labeled PCR products (7 to 176 μg DNA/ml was used successfully, with an optimal DNA concentration determined to be 20 to 25 μg/ml) was mixed with 2 μl of a biotin-labeled probe (20 pmol), heated at 95°C for 5 min, and then incubated at 50 to 58°C for 5 min for annealing. After cooling at 4°C for 1 min, 200 μl of blocking buffer (phosphate-buffered saline [PBS]-0.05% Tween-0.1% bovine serum albumin) was added and transferred to the well of a streptavidin-coated microtiter strip (Roche) for 30 min at 37 °C. After four washes with PBS-0.1% Tween, 200 μl of working solution of conjugated anti-digoxigenin-peroxidase Fab fragments (150 U/vial; Roche, Indianapolis, IN) was added to each well and incubated at 37°C for 60 min. The stock antibody was dissolved in 1 ml of water, aliquoted, and stored at −80°C. The optimal working solution was titrated, and the results ranged from a 1:10,000 to a 1:14,000 dilution from the stock solution. After four washes and the addition of 200 μl of 3,3′,5′,5-tetramethylbenzidine substrate for 5 to 30 min (Sigma-Aldrich, St. Louis, MO), the reaction was stopped after adding 100 μl of 0.5 M H2SO4. The optical density (OD) value was read at 450 nm in an ELISA plate reader. RT-PCRs containing no template RNA were used as controls for measuring background OD.
Nucleotide sequence accession numbers.
Sequences identified in the course of this study were submitted to GenBank under accession numbers DQ138312, DQ138313, DQ390224, and DQ138314 (Table 1).
RESULTS
Cutoff values.
The OD values of all negative controls tested (uninfected and unrelated virus infected) were in the range of 0.08 to 0.20 (Table 2, Table 3, and Table 6). The mean OD ± standard deviation was 0.13 ± 0.03. Based on previous reports (7, 15) and our data, an OD value more than 2 standard deviations above the highest negative control value (0.2) or >0.26 was considered positive; however, tests on samples with marginally positive OD values were repeated. We recommend that at least five RT-PCR negative controls (without template RNA) be included in each test to determine cutoff values.
TABLE 2.
Virus samples tested with the RT-PCR-ELISA
| Sample identification | Virus species, subtype, or strain (probe)a | Type of sampleb | Viral titer before RNA extraction (log10 PFU/ml) | Mean OD450 ± SD |
|---|---|---|---|---|
| 212863 | VEEV ID (A) | Human serum | 3.7 | 0.38 ± 0.02 |
| 213391 | VEEV ID (A) | Human serum | 4.8 | 2.04 ± 0.07 |
| SW37 | VEEV IC (A) | Horse serum | 3.5 | 0.76 ± 0.27 |
| SW39 | VEEV IC (A) | Horse serum | 4.5 | 0.67 ± 0.06 |
| Hamster 1 | VEEV ID (A) | Hamster blood | 2.3 | 0.31 ± 0.00 |
| Hamster 2 | VEEV ID (A) | Hamster blood | 5.7 | 1.20 ± 0.07 |
| Hamster 3 | VEEV ID (A) | Hamster blood | 5.2 | 2.00 ± 0.03 |
| Hamster 4 | Neg. control (A, B, C, D) | Hamster blood | Negative | 0.15 ± 0.02 |
| Mouse 4 | EEEV (B), S. Am | Mouse blood | 4.6 | 0.76 ± 0.05 |
| Mouse 5 | EEEV (B), S. Am | Mouse blood | 4.2 | 1.18 ± 0.07 |
| GP 4 | VEEV IC (A) | Guinea pig kidney | 6.6 | 2.88 ± 1.12 |
| GP 5 | VEEV ID (A) | Guinea pig kidney | 5.0 | 1.18 ± 0.16 |
| GP1-3908 | VEEV IC (A) | PE guinea pig brain | Unknown | 0.89 ± 0.10 |
| WEE-AH1 K | WEEV (C) | PE mouse kidney | Unknown | 0.69 ± 0.03 |
| FL79-AH 11 | EEEV (B), N. Am | PE mouse brain | Unknown | 0.28 ± 0.02 |
| FL-79-AH 13 | EEEV (B), N. Am | PE mouse brain | Unknown | 0.20 ± 0.15 |
| Animal tissue | Neg. control (A, B, C, D) | PE mouse brain | Negative | 0.14 ± 0.04 |
| Mosquito 1 | VEEV IC (A) | Mosquito pool | 5.0 | 3.00 ± 0.08 |
| Mosquito 2 | WEEV (C) | Mosquito pool | 5.4 | >4.00 |
| Mosquito 3 | N. Am-EEE (B) | Mosquito pool | 4.5 | 1.73 ± 0.01 |
| Mosquito 4 | Mayaro (D) | Mosquito pool | 5.4 | 1.28 ± 0.07 |
| Mosquito 5 | Neg. control (A, B, C, D) | Mosquito pool | Negative | 0.08 ± 0.02 |
| 66637 | VEEV ID (A) | Cell culture medium | 5.0 | 1.74 ± 0.40 |
| TC-83 | VEEV IAB | Cell culture medium | 2.7 | 0.56 ± 0.10 |
| Medium | Neg. control (A, B, C, D) | Cell culture medium | Negative | 0.10 ± 0.01 |
Probes used: A, VEEV-409; B, EEEVN-275; C, WEEV-263; D, MAYV-338. Neg., negative; S. Am., South American; N. Am., North American.
PE, paraffin embedded.
TABLE 3.
Comparison of PCR amplification cycles and DNA input with RT-PCR-ELISAa
| Viral titer before RNA extraction (PFU/ml) | PCR product input (μl) | OD450 value with the indicated number of PCR amplification cycles
|
||
|---|---|---|---|---|
| 30 | 35 | 40 | ||
| 5.0 × 102 | 4 | 0.22 | 0.30 | 0.28 |
| 8 | 0.22 | 0.39 | 0.37 | |
| 5.0 × 103 | 4 | 0.25 | 0.33 | 0.54 |
| 8 | 0.61 | 0.68 | 1.01 | |
| 5.0 × 104 | 4 | 1.20 | 1.42 | 1.28 |
| 8 | 1.55 | 1.99 | 1.78 | |
| Negative control | 4 | 0.13 | 0.15 | 0.12 |
| 8 | 0.14 | 0.18 | 0.18 | |
VEEV strain TC-83 was used and detected by ELISA with probe VEEV-409.
TABLE 6.
Specificity of alphavirus species probes in RT-PCR-ELISA
| Virus species | Strain | OD450 value with indicated probea
|
||||
|---|---|---|---|---|---|---|
| WEEV-263 | EEENV-275 | MAYV-338 | SINV-284 | VEEV-409 | ||
| Western equine encephalitis | CO92 | 1.34 | 0.12 | 0.11 | 0.14 | 0.10 |
| Western equine encephalitis | 93A27 | 0.86 | 0.13 | 0.10 | 0.13 | 0.10 |
| Highland J | B-230 | 0.12 | 0.11 | 0.10 | 0.17 | 0.10 |
| Sindbis | TR339 | 0.13 | 0.11 | 0.12 | 1.49 | 0.14 |
| Eastern equine encephalitis | FL93-939 | 0.11 | 0.73 | 0.10 | 0.11 | 0.12 |
| Mayaro | 394881 | 0.20 | 0.11 | 0.64 | 0.12 | 0.10 |
| Ross River | NB-5092 | 0.13 | 0.11 | 0.12 | 0.11 | 0.18 |
| VEE | TC-83 | 0.13 | 0.11 | 0.10 | 0.17 | 1.62 |
| EVE | Fe37c | NT | 0.10 | 0.10 | NT | 4.00 |
| Mucambo (VEE-IIIA) | BeAn-8 | 0.11 | 0.12 | 0.11 | NT | 0.10 |
| Tonate (VEE-IIIB) | CaAn 410d | 0.12 | 0.12 | 0.11 | 0.14 | 0.10 |
| Mucambo (VEE-IIIC) | 71D-1252 | 0.10 | 0.11 | 0.13 | NT | 0.17 |
| Mucambo (VEE-IIID) | FSL190 | 0.10 | 0.10 | 0.11 | NT | 0.11 |
| West Nile | NY785-99 | 0.17 | 0.14 | 0.18 | 0.15 | 0.17 |
| Dengue-2 | P8-377 | 0.16 | 0.19 | 0.13 | 0.18 | 0.18 |
Homologous reactions are indicated by bold numbers.
Sensitivity.
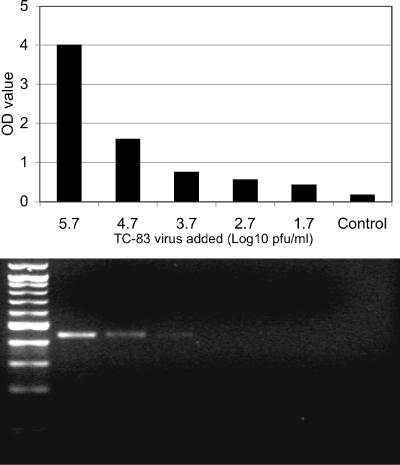
The two degenerate PCR primers described by Pfeffer et al. (16) amplified all of the alphaviruses tested and yielded single, 434-bp amplicons. To test the sensitivity of the assay, a stock of the TC-83 VEEV vaccine strain was serially 10-fold diluted and used to spike human serum. Serial dilutions of 140-μl volumes containing 52 to 5.2 × 105 PFU per ml were used for RNA extraction, and one-fifth of the RNA was used for each RT-PCR. The amplicons derived from samples with titers of 5.2 × 104 to 5.2 × 105 PFU/ml (RNA extracted from 1.5 × 103 to 1.5 × 104 PFU) were readily visible in an ethidium bromide-stained agarose gel. However, the detection of viral RNA from smaller amounts of virus (≤150 PFU) was inconsistent (Fig. 1).
FIG. 1.
VEEV strain TC-83 was diluted in human serum at concentrations of 52 to 5.2 × 105 PFU/ml. A 140-μl volume of diluted virus was used for RNA extraction and one-fifth of the RNA was used for RT-PCR. Upper panel, corresponding lane samples tested by ELISA detection using probe IAB-TRD-344. Lower panel, a 4-μl volume of each PCR amplicon was loaded onto a 1.5% agarose gel.
The sensitivity of the RT-PCR was increased more than 10-fold using ELISA detection. A positive ELISA absorbance value was consistently obtained from the sample containing 5.2 × 102 PFU/ml of virus (sample TC-83, RNA extracted from 15 PFU) (Table 2). We also tested some clinical samples containing low viral concentrations ranging from 2 × 102 to 4 × 106 PFU/ml (Table 2). One human serum, no. 212863, and one equine serum, no. SW37, were each negative by gel detection but were detected by use of the ELISA.
We further compared the sensitivity of our assay with that of the seminested PCR described by Pfeffer et al. (16). Our unnested assay using ELISA detection was less sensitive than the seminested PCR for low concentrations of virus (<5.2 × 102 PFU/ml). However, the sensitivity of our assay was dependent on the amount of PCR product used in the ELISA. The detection of low concentrations of alphaviral RNA could be improved by increasing the numbers of PCR amplification cycles to 35 to 40 or by using a larger proportion of PCR products (>4 μl) in the ELISA reactions (Table 3).
VEEV subtype-specific probes.
All GenBank nsP1 sequences from VEE complex viruses were aligned to identify conserved and variable regions for probe design. Nucleotide positions 344 to 357 (strain TRD genome positions) were selected based on the presence of 1 to 4 variable nucleotides in pairwise comparisons, and initial probes were designed to determine how many target sequence nucleotide differences were needed to generate subtype and genotype specificity (Table 4; Fig. 2). Optimal annealing temperatures were tested from 45 to 59°C, and 5 to 20 pmol of each probe was used. Four microliters of PCR product (20 to 25 μg DNA/ml) and 20 pmol of probe incubated at 50°C were determined to be optimal for infected cell culture supernatants, and 10 to 20 μl of amplicons (20 to 25 μg DNA/ml) was optimal for other samples. Each VEE complex probe was tested initially with viruses within subtype I. The RNA extracted from SIN virus and from uninfected cell culture supernatants was used as a negative control. The ELISA results indicated that probes with 1-nt differences relative to the target cross-reacted, but probes with ≥2-nt differences did not bind to amplicons (Fig. 2). Designing probes specific to a given VEEV subtype (e.g., IAB versus IC versus ID) proved difficult because these subtypes do not represent distinct genetic lineages; the epidemic subtypes IAB and IC evolved from one of several ID lineages (18, 19). However, subtype IE, which is monophyletic (18, 19), was clearly distinguished from the subtype IAB, IC, and ID strains.
TABLE 4.
VEE complex subtype-specific probes
| Probea | Nucleotide sequenceb | No. of nucleotide differences relative to strain TRD |
|---|---|---|
| IAB-TRD-344 | AAGAAAAACTGTAAGGAAATA | |
| IC | AAGAAAAATTGCAAGGAAATA | 2 |
| ID-66637 | AAGAAAAATTTCAAGGAAATA | 3 |
| ID-Mac87 | AAGAAAAACTGCAAGGAAATA | 1 |
| IE-347 | AAGAACTGTAAAGAGATTACA | 4 |
| III-407 | GTCATGGAGGACCC | 3 |
Numbering is according to the 5′ genome position of VEEV strain TRD.
Underlined nucleotides (except for the III-407 probe, which is not homologous to the others) are different from those of the homologous IAB-TRD-344 probe.
FIG. 2.
Results of detection of RT-PCR products by ELISA. The RT-PCRs were performed using VEE subtype I viruses (Table 1) as described in Materials and Methods, and cell culture medium and SIN virus were used as negative controls. All digoxigenin-labeled PCR amplicons were hybridized with TRD, Mac87, VEE IC, ID-66637, and VEE-IE biotin-labeled probes and detected using conjugated anti-digoxigenin-peroxidase as described in Materials and Methods, using an ELISA format. Names above graphs indicate probes used for hybridization.
Probe VEE III-407 was designed for the detection of all VEE complex subtype III viruses. This 14-mer (Table 4) had mismatches of 3 nt relative to strain TRD and all other VEEVs in subtype I. The VEE III-407 probe reacted with only the viruses in VEE complex subtype III, including the IIIA-Mucambo, IIIB-Tonate, IIIC, and IIID strains, but not with VEEV subtype I and Everglades virus (EVEV) (subtype II), EEEV strain FL93-939, WEEV strain CO92, or MAYV strain BeH504378 (Fig. 3).
FIG. 3.
Results of detection of RT-PCR products by ELISA using the VEE III-407 probe. The RT-PCRs were performed using VEE subtype I and III viruses as well as other alphaviruses outside the VEE complex (Table 1) as described in Materials and Methods. A 4-μl volume of each digoxigenin-labeled PCR amplicon was reacted with the VEE III-407 biotin-labeled probe and detected using conjugated anti-digoxigenin-peroxidase as described in Material and Methods, using an ELISA format. Error bars represent standard deviations.
Species probes.
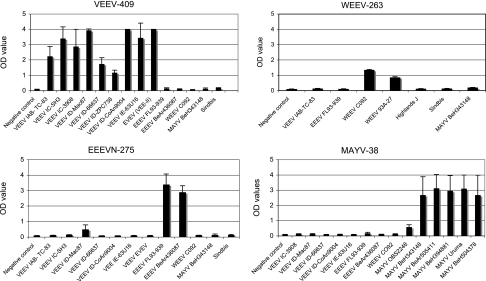
The VEE complex species-specific probes (VEEV-409 probe for subtype I, species VEEV; VEE-III-407 probe for subtype III, species Mucambo/Tonate) exhibited no interspecific cross-reactivity except for EVEV (subtype II strain Fe37c), which reacted with the VEEV-409 probe (Fig. 4). However, as noted previously, EVEV is a subtype ID VEEV variant when considered phylogenetically (17, 18).
FIG. 4.
Results of detection of RT-PCR products by ELISA using species-specific probes. The RT-PCRs were performed using different alphaviruses (Table 1) as described in Materials and Methods. A 4-μl volume of each digoxigenin-labeled PCR amplicon was reacted with alphavirus species-specific probe VEEV-409, WEEV-263, EEENV-275, or MAYV-338 (Table 5) and detected with conjugated anti-digoxigenin-peroxidase (as described in Materials and Methods) using an ELISA format. Error bars represent standard deviations.
To find suitable probes for differentiating alphavirus species in the other antigenic complexes, the following nsP1 sequences were aligned: EEEV (six strains), WEEV (three strains), Highland J, Fort Morgan, Aura, Whataroa, Sindbis, MAYV (seven strains), Semliki Forest, O'nyong-nyong, Ross River, and the VEE complex sequences described above. Probes were designed by first identifying relatively conserved sequence regions, followed by manually selecting a region with a ≥3-nt variation among species sequences. Between nucleotide positions 188 and 569 (numbering according to the TRD strain of VEEV), the sequences of probes VEEV-409, WEEV-263, and MAYV-338 were identical to the target sequences of VEEV, WEEV, and MAYV, respectively (Table 5). The sequence target of probe EEEVN-275 had a 1-nt mismatch between North and South American EEEV strains. All of the species probes differed by sufficient numbers (≥3) of nucleotides to react specifically with their target species sequences. The probes were tested with eight VEEV strains, one EVEV strain (Fe37c), and four VEE complex subtype III strains as well as two North American and two South American EEEV strains, two WEEV strains, and six MAYV strains. To simplify the assay, one annealing temperature of 58°C was used. All probes detected amplicons derived from their corresponding viruses, with no cross-activity observed except for VEEV subtype ID strain Mac87, which exhibited low cross-reactivity with the EEEVN-275 probe (Fig. 4). A blue color developed in all positive samples and could be visualized by eye or by using ELISA plate-reading instrumentation. The sequence alignment of strain Mac87 with probe EEEVN-275 showed mismatches of only 2 nt (Table 5), explaining the low level of cross-reactivity.
TABLE 5.
Probes targeting alphavirus species-specific sequences
| Probea | Nucleotide sequence | Predicted annealing temp (°C)b |
|---|---|---|
| VEEV-409 | GTCATGAGCGACCCTGA | 61.1 |
| EEEVN-275 | AAGTACCACTGTATTTGCCC | 58.0 |
| MAYV-338 | TGAGGACCCAGAGCGTCTGC | 70.3 |
| WEEV-263 | AGGTACTTTGGAAGTGTAGCAG | 58.0 |
| SINV-284 | TTCCGAGCACCAGTATCATTG | 64.8 |
Numbering is according to the 5′ genome position of VEEV strain TRD.
All probes were tested at an annealing temperature of 58°C.
Ability of alphavirus species probes to detect viruses from different sources.
Because alphaviruses replicate to high titers in most cell lines, our highest OD values were observed with cell culture samples (Table 2; Fig. 2 to 4). However, because most alphaviruses are zoonotic and mosquito borne, surveillance often relies on assays of field-collected mosquitoes and human and animal samples to monitor epizootic/epidemic circulation. Therefore, we tested our assays using other types of samples.
Humans infected with VEEV typically develop peak viremia levels from 2 to 6 log10 PFU/ml (25) (S. C. Weaver and P. V. Aguilar, unpublished data). We tested two serum samples collected within 3 days of the onset of acute illness from isolates of natural infections with VEEV subtype ID (6). Sample 213391 had a VEE viremia titer of 6.3 log10 PFU/ml. It tested positive by ELISA at 4.8 log10 PFU/ml with probe VEEV-409 after PCR amplification from a 1:28 dilution used for RNA extraction (Table 2). The other human sample, no. 212863 (viremia titer 4.2 log10 PFU/ml; 3.7 log10 assayed from a 1:3 serum dilution for RNA extraction), also tested positive. A serum sample from a horse experimentally infected with VEEV subtype IC strain 3908, no. SW37, with a viremia titer at 3.5 log10 PFU/ml, also tested positive by ELISA. However, PCR products from the latter two sera were invisible using agarose gel electrophoresis. Blood collected from hamsters and mice infected with VEEV subtype ID strains, with titers from 2.3 to 5.7 log10 PFU/ml, diluted 1:10 in MEM containing 2% FBS, all tested positive by ELISA (Table 2).
The RT-PCR-ELISA was also highly sensitive for detecting alphaviruses in mosquito pools prepared by mixing one infected mosquito with 49 uninfected ones (Table 2). In addition, infected vertebrate tissues, including those from frozen guinea pig kidney and fixed, paraffin-embedded animal organs, tested positive. The only exception was a paraffin-embedded sample infected with EEEV, sample FL AH 13, which was negative in the assay (Table 2).
Cross-reactivity.
We further examined potential cross-reactivity of our assays using different alphavirus species. The PCR products amplified from Highland J virus in the WEE complex, the Tonate virus in the VEE complex, and the Ross River virus in the Semliki Forest complex were also included. Each amplicon was tested with each species probe, and no cross-reactivity was detected (Table 6). In addition, RNAs from West Nile virus strain NY-785-99 and dengue virus type 2 strain P8-377 were tested as negative controls using the alphavirus reactions, and no reactivity was detected (Table 6).
DISCUSSION
Currently, no simple, rapid, and cost-effective diagnostic methods are available for detecting and distinguishing alphaviruses of different species, subtypes, and genotypes. Because minor genetic differences among strains can have profound epidemiological implications, such as those resulting in enzootic versus epizootic (equine amplification-competent) VEEV strains (21), rapid differentiation of arbovirus species, subtypes, and genotypes is important for surveillance and disease control. Traditionally, alphavirus diagnostics rely on virus isolation, which is usually possible from human sera within 3 days of disease onset; throat swabs obtained 2 to 4 days after acute onset also often contain infectious alphavirus (5, 6). However, virus isolation from mice or cell cultures generally requires an incubation of at least 1 to 2 days, delaying diagnosis. In addition, safe isolation of many of these viruses requires biosafety level 3 facilities and procedures that are not available in many locations of public health labs and in developing countries. Although antibody assays are generally faster and can be quite specific, seroconversion generally occurs at least 4 to 5 days after infection, delaying diagnosis. Nucleic acid sequence-based amplification assay and real-time PCR are highly sensitive and suitable for arbovirus surveillance; however, not all routine diagnostic laboratories have the equipment and expertise to perform these assays and interpret data.
To overcome some of these diagnostic limitations, we developed an assay that is based on the detection of alphaviral RNA by RT-PCR using two degenerate, genus-specific primers developed previously (16). Species- and subtype-specific probes and a simple ELISA step were added to increase sensitivity, and specificity, and to simplify interpretation. The assay was completed in 6 to 7 h, including RNA extraction. Alphaviruses were detected and correctly identified from acute blood samples collected from two naturally infected humans and from experimentally infected animals, from frozen and paraffin-embedded animal tissues, and from mosquito pools. While the sensitivity of the RT-PCR-ELISA (ca. 15 PFU) was slightly lower than that of virus isolation, we believe that the increased speed (6 to 7 h versus at least 1 to 2 days) compensates for this difference. Also, serologic and/or further genetic tests are required after virus isolation to determine the etiology, requiring additional time. Because viremia precedes seroconversion after alphavirus infections, our assay should be more useful during the acute phase of infection, when control measures such as equine vaccination or mosquito control may be critical. It is likely that the sensitivity of our assay could be further improved by increasing the number of PCR amplification cycles, by increasing the amount of PCR product in the ELISA (Table 3), or by changing the ratio of dTTP to digoxigenin-11-dUTP (19:1) to 18:2 or more. However, there would probably be an increased risk of false positives if samples were contaminated with small amounts of virus or nucleic acids in the laboratory. Despite the presence of cDNA clones in our laboratory for all of the alphaviruses tested, we did not encounter false positives in control reactions.
Our studies indicate that mismatches of at least 2 nucleotides between probes and target sequences are needed to avoid cross-reactions. In one instance, two different viruses (EEEV and the VEEV Mac87 strain) exhibited low cross-reactivity (EEEV assay) due to the presence of a 2-nt mismatch. However, the inverse assay using the VEEV-409 probe did not cross-react, allowing for an accurate discrimination of these two viruses. A limitation of the assay is in distinguishing among VEEV subtypes IAB, IC, and ID (Fig. 2). This is not surprising because these subtypes do not represent genetic lineages or clades; the epidemic subtype IAB and IC strains are believed to have arisen from one of several enzootic, subtype ID lineages (18, 19). Therefore, sequencing of the nsP1 amplicons may be required to assess the epidemic potential of VEEV strains.
In conclusion, the RT-PCR-ELISA assay presented here is simple, fast, specific, and sensitive for the detection and identification of all important alphavirus human pathogens in the New World. It can be performed easily in ordinary clinics and laboratories, without the need to replicate and isolate potentially dangerous viruses requiring high containment. Because the ELISA results can be read by eye, the only instruments needed for the assay are a thermal cycler and a microcentrifuge. With appropriate primer and probe design, this assay could also be applied to other important arboviruses, such as the West Nile, dengue, yellow fever, St. Louis encephalitis, and Japanese encephalitis viruses.
Acknowledgments
We thank James Olson and Evelia Quiroz for providing sera and Wenli Kang for technical assistance.
This work was supported by a grant from NIAID through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research, NIH grant number U54 AI057156, by NIH grant 48807, and by NIH contract N01-AI25489. S.P. was supported by NIH K08 grant AI059491. I.P.G. was supported by the CDC training grant T01/CCT622892, and P.V.A. was supported by the James W. McLaughlin Fellowship Fund.
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Alexandersen, S., M. A. Forsyth, S. M. Reid, and G. J. Belsham. 2000. Development of reverse transcription-PCR (oligonucleotide probing) enzyme-linked immunosorbent assays for diagnosis and preliminary typing of foot-and-mouth disease: a new system using simple and aqueous-phase hybridization. J. Clin. Microbiol. 38:4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaty, B. J., C. H. Calisher, and R. E. Shope. 1989. Arboviruses, p. 797-855. In N. J. Schmidt and R. W. Emmons (ed.), Diagnostic procedures for viral, rickettsial and chlamydial infections, 6th ed. American Public Health Association, Washington, D.C.
- 3.Calisher, C. H., A. O. el-Kafrawi, M. I. Al-Deen Mahmud, A. P. Travassos da Rosa, C. R. Bartz, M. Brummer-Korvenkontio, S. Haksohusodo, and W. Suharyono. 1986. Complex-specific immunoglobulin M antibody patterns in humans infected with alphaviruses. J. Clin. Microbiol. 23:155-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callens, M., and K. De Clercq. 1999. Highly sensitive detection of swine vesicular disease virus based on a single tube RT-PCR system and DIG-ELISA detection. J. Virol. Methods 77:87-99. [DOI] [PubMed] [Google Scholar]
- 5.Clarke, D. H. 1961. Two nonfatal human infections with the virus of eastern encephalitis. Am. J. Trop. Med. Hyg. 10:67-70. [DOI] [PubMed] [Google Scholar]
- 6.Dietz, W. H., Jr., P. H. Peralta, and K. M. Johnson. 1979. Ten clinical cases of human infection with Venezuelan equine encephalomyelitis virus, subtype I-D. Am. J. Trop. Med. Hyg. 28:329-334. [DOI] [PubMed] [Google Scholar]
- 7.Forsyth, M. A., S. Parida, S. Alexandersen, G. J. Belsham, and T. Barrett. 2003. Rinderpest virus lineage differentiation using RT-PCR and SNAP-ELISA. J. Virol. Methods 107:29-36. [DOI] [PubMed] [Google Scholar]
- 8.Greene, I. P., S. Paessler, L. Austgen, M. Anishchenko, A. C. Brault, R. A. Bowen, and S. C. Weaver. 2005. Envelope glycoprotein mutations mediate equine amplification and virulence of epizootic Venezuelan equine encephalitis virus. J. Virol. 79:9128-9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin, D. E. 2001. Alphaviruses, p. 917-962. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, New York, N.Y.
- 10.Hawley, R. J., and E. M. Eitzen, Jr. 2001. Biological weapons—a primer for microbiologists. Annu. Rev. Microbiol. 55:235-253. [DOI] [PubMed] [Google Scholar]
- 11.Hodneland, K., and C. Endresen. 2006. Sensitive and specific detection of Salmonid alphavirus using real-time PCR (TaqMan). J. Virol. Methods 131:184-192. [DOI] [PubMed] [Google Scholar]
- 12.Kinney, R. M., B. J. Johnson, J. B. Welch, K. R. Tsuchiya, and D. W. Trent. 1989. The full-length nucleotide sequences of the virulent Trinidad donkey strain of Venezuelan equine encephalitis virus and its attenuated vaccine derivative, strain TC-83. Virology 170:19-30. [DOI] [PubMed] [Google Scholar]
- 13.Lambert, A. J., D. A. Martin, and R. S. Lanciotti. 2003. Detection of North American eastern and western equine encephalitis viruses by nucleic acid amplification assays. J. Clin. Microbiol. 41:379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKnight, K. L., D. A. Simpson, S. C. Lin, T. A. Knott, J. M. Polo, D. F. Pence, D. B. Johannsen, H. W. Heidner, N. L. Davis, and R. E. Johnston. 1996. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J. Virol. 70:1981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milne, S. A., S. Gallacher, P. Cash, and A. J. Porter. 2006. A reliable RT-PCR-ELISA method for the detection of infectious pancreatic necrosis virus (IPNV) in farmed rainbow trout. J. Virol. Methods 132:92-96. [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer, M., B. Proebster, R. M. Kinney, and O. R. Kaaden. 1997. Genus-specific detection of alphaviruses by a semi-nested reverse transcription-polymerase chain reaction. Am. J. Trop. Med. Hyg. 57:709-718. [DOI] [PubMed] [Google Scholar]
- 17.Powers, A. M., A. C. Brault, Y. Shirako, E. G. Strauss, W. Kang, J. H. Strauss, and S. C. Weaver. 2001. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 75:10118-10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers, A. M., M. S. Oberste, A. C. Brault, R. Rico-Hesse, S. M. Schmura, J. F. Smith, W. Kang, W. P. Sweeney, and S. C. Weaver. 1997. Repeated emergence of epidemic/epizootic Venezuelan equine encephalitis from a single genotype of enzootic subtype ID virus. J. Virol. 71:6697-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salas, R. A., C. Z. Garcia, J. Liria, R. Barrera, J. C. Navarro, G. Medina, C. Vasquez, Z. Fernandez, and S. C. Weaver. 2001. Ecological studies of enzootic Venezuelan equine encephalitis in north-central Venezuela, 1997-1998. Am. J. Trop. Med. Hyg. 64:84-92. [DOI] [PubMed] [Google Scholar]
- 20.Tsai, T. F., S. C. Weaver, and T. P. Monath. 2002. Alphaviruses, p. 1177-1210. In D. D. Richman, R. J. Whitley, and F. G. Hayden (ed.), Clinical virology. ASM Press, Washington, D.C.
- 21.Wang, E., R. Barrera, J. Boshell, C. Ferro, J. E. Freier, J. C. Navarro, R. Salas, C. Vasquez, and S. C. Weaver. 1999. Genetic and phenotypic changes accompanying the emergence of epizootic subtype IC Venezuelan equine encephalitis viruses from an enzootic subtype ID progenitor. J. Virol. 73:4266-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver, S. C. 2001. Eastern equine encephalitis, p. 151-159. In M. W. Service (ed.), The encyclopedia of arthropod-transmitted infections. CAB International, Wallingford, United Kingdom.
- 23.Weaver, S. C., C. Ferro, R. Barrera, J. Boshell, and J. C. Navarro. 2004. Venezuelan equine encephalitis. Annu. Rev. Entomol. 49:141-174. [DOI] [PubMed] [Google Scholar]
- 24.Weaver, S. C., T. K. Frey, H. V. Huang, R. M. Kinney, C. M. Rice, J. T. Roehrig, R. E. Shope, and E. G. Strauss. 2005. Togaviridae, p. 999-1008. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy, VIIIth report of the ICTV. Elsevier/Academic Press, London, United Kingdom.
- 25.Weaver, S. C., R. Salas, R. Rico-Hesse, G. V. Ludwig, M. S. Oberste, J. Boshell, R. B. Tesh, et al. 1996. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. Lancet 348:436-440. [DOI] [PubMed] [Google Scholar]