Abstract
Nosocomial transmission of Neisseria meningitidis has only rarely been reported. Here, we present a significant spatiotemporal association of two cases of invasive meningococcal disease identified by retrospective cluster analysis with the program SaTScan. The most likely epidemiological link was simultaneous hospitalization, resulting in indirect nosocomial transmission.
CASE REPORT
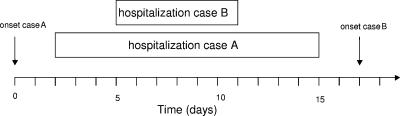
Invasive meningococcal disease (IMD) continues to be a major health threat for children and adolescents. In industrialized countries, up to 10% of cases of IMD may occur in clusters (1, 3, 5, 8, 12). Two cases of IMD with a significant spatiotemporal association were identified by retrospective data analysis using the software SaTScan (version 5.1.1), developed by Martin Kulldorff of the National Cancer Institute and Farzad Mostashari of the New York Department of Health and Mental Hygiene (6). Case A, a 17-year-old male apprentice, was admitted to a small regional pediatric hospital with meningitis 1 day after the onset of illness. Three days after his hospitalization, case B, an 11-year-old boy, was admitted to the same ward for immunotherapy of allergy and stayed for 6 days. He developed IMD 6 days after discharge from the hospital. Figure 1 summarizes the sequence of events. In-depth questioning by the local health authorities did not reveal any social links between the patients or their relatives. The two patients were never members of the same sports club, music group, or other organization. Furthermore, they had not attended any common event, concert, or carnival procession, the last being common at that time of year. The places of residence of the two patients were 17 km apart and located within the same rural district in the federal state of Thuringia. Neisseria meningitidis was isolated from the cerebrospinal fluid of both patients. Fine typing revealed the two isolates to be identical: the two cases were caused by serogroup B meningococci with the PorA type P1.18,25-1 and the FetA type F5-1 (10, 11). The multilocus sequence type (7) was sequence type 283 (ST-283) (clonal complex ST-269).
FIG. 1.
Sequence of events illustrating the onset of IMD and the overlap of the hospital stays of the two cases.
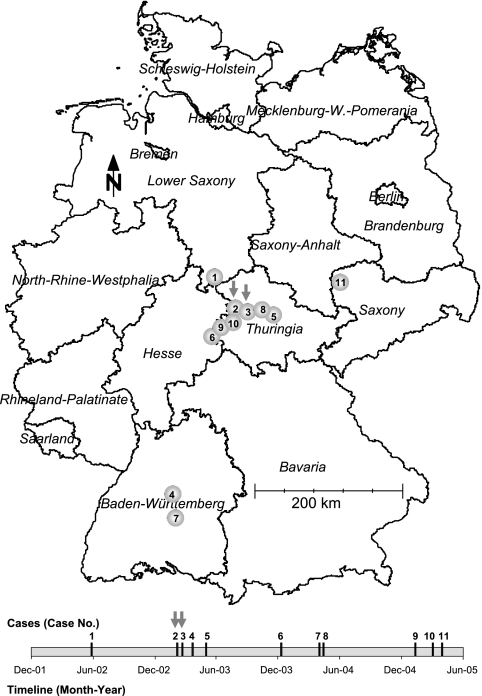
In Germany, ST-283 meningococci with the fine type B:P1.18,25-1:F5-1 caused disease in 11 persons between December 2001 and June 2005 (42 months), according to the surveillance data of the National Reference Centre for Meningococci. Figure 2 shows a geographic representation of the 11 cases. There was a spatial accumulation of this particular fine type in Thuringia, which suggests an enhanced carriage and transmission rate of the clone in that area. The total incidence of cases due to B:P1.18,25-1:F5-1 in Thuringia was low during the observation period (0.06 cases/100,000 population/year) but more than 10-fold higher than in the rest of Germany. As shown in Fig. 2, there were no cases of B:P1.18,25-1:F5-1 disease in spatial proximity to the places of residence of the two patients in 2002. Three further cases occurred in 2003: one in the federal state of Baden-Wuerttemberg in March, one in another rural district of Thuringia in May, and a third in the federal state of Hesse in December. These three cases were neither spatially nor temporally linked to the cases reported here.
FIG. 2.
Eleven cases of IMD caused by meningococci with the fine type B:P1.18,25-1:F5-1 that occurred between December 2001 and June 2005. The map approximates the geographic locations of the places of residence. The temporal succession of the cases is illustrated at the bottom. Cases 2 and 3 are the subjects of this report and are marked with gray arrows. They are referred to in the text as cases A and B, respectively.
Since in most cases of IMD, disease develops within 1 week after strain acquisition, infection of case B may have occurred at the hospital. We cannot rule out the possibility that the patients or their relatives met on the premises of the hospital. However, transmission between patients seems unlikely because antibiotic treatment of patient 1 should have rendered him noncontagious before the second patient was admitted. Since only two or three nurses were on duty per shift, it is conceivable that a single nurse looked after both patients. Thus, indirect transmission of the bacteria might have occurred via hospital staff.
The results of a retrospective fine-type-specific cluster analysis employing the software SaTScan (see above) prompted the epidemiological investigation of the cases presented here. Briefly, the project included 1,616 cases of IMD in Germany for which geographic, temporal, serogroup, and PorA/FetA fine types were available over a period of 42 months (3a). For the cluster reported here, SaTScan analysis yielded quantitative evidence for clustering with an age-adjusted P value of 0.01 in a retrospective scan using a 30-day window and a maximum population size set at 7% of the German population. The population size of the cluster area was 142,595 as assessed by SaTScan analysis.
Nosocomial transmission of meningococcal disease between patients has rarely been reported. One report postulated that IMD due to serogroup Y meningococci in two oncology patients was due to nosocomial transmission (2). However, further fine typing was not undertaken in that study, dating back to the 1970s. A Danish report demonstrated C:2a disease in a nurse and two patients sharing the same room in a hospital (9). Carriage of C:2a was significantly elevated in staff from the department, indicating spread of the virulent clone. Moreover, secondary cases of IMD among health care workers have been reported (4).
The epidemiological link proposed in this report is a plausible hypothesis to explain the clustering. Nevertheless, the following limitations of the study must be taken into account. (i) An asymptomatic health care worker colonized with B:P1.18,25-1:F5-1 could not be looked for retrospectively. (ii) Data on the prevalence of B:P1.18,25-1:F5-1 meningococci among healthy carriers in Thuringia are not available. (iii) From an epidemiological viewpoint, detailed information regarding the identities of health care workers serving both patients and social contacts of visitors of the two patients at the hospital, as well as devices and facilities used by both patients, were not available retrospectively. (iv) The National Reference Centre for Meningococci receives diagnostic material or isolates from approximately 65% of the reported cases in Germany. Underreporting might therefore have interfered with our analysis. In 2003, however, only the two cases of IMD presented here were reported by the relevant district to the Robert Koch-Institute in Berlin.
Despite these limitations and remaining uncertainties, this report exemplifies how the combination of DNA sequence-based fine typing and computer-assisted cluster analysis can lead to the identification of plausible epidemiological links between cases with identical fine types. As a practical consequence, the report highlights the importance of thorough hygiene measures during the initial treatment phase of IMD, not only to avoid secondary cases of IMD among health care workers, but also to prevent their asymptomatic colonization, resulting in possible subsequent transmission to further susceptible hosts.
Acknowledgments
Financial support for this study was provided by the German Ministry of Health via the Robert Koch-Institute as part of the funding of the National Reference Centre for Meningococci (Matthias Frosch is head of the Reference Centre; grant number ZV2-1369-237).
We are indebted to Christine Meinhardt for expert technical assistance and to Dag Harmsen, Münster, Germany, for helpful discussions. The developers of SaTScan are gratefully acknowledged for making the software freely available. Wiebke Hellenbrand (Berlin, Germany) is gratefully acknowledged for invaluable comments on the manuscript.
Footnotes
Published ahead of print on 30 August 2006.
REFERENCES
- 1.Block, C., M. Roitman, B. Bogokowsky, S. Meizlin, and P. E. Slater. 1993. Forty years of meningococcal disease in Israel: 1951-1990. Clin. Infect. Dis. 17:126-132. [DOI] [PubMed] [Google Scholar]
- 2.Cohen, M. S., A. C. Steere, R. Baltimore, A. von Graevenitz, E. Pantelick, B. Camp, and R. K. Root. 1979. Possible nosocomial transmission of group Y Neisseria meningitidis among oncology patients. Ann. Intern. Med. 91:7-12. [DOI] [PubMed] [Google Scholar]
- 3.Cooke, R. P., T. Riordan, D. M. Jones, and M. J. Painter. 1989. Secondary cases of meningococcal infection among close family and household contacts in England and Wales, 1984-7. BMJ 298:555-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Elias, J., D. Harmsen, H. Claus, W. Hellenbrand, M. Frosch, and U. Vogel. Spatiotemporal analysis of invasive meningococcal disease, Germany. Emerg. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]
- 4.Gilmore, A., J. Stuart, and N. Andrews. 2000. Risk of secondary meningococcal disease in health-care workers. Lancet 356:1654-1655. [DOI] [PubMed] [Google Scholar]
- 5.Jackson, L. A., A. Schuchat, M. W. Reeves, and J. D. Wenger. 1995. Serogroup C meningococcal outbreaks in the United States. An emerging threat. JAMA 273:383-389. [PubMed] [Google Scholar]
- 6.Kulldorff, M. 1997. A spatial scan statistic. Commun. Stat. 26:1481-1496. [Google Scholar]
- 7.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivares, R., and B. Hubert. 1992. Clusters of meningococcal disease in France (1987-1988). Eur. J. Epidemiol. 8:737-742. [DOI] [PubMed] [Google Scholar]
- 9.Riewerts Eriksen, N. H., F. Espersen, L. Laursen, P. Skinhoj, N. Hoiby, and I. Lind. 1989. Nosocomial outbreak of group C meningococcal disease. BMJ 298:568-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell, J. E., K. A. Jolley, I. M. Feavers, M. C. Maiden, and J. Suker. 2004. PorA variable regions of Neisseria meningitidis. Emerg. Infect. Dis. 10:674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson, E. A., I. M. Feavers, and M. C. Maiden. 2003. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology 149:1849-1858. [DOI] [PubMed] [Google Scholar]
- 12.Zangwill, K. M., A. Schuchat, F. X. Riedo, R. W. Pinner, D. T. Koo, M. W. Reeves, and J. D. Wenger. 1997. School-based clusters of meningococcal disease in the United States. Descriptive epidemiology and a case-control analysis. JAMA 277:389-395. [PubMed] [Google Scholar]