Abstract
Forty-one clinical isolates of group A streptococcus (GAS) were recovered in Poland from patients with severe invasive infections and were analyzed by phenotypic and genotypic techniques. All isolates were characterized by determining their susceptibilities to antimicrobial agents and by determining their types by pulsed-field gel electrophoresis, multilocus sequence typing, emm typing, and the detection of five streptococcal pyrogenic exotoxin genes (speA, speB, speC, speF, ssa). The isolates studied were fully susceptible to penicillin G, levofloxacin, quinupristin-dalfopristin, and linezolid. Resistance to tetracycline, chloramphenicol, and erythromycin was detected in 46.3, 12.1, and 9.8% of the isolates, respectively. A total of 23 different emm sequence types were identified, of which emm1 and emm12 (19.5% each) were the most common, followed by emm81, emm44/61, and emm85. All the emm1 isolates had the speA2 allele. Twenty-three unrelated sequence types (STs) were identified, with the most frequent STs, ST28 and ST36, corresponding to emm1 and emm12, respectively. Six newly found STs (STs 375, 376, 377, 378, 379, and 385) corresponded to emm types 74, 102, 77, 76, 84 and 63, respectively. The emm1 type and the presence of speA2 gene were associated with the severity of GAS infections. This work presents the first molecular study on Polish invasive GAS isolates.
The past two decades have witnessed a worldwide resurgence in invasive group A streptococcus (GAS) disease, which includes various clinical syndromes, such as bacteremia, septic arthritis, pneumonia, peritonitis, puerperal sepsis, necrotizing fasciitis (NF), meningitis, and streptococcal toxic shock syndrome (STSS). These rapidly progressing infections are associated with high morbidity and mortality rates, even in patients receiving appropriate antimicrobial therapy (4, 7, 25). The extracellular pyrogenic exotoxins (SpeA, SpeB, SpeC), mitogenic factor (SpeF), and streptococcal superantigen (SSA), together with the surface-located M protein, play a major role in the pathogenesis of invasive GAS infections. The M protein, encoded by the emm gene, provides the basis for the identification of different GAS M types as a tool for epidemiological analyses (4, 11). Recently, classical serologic M typing in many laboratories has been replaced by molecular typing based on the sequencing of the 5′ region of the emm gene, and over 160 different emm genotypes are currently recognized (data available at ftp://ftp.cdc.gov/pub/infectious_diseases/biotech/tsemm/). The majority of GAS outbreaks studied worldwide so far have been caused predominantly by strains of emm types 1, 3, 12, and 28 (4, 7, 16). Pulsed-field gel electrophoresis (PFGE) of macrorestricted bacterial DNA and multilocus sequence typing (MLST) represent other important tools for discrimination among GAS strains (10, 11, 17).
The invasive GAS cases that occur in Poland pose a serious hazard to public health. GAS isolates from patients with invasive forms of disease were sent by local laboratories to the National Institute of Public Health for further species confirmation and identification of toxin genes. The present study constitutes the first one to describe the properties of the invasive Polish GAS isolates in terms of their susceptibilities to antimicrobial agents, emm types, sequence types (STs), and PFGE profiles and to analyze their virulence gene distribution.
(This work was presented in part at the 15th European Congress of Clinical Microbiology and Infections Diseases, Copenhagen, Denmark [abstr. P1166].)
MATERIALS AND METHODS
Bacterial strains and patients characteristics.
Forty-one GAS isolates from patients with severe symptoms of invasive GAS disease episodes were collected at 17 different hospitals distributed in different parts of Poland and sent to the National Institute of Public Health between 1997 and 2005. The criteria used to define severe GAS infection were in accordance with those described by The Working Group on Severe Streptococcal Infection (31). These isolates were recovered from blood (n = 25), pus (n = 5), wounds (n = 6), peritoneal fluid (n = 2), synovial fluid (n = 2), and pleural fluid (n = 1). Isolates were reidentified by standard procedures with a commercially available agglutination test kit (Streptex; Murex Biotech Ltd., United Kingdom) and the pyrrolidonyl-arylamidase test (PYR 50 test kit; Remel Inc., Lenexa, KS).
Patients' data were collected on a specially prepared questionnaire that included information on demographic characteristics (age, sex), underlying conditions, clinical manifestation, and the outcome of illness. The case-fatality ratio (CFR) was calculated on the basis of the number of cases with known outcomes.
Antimicrobial susceptibility testing.
MICs were determined according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) by the standard microdilution method (21). Streptococcus pneumoniae strain ATCC 49619 was included for quality control purposes. For all isolates, susceptibility to the following antimicrobial agents was tested: penicillin G, erythromycin, tetracycline, and chloramphenicol (Sigma-Aldrich, Steinheim, Germany); clindamycin and linezolid (Pharmacia Upjohn, Inc., Kalamazoo, MI); spiramycin (Rhone-Poulenc Rorer, Collegeville, PA); and telithromycin, quinupristin-dalfopristin, and levofloxacin (Aventis Pharma, Romainville, France). The MIC breakpoints were interpreted according to the CLSI criteria (2). Breakpoints for spiramycin were those proposed by the French Society for Microbiology (3). All erythromycin-resistant isolates were assigned to one of the following macrolide, lincosamide, and streptogramin B (MLSB) phenotypes by the double-disk test described previously (27): inducible MLSB (iMLSB), constitutive MLSB (cMLSB), and efflux-mediated resistance (M phenotype).
PFGE analysis, MLST, emm typing, and detection of streptococcal pyrogenic exotoxin genes.
Chromosomal DNA was isolated from bacterial cultures, digested with the SmaI restriction enzyme (Fermentas, Vilnius, Lithuania), and electrophoresed in 1% pulsed-field certified agarose (Bio-Rad Laboratories, Hercules, CA) in a CHEF-DR III system (Bio-Rad) as described by Stanley et al. (24). PFGE patterns were compared with the use of Molecular Analyst software, version 1.12 (Bio-Rad), by using the unweighted pair group method with arithmetic means clustering method with the Dice coefficient and a position tolerance of 1.5%. In the resulting dendrogram, isolates with a genetic relatedness of >80% were considered to represent the same PFGE type, with the subtypes designated A1, A2, etc. Total bacterial DNA, which was subsequently used as a PCR template, was isolated by using a Genomic DNA Prep Plus kit (A&A Biotechnology, Gdańsk, Poland). For MLST, the internal fragments of seven housekeeping genes were amplified and sequenced with primers by following the protocol described by Enright et al. (10). To be considered related, isolates had to share at least five alleles of the seven loci. The Internet-accessible database (www.mlst.net) was used to assign the allele numbers and the STs to particular allelic profiles. The emm types for all isolates were determined by sequencing according to the recommendations of the Division of Bacterial and Mycotic Diseases, Centers for Disease Control and Prevention, and by using the emm sequence database (www.cdc.gov/nciod/biotech/strep). PCR was performed to detect the presence of the speA, speB, speC, speF, and ssa genes with primer pairs specific for each gene following previously described protocols (1, 28, 30). The alleles of the speA gene were identified by sequencing of the PCR product (20).
RESULTS
Patient characteristics and clinical features.
Our study analyzed 41 isolates obtained from patients with invasive GAS disease. The clinical manifestations observed in these patients included sepsis (16 patients), STSS (10 patients), erysipelas (6 patients), septic arthritis (4 patients), peritonitis (2 patients), and necrotizing fasciitis (3 patients). The majority of patients (60.4%) were male. In one case the age was not reported; the remaining 40 patients ranged in age from 1 to 88 years (median age, 46 years), although the majority of cases (67.5%) occurred in patients aged 26 to 60 years (median age, 45 years). The five pediatric patients (median age, 3 years; range, 1 to 14 years) accounted for 12.2% of all isolates, and the remaining eight patients (19.5%) were older than 65 years (median age, 74 years). Among the patients with STSS, seven were adults (median age, 43 years; range, 26 to 72 years) and three were children (range, 1 to 4 years). In the non-STSS group of patients, the median age was 46 years (range, 3 to 88 years). Predisposing factors were identified for 28 (68.2%) patients (Table 1), of whom 10 patients (35.7%) had more than one predisposing factor. The CFR was assessed for 37 (90.2%) patients, as the outcome was unknown in 4 cases. The overall CFR was 35.1%; however, it reached 50% for the 10 patients who developed STSS. Among patients without STSS, the CFR was 29.6%.
TABLE 1.
Predisposing factors among patients with invasive GAS infections
| Predisposing factor | No. (%) of patients |
|---|---|
| Predisposing factor(s) (one or more) | 28 (68.2) |
| Alcoholisma | 9 (32.1) |
| Surgical proceduresb | 8 (28.5) |
| Viral infectionsc | 4 (14.2) |
| Pneumonia | 3 (10.7) |
| Cancer | 2 (7.1) |
| Chronic renal insufficiency | 2 (7.1) |
| Cirrhosis of liver | 2 (7.1) |
| Scabies | 1 (3.5) |
| Epilepsy | 1 (3.5) |
| Postpartum status | 1 (3.5) |
| Hypothermia | 1 (3.5) |
Six patients had more than one predisposing factor.
Including appendectomy, postfrostbite transtibial amputation, thyroidectomy, cholecystectomy, and prosthetic joint replacement.
Including varicella-zoster virus, human immunodeficiency virus, rotavirus, and Epstein-Barr virus.
Susceptibilities to antimicrobial agents.
All isolates were fully susceptible to penicillin G, levofloxacin, quinupristin-dalfopristin, and linezolid. The highest proportion of resistance was found for tetracycline (46.3%). Resistance to chloramphenicol and erythromycin was seen in 12.1% and 9.8% of the isolates, respectively (Table 2). According to the double-disk test, two of four erythromycin-resistant isolates exhibited the iMLSB phenotype and the other two exhibited the cMLSB phenotype. All four erythromycin-resistant isolates were also resistant to chloramphenicol and tetracycline. Clindamycin and spiramycin retained good activity against all erythromycin-susceptible isolates. Susceptibility to telithromycin was found in all but the two isolates which manifested the cMLSB phenotype.
TABLE 2.
Susceptibilities of 41 invasive GAS isolates to 10 antimicrobial agents
| Antibiotic | MIC (μg/ml)
|
% Resistant | ||
|---|---|---|---|---|
| 50% | 90% | Range | ||
| Penicillin G | 0.008 | 0.015 | 0.008-0.015 | 0 |
| Erythromycin | 0.015 | 0.12 | 0.015->128 | 9.8 |
| Spiramycin | 0.25 | 0.5 | 0.03-128 | 4.9 |
| Clindamycin | 0.06 | 0.12 | 0.03-128 | 4.9 |
| Telithromycin | 0.015 | 0.03 | 0.015-16 | 4.9 |
| Q/Da | 0.5 | 0.5 | 0.12-1 | 0 |
| Tetracycline | 0.25 | 32 | 0.12-128 | 46.3 |
| Chloramphenicol | 2 | 2 | 2-32 | 12.1 |
| Levofloxacin | 0.5 | 0.5 | 0.25-1 | 0 |
| Linezolid | 1 | 1 | 0.25-1 | 0 |
Q/D, quinupristin-dalfopristin.
PFGE analysis, emm typing, and MLST.
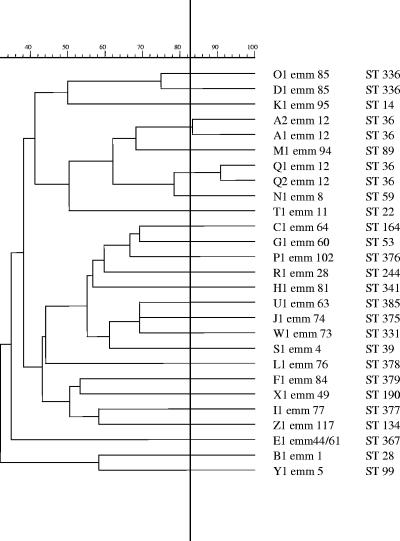
A total of 23 different emm sequence types were identified, of which emm1 and emm12 (19.5% each) were the most common, followed by emm81 (7.3%), emm44/61, and emm85 (4.9% each). Together, these five emm types accounted for 56% of the isolates studied. Among the 10 isolates from patients with STSS, 5 and 2 isolates were emm1 and emm85, respectively; the remaining 3 isolates were emm12, emm81, and emm94, respectively. The CFR among patients infected with emm1 GAS isolates was 50% and was four times higher than that among the group of patients with emm12 isolates. The four erythromycin-resistant isolates included two emm12 isolates with the cMLSB phenotype and two emm44/61 isolates with the iMLSB phenotype. MLST analysis revealed the presence of 23 unrelated STs (Table 3), which correlated very well with the emm types. The most frequent STs, ST28 and ST36 (eight isolates each), corresponded to emm1 and emm12, respectively. Six newly found STs (STs 375, 376, 377, 378, 379, and 385) corresponded to emm types 74, 102, 77, 76, 84, and 63, respectively. Twenty-seven different PFGE patterns were identified, and these constituted 25 types (Fig. 1). The two predominant types, types A (type A1, five isolates; type A2, one isolate) and B (eight isolates), comprised 36.6% of the isolates studied and were characteristic for isolates of emm12/ST36 and emm1/ST28, respectively. Most isolates with the same emm type and ST generally shared related chromosomal PFGE patterns. The exceptions to this were emm12/ST36 and emm85/ST336, which included more than a single PFGE type (types A1-A2 and Q1-Q2 and types D1 and O1, respectively; Table 3). The two predominant emm1/ST28 and emm12/ST36 types were isolates from patients in seven and six hospitals, respectively, recovered between 1998 and 2004. An epidemiological link between strains with the same PFGE/emm/ST type was observed for only two emm1/ST28 isolates, which were derived from two patients who were hospitalized in the same institution and who both developed STSS as a result of postoperative wound infections.
TABLE 3.
emm types, PFGE types, resistance profiles, MLST characteristics, toxin genes profiles, and clinical manifestations among 41 Polish invasive GAS isolates
| emm type | PFGE type | Resistance profilea | STb | MLST allelic profilec | No. of invasive isolates in DBd | Presence of toxin genes
|
Clinical manifestation (no. of isolates, no. of deaths) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| speAe | speB | speC | speF | ssa | |||||||
| 1 | B1 | 28 | 4-3-4-4-4-2-4 | 33 | 2 | + | − | + | − | STSS (5, 2), peritonitis (2, 1), sepsis (1, 1) | |
| 4 | S1 | 39 | 5-11-8-5-15-2-1 | 14 | − | + | + | + | + | Sepsis (1) | |
| 5 | Y1 | 99 | 33-30-7-5-5-26-3 | 6 | − | + | + | + | − | Sepsis (1) | |
| 8 | N1 | T | 59 | 13-2-8-19-1-3-4 | − | + | + | + | − | Erysipelas (1) | |
| 11 | T1 | T | 22 | 3-4-6-7-1-5-4 | 2 | − | + | + | + | − | Sepsis (1) |
| 12 | A1 | 36 | 5-2-2-6-6-2-2 | 6 | − | + | − | + | − | Septic arthritis (1), erysipelas (1), STSS (2, 1) | |
| 12 | A1 A2 | 36 | 5-2-2-6-6-2-2 | − | + | + | + | − | Erysipelas (1), septic arthritis (1) | ||
| 12 | Q1 Q2 | E S C Te T Ch | 36 | 5-2-2-6-6-2-2 | − | + | + | + | − | Septic arthritis (1), sepsis (1) | |
| 28 | R1 | T | 244 | 11-6-14-5-9-44-19 | − | + | − | + | − | Sepsis (1, 1) | |
| 44/61 | E1 | E T Ch | 367 | 4-2-3-11-17-3-61 | − | + | − | + | − | Erysipelas (1), sepsis (1) | |
| 49 | X1 | T Ch | 190 | 4-6-28-7-21-7-1 | 1 | 1 | + | + | + | + | Sepsis (1) |
| 60 | G1 | T | 53 | 11-6-22-7-9-2-17 | − | + | + | + | − | Sepsis (1) | |
| 63 | U1 | 385 | 78-53-52-5-81-68-4c | − | + | − | + | − | Sepsis (1) | ||
| 64 | C1 | T | 164 | 2-2-8-3-5-2-29 | − | + | − | + | − | Sepsis (1) | |
| 73 | W1 | 331 | 43-2-2-47-1-3-4 | 2 | + | − | + | − | Sepsis (1, 1) | ||
| 74 | J1 | T | 375 | 92-2-2-2-31-3-2 | − | + | − | + | − | Erysipelas (1) | |
| 76 | L1 | T | 378 | 11-6-3-6-6-27-46 | − | + | − | + | − | Sepsis (1) | |
| 77 | I1 | 377 | 4-2-2-11-34-3-2 | − | + | − | + | − | Sepsis (1, 1) | ||
| 81 | H1 | T | 341 | 91-2-65-7-1-3-60 | 2 | − | + | + | + | − | STSS (1, 1), NF (2, 1) |
| 84 | F1 | T | 379 | 12-21-17-5-5-3-7 | − | + | − | + | − | Sepsis (1) | |
| 85 | D1 O1 | T | 336 | 87-9-8-7-5-57-54 | 1 | − | + | + | + | − | STSS (1, 1), sepsis (1, 1) |
| 94 | M1 | 89 | 24-2-3-5-1-3-1 | 2 | − | + | + | + | − | STSS (1) | |
| 95 | K1 | T | 14 | 2-6-8-3-9-3-1 | 3 | − | + | − | + | − | Erysipelas (1) |
| 102 | P1 | T | 376 | 4-2-2-5-70-2-1 | − | + | + | + | − | NF (1, 1) | |
| 117 | Z1 | 134 | 54-24-14-4-9-2-2 | 1 | − | + | − | + | − | Septic arthritis (1) | |
C, clindamycin; Ch, chloramphenicol; E, erythromycin; S, spiramycin; T, tetracycline; Te, telithromycin.
Boldface type, new STs.
Underlined, new allele; the MLST allelic profile is in the order gki-gtr-murI-mutS-recP-xpt-yqiL.
Number of isolates reported to the MLST database (DB) as recovered from invasive disease-sterile site.
Numbers indicate the speA allele.
FIG. 1.
PFGE-based dendrogram of invasive Streptococcus pyogenes isolates and their emm types and STs.
Distribution of virulence genes.
The results of PCR amplification of streptococcal pyrogenic exotoxin genes showed that all the isolates possessed the chromosomal speB and speF genes (Table 3). The bacteriophage-encoded speA and speC genes were present in 24.4% (10/41) and 41.5% (17/41) of the isolates, respectively. The ssa gene was detected in only two isolates (4.9%), which were of emm4 and emm49. All eight of the emm1 isolates possessed the speA2 gene, which was also present in a single isolate of emm73/ST331. Generally, five exotoxin gene profiles could be distinguished in the isolates studied, with three predominant profiles, speA negative (speA−), speB positive (speB+), speC−, speF+, and ssa− (15 isolates); speA−, speB+, speC+, speF+, and ssa− (15 isolates); and speA2+, speB+, speC−, speF+, and ssa− (9 isolates). The presence of all four spe genes (with the profile speA1+ speB+, speC+, speF+, and ssa+) was observed in a single isolate of emm49/ST190. Among the STSS isolates, three different exotoxin profiles were found, with the predominance of the profile speA2+, speB+, speC−, speF+, ssa− (five isolates). None of the STSS isolates possessed the ssa gene. All eight emm1 isolates showed the presence of a single exotoxin gene profile (speA2+ speB+, speC−, speF+, and ssa−), whereas among the eight emm12 isolates, two toxin gene profiles were found.
DISCUSSION
Recently, cases of severe invasive GAS infections have been reported in all parts of the world (7, 16). These infections occurred both in previously healthy individuals and in patients with weakened immune systems caused by other medical conditions (4). While some studies reported that the risk of invasive GAS disease associated with alcoholism was low (12), others indicated that it is a possible predisposing factor (5, 32), similar to the observation in this study. However, it must be underlined that the majority of alcoholic patients in our study had two or more other risk factors. Surgical procedures constituted another known factor associated with the development of invasive GAS infections found in the current study. Several studies reported the occurrence of invasive GAS disease among elderly individuals with underlying medical problems, while others found the highest rates of these diseases among young children (5, 22, 26). In the present study more than a half of the patients with invasive disease were adults with a mean age of 43.5 years, which is in accordance with reports from the United States (14, 32) and Taiwan (15). The CFR of 35% for the total sample was much higher than the CFRs described by others in Canada (5, 29), the United States (22, 32), Sweden (26), Denmark (9), and Israel (19), where it ranged from 12% to 24%. As only strains from the sickest patients are referred to our laboratory, this may explain the higher CFR reported here. Similar to other studies, we found that the risk of death due to invasive GAS infection is greatest when STSS develops (5, 8, 9, 15). Moreover, we observed a high risk of death among patients with NF. We tested a broad panel of antimicrobial drugs, including those not recommended for the treatment of streptococcal infections, for the purpose of epidemiological investigations. In this study, all isolates were susceptible to penicillin, the drug of choice for the treatment of GAS infections, and exhibited very low MICs. However, penicillin was shown to be ineffective in several clinical studies as well as in some experimental models of GAS invasive infections in which toxins were involved in the pathogenesis of that clinical condition and when a particularly large number of organisms was present (4). Therefore, the use of clindamycin in combination with penicillin for the treatment of necrotizing fasciitis or STSS was suggested (7, 25) since clindamycin inhibits protein (toxin) synthesis. As the prevalence of erythromycin- and clindamycin-resistant isolates from severe GAS infections reported in many countries varies (4, 7, 13, 22), it is obvious that clindamycin should not be used alone until an isolate is shown to be sensitive to this agent. Recently, a significant increase in the MLSB phenotype was observed among clinical GAS isolates in Poland (27). In the present study, all the erythromycin-resistant isolates manifested the MLSB phenotype. In light of this observation, the use of clindamycin as a therapeutic option requires more attention.
The M protein constitutes a major virulence factor of GAS; certain emm types, mainly types 1, 3, 11, 12, and 28, were associated with STSS and other severe GAS infections (4, 7, 22). Apart from the most common types, types emm1 and emm12, which together accounted for 39% of the isolates, many other emm types were observed in our study. Such an emm type distribution is in general agreement with the type distribution of invasive GAS isolates in other European countries (6, 16). In addition, almost 15% of the isolates in this study represented emm types (types 64, 74, 84, 85, 95, and 117) which have rarely, if ever, been associated with severe GAS infections, while some other GAS types of increased invasiveness (emm3, emm18) were absent in this study. On the basis of epidemiological data demonstrating that the majority of noninvasive and invasive streptococcal infections are caused by a limited number of M types, a multivalent vaccine containing amino-terminal M-protein fragments from 26 different serotypes of GAS was recently developed in the United States (18). However, the vaccine would include the emm types of only 30.7% of the organisms identified in the present study, which reflects differences in the epidemiologies among various geographic locations.
Many reports on the pathogenesis of STSS and other invasive GAS diseases stressed the role of streptococcal toxins (pyrogenic exotoxins and superantigens), primarily SpeA, in the pathogenesis of GAS infections. The presence of toxin genes with different profiles and their relationships to emm types have been noted by many investigators (6, 9, 15, 30, 32). In our study, we observed the predominance of a single exotoxin gene profile in all eight emm1 isolates: speA positive and speC and ssa negative. In contrast, the eight emm12 isolates were characterized by two toxin gene profiles, and all lacked the speA gene. These findings are in accordance with the findings of previous studies from The Netherlands, Denmark, and Belgium (6, 9, 30) and support the strong association between invasive GAS isolates with the emm1 type and the presence of the speA gene. Moreover, we observed an association between the severity of GAS infection and the emm1 type, which was responsible for 50% of all STSS cases.
Until now, no data on the genetic diversity of Polish invasive GAS isolates have been available. This study revealed the presence of two widely distributed clones of emm1/ST28 and emm12/ST36 that accounted for almost 40% of all cases of invasive GAS disease, while the remaining isolates were highly diverse and each was typically found only once or twice. The emm1/ST28 clone was highly homogeneous, while the emm12/ST36 clone was further differentiated by susceptibility testing, PFGE, and exotoxin gene profiling. However, isolates of emm1 are significantly overrepresented in invasive disease; in the studies of pharyngeal GAS of Shulman et al., the prevalences of types 1 and 12 were comparable (23). Moreover, in a surveillance study conducted in the United States, infection with the emm1 GAS strain was a statistically significant predictor of the patient's death (22). In our study, the emm1/ST28 clone represented the major cause of STSS (50% of all the cases, two of which were fatal), and this form of the disease clearly dominated for this clone (of the eight isolates, five were associated with cases of STSS), further supporting the increased virulence of this clone. In contrast, some other clones, such as emm12/ST36, emm4/ST39, and emm28/ST244, occur predominantly in uncomplicated throat infections rather than in invasive disease (8).
In summary, the results obtained in this pilot study provide useful comparative data for future research, and the study constitutes the first molecular study on Polish invasive GAS isolates. While many questions about the pathogenesis and epidemiology of invasive GAS infections remain to be answered, our data support the association between severe invasive GAS diseases and emm1 GAS isolates bearing the speA2 gene.
Acknowledgments
We acknowledge the use of the GAS MLST database, which is located at Imperial College, London, United Kingdom, and which is funded by the Wellcome Trust.
We thank Stephen Murchan for editing of the manuscript.
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Chatellier, S., N. Ihendyane, R. G. Kansal, F. Khambaty, H. Basma, A. Norrby-Teglund, D. E. Low, A. McGeer, and M. Kotb. 2000. Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect. Immun. 68:3523-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing, fifteenth informational supplement; M100-S15. Cinical and Laboratory Standards Institute, Wayne, Pa.
- 3.Comité de l'Antibiogramme de la Société Française de Microbiologie. 1996. Report of the Comité de l'Antibiogramme de la Société Française de Microbiologie. Statement 1996 CA-SFM. Zone sizes and MIC breakpoints for non-fastidious organisms. Clin. Microbiol. Infect. 2:46-49. [PubMed] [Google Scholar]
- 4.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies, D. H., A. McGeer, B. Schwartz, K. Green, D. Cann, A. E. Simor, and D. E. Low. 1996. Invasive group A streptococcal infections in Ontario, Canada. N. Engl. J. Med. 335:547-554. [DOI] [PubMed] [Google Scholar]
- 6.Descheemaeker, P. F., M. Van Loock, P. Hauchecorne. P. Vandamme, and H. Goossens. 2000. Molecular characterisation of group A streptococci from invasive and noninvasive disease episodes in Belgium during 1993-1994. J. Med. Microbiol. 49:467-471. [DOI] [PubMed] [Google Scholar]
- 7.Efstratiou, A. 2000. Group A streptococci in the 1990s. J. Antimicrob. Chemother. 45:3-12. [DOI] [PubMed] [Google Scholar]
- 8.Ekelund, K., J. Darenberg, A. Norrby-Teglund, S. Hoffmann, D. Bang, P. Skinhøj, and H. B. Konradsen. 2005. Variations in emm type among group A streptococcal isolates causing invasive or noninvasive infections in a nationwide study. J. Clin. Microbiol. 43:3101-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekelund, K., P. Skinhøj, J. Madsen, and H. B. Konradsen. 2005. Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study. J. Clin. Microbiol. 43:1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enright, M. C., B. G. Spratt, A. Kalia, J. H. Cross, and D. B. Bessen. 2001. Multilocus sequence typing of Streptococcus pyogenes and the relationships between emm type and clone. Infect. Immun. 69:2416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facklam, R., B. Beall, A. Efstratiou, A. Efstratiou, V. Fischetti, D. Johnson, E. Kaplan, P. Kriz, M. Lovgren, D. Martin, B. Schwartz, A. Totolian, D. Bessen, S. Hollingshead, F. Rubin, J. Scott, and G. Tyrrell. 1999. emm typing and validation of provisional M types for group A streptococci. Emerg. Infect. Dis. 5:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Factor, S. H., O. S., Levine, B. Schwartz, L. H. Harrison, M. M. Farley, A. McGeer, and A. Schuchat. 2003. Invasive group A streptococcal disease: risk factors for adults. Emerg. Infect. Dis. 9:970-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho, P. L., D. R. Johnson, A. W. Y. Yue, D. N. C. Tsang, T. L. Que, B. Beall, and E. L. Kaplan. 2003. Epidemiological analysis of invasive and noninvasive group A streptococcal isolates in Hong Kong. J. Clin. Microbiol. 41:937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoge, C. W., B. Schwartz, D. F. Talkington, R. F. Breiman, E. M. MacNeill, and S. J. Englender. 1993. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. JAMA 269:384-389. [PubMed] [Google Scholar]
- 15.Hsueh, P.-R., J.-J. Wu, P.-J. Tsai, J.-W. Liu, Y.-C. Chuang, and K.-T. Luh. 1998. Invasive group A streptococcal disease in Taiwan is not associated with the presence of streptococcal pyrogenic exotoxin genes. Clin. Infect. Dis. 26:584-589. [DOI] [PubMed] [Google Scholar]
- 16.Jasir, A., and C. Schalén. 2005. Strep-EURO: progress in analysis and research into severe streptococcal disease in Europe, 2003-2004. Eurosurveillance 10:60-61. [DOI] [PubMed] [Google Scholar]
- 17.McGregor, K. F., B. G. Spratt, A. Kalia, A. Bennett, N. Bilek, B. Beall, and D. E. Bessen. 2004. Multilocus sequence typing of Streptococcus pyogenes representing most known emm types and distinctions among subpopulation genetic structures. J. Bacteriol. 186:4285-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil, S. A., S. A. Halperin, J. M. Langley, B. Smith, A. Warren, G. P. Sharratt, D. M. Baxendale, M. A. Reddish, M. C. Hu, S. D. Stroop, J. Linden, L. F. Fries, P. E. Vink, and J. B. Dale. 2005. Safety and immunogenicity of 26-valent group A Streptococcus vaccine in healthy adult volunteers. Clin. Infect. Dis. 41:1114-1122. [DOI] [PubMed] [Google Scholar]
- 19.Moses, A. E., S. Goldberg, Z. Korenman, M. Ravins, E. Hanski, and M. Shapiro. 2002. Invasive group A streptococcal infections, Israel. Emerg. Infect. Dis. 8:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musser, J. M., A. R. Hauser, M. H. Kim, P. M. Schlievert, K. Nelson., and R. K. Selander. 1991. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases. Clonal diversity and pyrogenic exotoxin expression. Proc. Natl. Acad. Sci. USA 88:2668-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 2002. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standards, 5th ed. M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.O'Brien, K. L., B. Beall, N. L. Barrett, P. R. Cieslak, A. Reingold, M. M. Farley, R. Danila, E. R. Zell, R. Facklam, B. Schwartz, and A. Schuchat. 2002. Epidemiology of invasive group A Streptococcus disease in United States, 1995-1999. Clin. Infect. Dis. 35:268-276. [DOI] [PubMed] [Google Scholar]
- 23.Shulman, S. T., R. R. Tanz, W. Kabat, K. Kabat, E. Cederlund, D. Patal, Z. Li, V. Sakota, J. B. Dale, B. Beal, and U.S. Streptococcal Surveillance Group. 2004. Group A streptococcal pharyngitis serotype surveillance in North America, 2000-2002. Clin. Infect. Dis. 39:325-332. [DOI] [PubMed] [Google Scholar]
- 24.Stanley, J., D. Linton, M. Desai, A. Efstratiou, and R. George. 1995. Molecular subtyping of prevalent M serotypes of Streptococcus pyogenes causing invasive disease. J. Clin. Microbiol. 33:2850-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens, D. L. 1992. Invasive group A streptococcus infections. Clin. Infect. Dis. 14:2-13. [DOI] [PubMed] [Google Scholar]
- 26.Svensson, N., S. Öberg, B. Henriques, S. Holm, G. Källenius, V. Romanus, and J. Giesecke. 2000. Invasive group A streptococcal infections in Sweden in 1994 and 1995: epidemiology and clinical spectrum. Scand. J. Infect. Dis. 32:609-614. [DOI] [PubMed] [Google Scholar]
- 27.Szczypa, K., E. Sadowy, R. Izdebski, and W. Hryniewicz. 2004. A rapid increase in macrolide resistance in Streptococcus pyogenes isolated in Poland during 1996-2002. J. Antimicrob. Chemother. 54:828-831. [DOI] [PubMed] [Google Scholar]
- 28.Tyler, S. D., W. M. Johnson, J. C. Huang, F. E. Ashton, G. Wang, D. E. Low, and K. R. Rozee. 1992. Streptococcal erythrogenic toxin genes: detection by polymerase chain reaction and association with disease in strains isolated in Canada from 1940 to 1991. J. Clin. Microbiol. 30:3127-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyrrell, G., M. Lovgren, B. Forwick, N. P. Hoe, J. M. Musser, and J. A. Talbot. 2002. M types of group A streptococcal isolates submitted to the National Centre for Streptococcus (Canada) from 1993 to 1999. J. Clin. Microbiol. 40:4466-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlaminckx, B. J. M., E. M. Mascini, J. Schellekens, L. M. Schouls, A. Paauw, A. C. Fluit, R. Novak, J. Verhoef, and F. J. Schmitz. 2003. Site-specific manifestations of invasive group A streptococcal disease: type distribution and corresponding patterns of virulence determinants produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 41:4941-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Working Group on Severe Streptococcal Infections. 1993. Defining the group A streptococcal toxic shock syndrome. JAMA 269:390-391. [PubMed] [Google Scholar]
- 32.Zurawski, C. A., M. S. Bardsley, B. Beall, J. A. Elliott, R. Facklam, B. Schwartz, and M. M. Farley. 1998. Invasive group A streptococcal disease in metropolitan Atlanta: a population-based assessment. Clin. Infect. Dis. 27:150-157. [DOI] [PubMed] [Google Scholar]