Abstract
In this study, we compared a recently developed PCR-restriction fragment length polymorphism (PCR-RFLP) assay with pulsed-field gel electrophoresis (PFGE) using three different Shiga toxin-producing Escherichia coli (STEC) strains to understand whether repeated subculture in vitro and prolonged storage at room temperature affect the RFLP patterns of STEC. The PFGE profiles of the STEC strains changed by 1 to 8 fragments after repeated subculture and prolonged storage; one strain was no longer clonal after repeated subculture compared to the original isolate according to the Tenover criteria. In contrast, RFLP patterns obtained by PCR-RFLP were identical after repeated subculture and prolonged storage. These data clearly indicate that the PCR-RFLP assay which is based on the diversity of region V, a regulatory region of Stx-phage, was not affected by repeated subculture and prolonged storage and is a more practical and reliable method for molecular typing of STEC strains.
Shiga toxin-producing Escherichia coli (STEC) is a serious public health concern worldwide (8). This pathogen causes diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome (19, 33). Shiga toxin produced by STEC has been considered a prime virulence factor (27). Shiga toxin is classified into two groups, Stx1 and Stx2, on the basis of immunological properties (19, 27, 32). Both Sxt1 and Stx2 are encoded on a lambda-like bacteriophage, the so-called Stx phage (19, 27, 33). Though more than 100 serotypes of STEC have been isolated from humans (2), O157:H7 is the most predominant serotype isolated from sporadic cases and outbreaks (11, 38). In addition to causing large outbreaks (8), STEC O157:H7 is associated with life-threatening complication of hemolytic-uremic syndrome (19, 33). Since most of the food poisonings due to STEC are related to the consumption of beef or beef products, cattle have been considered a major reservoir of STEC (9). However, other vehicles, such as contaminated water, vegetables, and fruits, have been increasingly recognized as an infection source of STEC (1, 7, 36). Person-to-person transmission is also reported to be an important route of infection (11, 29).
Several molecular typing methods, such as ribotyping, random amplified polymorphic DNA-PCR, and pulsed-field gel electrophoresis (PFGE), have been utilized for molecular epidemiological studies (10, 37). Among them, PFGE is the most commonly used molecular typing method to identify the possible source and route of infection of the pathogen, and it has been used for a variety of pathogens, including STEC, because of its high discriminatory power to reveal clonal relationships among the isolates (5, 35). However, PFGE has some limitations, like expense, equipment required, and limitations on the number of isolates that can be analyzed simultaneously. The PFGE profiles of some strains cannot be resolved due to strong DNase activity or degradation by free radical or peroxide produced during electrophoresis, resulting in smeared profiles (21, 30). In addition, variation in PFGE patterns has been reported for STEC strains that are passaged through the bovine or human gastrointestinal tract (4, 18). Changing PFGE patterns have also been reported after subculture of STEC strains in vitro (13). To overcome such problems, we recently developed a rapid and simple DNA fingerprinting method for molecular epidemiological analysis of STEC strains (30). The PCR-restriction fragment length polymorphism (PCR-RFLP) assay exploits the nucleotide sequence diversity within the region V defined as the regulatory region of Stx phage.
In the present study, we evaluated the usefulness of the newly developed PCR-RFLP assay compared to PFGE to ascertain clonal lineages among STEC strains that were either subcultured repeatedly in vitro or stored at room temperature for a prolonged period of time.
MATERIALS AND METHODS
Bacterial strains and growth media.
Three wild-type STEC O157:H7 strains, RIMD0509952, EC1015, and 83-1386, harboring both stx1 and stx2 genes, were included in this study. Strain RIMD0509952, the so-called Sakai strain, was isolated during the Sakai outbreak in 1996 (12, 23, 38). Strain EC1015 was isolated during the Okayama outbreak in 1996 (38), and strain 83-1386 was isolated from a patient with hemorrhagic colitis in Canada (16). E. coli strain C600 was used as the negative control. E. coli strains were grown either on heart infusion agar (HIA), L agar, or in L broth.
Chemicals and enzymes.
Chemicals were purchased either from Nacalai Tesque (Kyoto, Japan), Wako Pure Chemicals (Tokyo, Japan), or Sigma Chemical Co. (St. Louis, MO). Restriction enzymes, Takara long and accurate (LA) Taq, and LA-PCR kit version 2 were purchased from Takara Bio Inc. (Shiga, Japan). Bacto tryptone, yeast extract, and HIA used for preparation of media were purchased from DIFCO Laboratories (Detroit, MI). Pulsed-field certified agarose, low-melt preparative grade agarose, and SEAKEM HGT agarose were from either Bio-Rad (Hercules, CA) or Takara Bio, Inc. (Shiga, Japan). Molecular weight markers were purchased from Bio-Rad (Hercules, CA), Takara Bio, Inc. (Shiga, Japan), or Invitrogen Corp. (Carlsbad, CA).
Repeated subculture and prolonged storage.
The repeated in vitro subculture or prolonged storage was performed following a method described previously (13) with minor modifications. Briefly, each bacterial strain, kept on Dorset medium or as glycerol stock, was inoculated onto an L agar plate, and 14 well-isolated colonies were randomly picked and subjected to PFGE and PCR-RFLP. The colony with the most dominant PFGE fragment pattern was repeatedly subcultured on the slant medium of HIA during a 25-week period at 3- to 4-day intervals, and growth was achieved by incubating slant culture at 37°C. Fourteen colonies were randomly selected after every 10 subcultures and were analyzed by PFGE as well as PCR-RFLP assay. To evaluate the effect of prolonged storage, the same colonies used for the repeated subculture were inoculated on a HIA slant media and kept at room temperature for 5 weeks. The subculture was repeated 4 times, up to a total 25-week period. A loopful of the culture on the medium was streaked onto a separate HIA plate at the end of every 5-week storage interval and incubated at 37°C for 18 h. Fourteen colonies were randomly selected and were analyzed for alteration, if any, of PFGE profile or of the PCR-RFLP patterns.
PFGE.
PFGE was performed following essentially the same method as described previously (30). Total genomic DNA was isolated in an agarose-embedded form and was subjected to enzymatic digestion with 50 U of XbaI. The agarose plug containing completely digested genomic DNA was placed into the preformed wells of an agarose gel. Electrophoretic separation of the DNA fragments was achieved through the contour-clamped homogenous electric field method on a CHEF Mapper system (Bio-Rad) using 0.5× TBE buffer (45 mM Tris-HCl, 45 mM boric acid, 1.0 mM EDTA, pH 8.0). Run conditions were generated by the autoalgorithm mode of the CHEF Mapper PFGE system for the sizes ranging between 20 and 300 kb, and PFGE was performed for 40.24 h. PFGE profiles of the wild-type E. coli strains as well as their passaged/stored derivative strains were visualized under UV after staining with ethidium bromide, and the pulsotypes were recorded digitally using the Gel-Doc 2000 system (Bio-Rad).
PCR.
The multiplex PCR for the detection of the stx1 and stx2 genes was performed following the method described previously (20). The PCR amplicons were subjected to 2% agarose gel electrophoresis, followed by staining with ethidium bromide. The bands were visualized and recorded digitally using Gel-Doc 2000 (Bio-Rad).
PCR-RFLP.
LA-PCR was performed using a primer set targeted to region V in Stx phage as described previously (30). The PCR products were analyzed by 0.4% agarose gel electrophoresis using HGT agarose and/or by field inversion gel electrophoresis using 1.0% pulsed-field certified agarose gel in 0.5× TBE (45 mM Tris-HCl, 45 mM boric acid, 1.0 mM EDTA, pH 8.0) buffer for 26 h, followed by staining with ethidium bromide. PCR amplicons were enzymatically cleaved either with BglI or EcoRV. The digested DNA materials were then analyzed by 1.5% agarose gel electrophoresis and/or by field inversion gel electrophoresis using 1.0% pulsed-field certified agarose gel in 0.5× TBE buffer for 18 h, followed by staining with ethidium bromide. The electrophoretic patterns of the digested material (PCR-RFLP) were recorded digitally using a gel documentation system (Gel-Doc 2000; Bio-Rad).
RESULTS
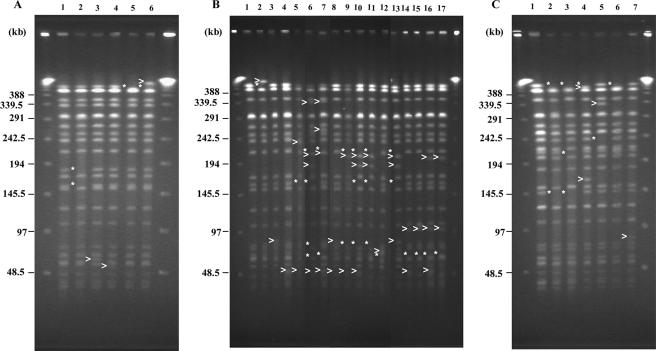
The PFGE results with STEC strains passaged in vitro are summarized in Table 1 and Fig. 1. All of the STEC strains were passaged in vitro for a total period of 25 weeks. STEC strains used in this study were kept on Dorset medium at room temperature or as glycerol stock at −80°C for almost 8 years. The PFGE profiles of the test strains were analyzed prior to beginning the experiments, and the most dominant profile was considered the original pattern (Table 1). From Table 1, it is clear that two (I-a and I-b), three (II-a, II-b, and II-c), and four (III-a, III-b, III-c, and III-d) PFGE profiles were identified among STEC O157:H7 strains RIMD0509952, EC1015, and 83-1386, respectively, when examined just after reviving from the stock. Among 14 colonies of RIMD0509952, 9 colonies showed identical XbaI PFGE profiles, termed I-a. On the other hand, the remaining 5 colonies showed identical PFGE profiles but differed by 2 bands from that of the I-a profile. The second type of PFGE profile was named I-b (Table 1). Interestingly, for strain EC1015, 6 colonies displayed identical PFGE profiles, termed II-a, whereas another 5 and 3 colonies displayed PFGE profiles termed II-b and II-c that differed by 2 and 1 bands, respectively, from the II-a profile. In the case of strain 83-1386, however, 10 colonies displayed a single type of PFGE profile, termed III-a, whereas the remaining 2, 1, and 1 colonies showed different PFGE patterns (III-b, III-c, and III-d) differing by 2, 3, and 3 bands, respectively, from III-a. Therefore, strains with the most dominant PFGE profile (I-a, II-a, and III-a) were selected for use as representative strains for further subculture experiments. Although the colonies displaying PFGE types other than I-a, II-a, and III-a were not used in the passage experiments, these different PFGE profiles were also used to refer PFGE types while comparing the PFGE profiles of the strains derived after passage. One of the 14 selected colonies among the strains derived after passage of RIMD0509952 (Sakai strain) showed variations in the PFGE profile (I-b and I-c) after the 20th and 30th subculture, although its parental type was I-a. For strain 83-1386, after 10 subcultures, two different PFGE profiles (III-d and III-e) were noted. On the other hand, the selected 14 colonies of strain EC1015 showed variations even from the first subculture (Table 1). Surprisingly, no strain that retained the original PFGE profile remained after 20 subcultures and, 2 and 6 of the passaged colonies showed 8 fragment differences in the PFGE profile compared to the original profile after the 40th and 50th subcultures, respectively. PFGE analysis revealed generation of I-b, II-b, and III-d profiles among the strains derived after passage, and these profiles were present among certain colonies of nonpassaged strains (Table 1). Apart from these three profiles, generation of I-c, II-d, II-e, II-f, II-g, and III-e type profiles were also evident among the strains derived after passage. The results obtained by PFGE assay with prolonged storage at room temperature of STEC strains are summarized in Table 2 and Fig. 1. Occurrence of patterns different from the original pattern was limited to 3 fragments at most in the cases of strains RIMD0509952 and 83-1386. However, in the case of strain EC1015, 13 variations of PFGE fragment patterns with 1 to 6 band differences were obtained. Particularly, at the end of 15 weeks of incubation, no original PFGE fragment patterns were recovered.
TABLE 1.
XbaI-PFGE profiles of wild-type STEC O157:H7 and isolates derived through repeated in vitro passages for a 25-week period at intervals of 3 to 4 days
| Strain and PFGE profile | No. of fragment differences from original pattern | No. of colonies showing identity to PFGE profile following no. of subcultures:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Presubculture | 1 | 10 | 20 | 30 | 40 | 50 | ||
| RIMD0509952 (Sakai strain) | ||||||||
| I-a | 0a | 9 | 14 | 14 | 13 | 13 | 14 | 14 |
| I-b | 2 | 5 | 0 | 0 | 0 | 1 | 0 | 0 |
| I-c | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| EC1015 | ||||||||
| II-a | 0a | 6b | 13 | 7 | 0 | 0 | 0 | 0 |
| II-b | 2 | 5c | 1 | 0 | 0 | 0 | 0 | 0 |
| II-c | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| II-d | 1 | 0 | 0 | 6 | 14 | 14 | 12 | 0 |
| II-e | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| II-f | 8 | 0 | 0 | 0 | 0 | 0 | 2 | 6 |
| II-g | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| 83-1386 | ||||||||
| III-a | 0a | 10 | 14 | 12 | 14 | 14 | 14 | 14 |
| III-b | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| III-c | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| III-d | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| III-e | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
Original pattern.
One of the 6 strains did not contain the stx2 gene.
One of the 5 strains did not contain the stx2 gene.
FIG. 1.
PFGE patterns of XbaI-digested fragments obtained when wild-type and successive-passage-derived STEC strains RIMD0509952 (A), EC1015 (B), and 83-1385 (C). (A) PFGE profiles obtained with STEC O157:H7 strain RIMD0509952 and its passage-derived strains. Lanes: 1, I-a (original pattern); 2, I-b; 3, I-c; 4, I-d; 5, I-e; 6, I-f. (B) PFGE profiles obtained with STEC O157:H7 strain EC1015 and its passage-derived strains. Lanes: 1, II-a (original pattern); 2, II-b; 3, II-c; 4, II-d; 5, II-e; 6, II-f; 7, II-g; 8, II-h; 9, II-i; 10, II-j; 11, II-k; 12, II-l; 13, II-m; 14, II-n; 15, II-o; 16, II-p; 17, II-q. (C) PFGE profiles obtained with STEC O157:H7 strain 83-1386 and its passage-derived strains. Lanes: 1, III-a (original pattern); 2, III-b; 3, III-c; 4, III-d; 5, III-e; 6, III-f; 7, III-g. Lambda ladders were used as molecular size markers. Positions of DNA fragments with known molecular masses are indicated. Parentheses and asterisks represented additional and lost bands, respectively.
TABLE 2.
XbaI-PFGE profiles of wild-type STEC O157:H7 strains and their derived isolates revived after prolonged storage at room temperature for a total period of 25 weeks, with subcultures performed at every 5-week interval
| Strain and PFGE profile | Typical no. of fragment differences from original pattern | No. of colonies showing identity to PFGE profile following storage for no. of wks:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Presubculture | 0 | 5 | 10 | 15 | 20 | 25 | ||
| RIMD0509952 | ||||||||
| I-a | 0a | 9 | 14 | 14 | 14 | 14 | 11 | 14 |
| I-b | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| I-d | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| I-e | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| I-f | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| EC1015 | ||||||||
| II-a | 0a | 6b | 13 | 9 | 8 | 0 | 9 | 9 |
| II-b | 2 | 5c | 1 | 0 | 0 | 0 | 0 | 0 |
| II-c | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| II-d | 1 | 0 | 0 | 4 | 6 | 3 | 2 | 3 |
| II-h | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| II-i | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| II-j | 6 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| II-k | 5 | 0 | 0 | 0 | 0 | 6 | 1 | 0 |
| II-l | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| II-m | 5 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| II-n | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| II-o | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| II-p | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| II-q | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 83-1386 | ||||||||
| III-a | 0a | 10 | 14 | 13 | 14 | 14 | 10 | 14 |
| III-b | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| III-c | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| III-d | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| III-f | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| III-g | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
Original pattern.
One of the 6 strains did not contain the stx2 gene.
One of the 5 strains did not contain the stx2 gene.
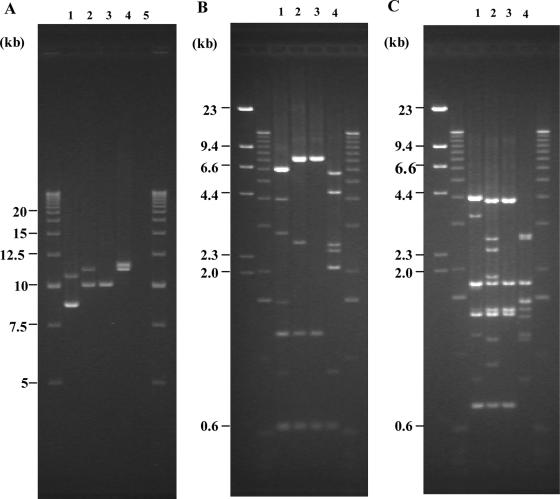
The same sets of the parental and passage-derived strains generated either from successive passages or revived from storage were included for analysis by PCR-RFLP assay. The results obtained with PCR-RFLP assay are summarized in Tables 3 and 4. All colonies except for 2 colonies of strain EC1015 analyzed prior to the repeated subculture experiment showed identical RFLP patterns by PCR-RFLP. All of the colonies of RIMD0509952 produced 2 amplicons of approximately 8.5 and 10.5 kb in size (Fig. 2A, lane 2) regardless of their PFGE profile type. Furthermore, BglI digest of the amplicons produced seven bands with sizes ranging from 560 bp to 6.2 kb (Fig. 2B, lane 3), while EcoRV digest of the amplicons gave nine bands with sizes ranging from 630 bp to 4.0 kb (Fig. 2C, lane 3). In the case of strain 83-1386, all 14 colonies produced 2 amplicons of approximately 11.0 and 11.5 kb in size, regardless of PFGE profiles of the parental strains (Fig. 2A, lane 5). BglI digest of the amplicons gave 7 bands whose sizes ranged from 560 bp to 6.0 kb (Fig. 2B, lane 6), while EcoRV digest of the amplicons gave 9 bands whose sizes ranged from 820 bp to 2.7 kb (Fig. 2C, lane 6). In the case of strain EC1015, however, 12 of the 14 colonies produced 2 amplicons of approximately 9.6 and 11.0 kb in size (Fig. 2A, lane 3). BglI digest of the amplicons gave 5 bands whose sizes ranged from 560 bp to 7.5 kb (Fig. 2B, lane 4), while EcoRV digest of the amplicons gave 10 bands whose sizes ranged from 630 bp to 3.7 kb (Fig. 2C, lane 4). The remaining 2 colonies of the 14 selected colonies produced single amplicons of approximately 9.6 kb in size. BglI digest of the amplicon gave 3 bands whose sizes ranged from 560 bp to 7.5 kb (Fig. 2B, lane 5), while EcoRV digest of the amplicon gave 5 bands whose sizes ranged from 630 bp to 3.7 kb (Fig. 2C, lane 5). A multiplex PCR test for the detection of the stx1 and stx2 genes indicated that these two colonies were positive for only the stx1 gene but not stx2 gene. The results of the multiplex PCR test in conjunction with the PCR-RFLP assay indicate that Stx2 phage might be lost during long-term storage.
TABLE 3.
PCR-RFLP fragment patterns obtained with three wild-type STEC O157:H7 strains and isolates derived after repeated subculture for a period of 25 weeks at intervals of 3 to 4 days
| Strain and PCR-RFLP profile | No. of colonies showing identity to PCR-RFLP profile following no. of subcultures:
|
||||||
|---|---|---|---|---|---|---|---|
| Presubculture | 1 | 10 | 20 | 30 | 40 | 50 | |
| RIMD0509952 (Sakai strain), I-A | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| EC1015 | |||||||
| II-A | 12 | 14 | 14 | 14 | 14 | 14 | 14 |
| II-B | 2a | 0 | 0 | 0 | 0 | 0 | 0 |
| 83-1386, III-A | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
TABLE 4.
PCR-RFLP fragment patterns obtained with three wild-type STEC O157:H7 strains and their derivative strains revived after prolonged storage at room temperature for a total period of 25 weeks and with subcultures at every 5-week interval
| Strain and PCR-RFLP profile | No. of colonies showing identity to PCR-RFLP profile following storage for no. of wks:
|
||||||
|---|---|---|---|---|---|---|---|
| Presubculture | 0 | 5 | 10 | 15 | 20 | 25 | |
| RIMD0509952 (Sakai strain), I-A | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| EC1015 | |||||||
| II-A | 12 | 14 | 14 | 14 | 14 | 14 | 14 |
| II-B | 2a | 0 | 0 | 0 | 0 | 0 | 0 |
| 83-1386, III-A | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
FIG. 2.
(A) Agarose gel electrophoresis patterns of PCR amplicons obtained against region V of Stx phage. Lanes: 1, I-A (RIMD0509952, original pattern); 2, II-A (EC1015, original pattern); 3, II-B (EC1015, lost Stx2 phage pattern); 4, III-A (83-1386, original pattern); 5, C600. Ladders (2.5 kb) were used as molecular mass markers on both sides of the gel. (B and C) BglI digest (B) and EcoRV digest (C) of LA-PCR products obtained from region V in STEC O157:H7 strains RIMD0509952, EC1015, and 83-1386. Lanes: 1, I-A (RIMD0509952, original pattern); 2, II-A (EC1015, original pattern); 3, II-B (EC1015, lost Stx2 phage pattern); 4, III-A (83-1386, original pattern). Lambda-HindIII digest and 1-kb ladders were used as molecular mass markers on both sides of each gel. Positions of DNA fragments with known molecular masses are indicated.
DISCUSSION
PFGE profile-based analysis to determine clonal relatedness among strains is based on similarity within the genome and is considered a powerful tool for reflecting genetic diversity that occurred over a long evolutionary period. However, alteration in the PFGE profile of a single isolate induced by repeated subcultures has been reported for several bacterial species (6, 24, 26). Iguchi et al. (13) reported that repeated subculture in vitro and prolonged storage of STEC O157:H7 caused change in the PFGE profile. The occurrence of genetic changes as demonstrated by the change in PFGE profiles among STEC strains after passages through the bovine and human gastrointestinal tracts have also been described previously (4, 18). Therefore, it would be difficult for unambiguous determination of clonal relatedness among the STEC strains by PFGE alone.
In the present study, we evaluated the usefulness and reliability of the PCR-RFLP assay compared to PFGE. As shown in Tables 1 and 2, a total of 27 patterns different from the original PFGE patterns were observed through repeated subcultures and prolonged storage of three STEC O157:H7 strains. Among these strains, most variations in PFGE profiles were observed in strain EC1015, indicating that the genomic stability of STEC strains varies from strain to strain. According to the Tenover criteria, two isolates are considered to be two different clones if more than 7 bands are different by PFGE analysis (34). Our data are similar to the observed alteration in PFGE profiles of STEC strains as reported by Iguchi et al. (13). In their report, the variations of PFGE patterns caused by repeated subculture and prolonged storage of a strain considered clonal did not invalidate the lineage of the STEC strains (13). This study, however, showed that a PFGE fragment pattern, designated II-f, obtained after the 40th and 50th subcultures of strain EC1015 differed by 8 fragments compared to the original one (II-a). Therefore, it is evident that changes of more than 7 bands, even for a strain considered clonal, could be detected following repeated subculture in vitro, and such changes were not reported by Iguchi et al. (13). Such changes would result in the misinterpretation of some of the passage-derived STEC strains as strains of different clonal origins.
Although the exact mechanisms of how the change in PFGE pattern occurs is still unclear, point mutations, insertions, or deletions of DNA or internal reshuffling through recombination could be involved. Iguchi et al. (15) recently found that intrachromosomal recombination between homologous prophage regions could also be involved in change of PFGE fragment patterns. This phenomenon was designated inversion, and 4 bands changed in this case. LeClerc et al. (22) reported that high mutation frequencies among E. coli O157:H7 strains might be related to defects in methyl-directed mismatch repair. Whole-genome PCR scanning assay by Ohnishi et al. (28) demonstrated that prophages, including Stx phages, exhibited extensive structural and positional diversity, implying that variation of bacteriophages is a major factor in generating genomic diversity among the O157 lineage. Indeed, it was demonstrated that loss (3, 4, 17, 25) or insertion (14) of Stx2 phage could cause changes in PFGE profiles of STEC strains. Given the several reports on the instability of Stx2 phage by several researchers (17, 25), alteration of PFGE profiles due to loss of Stx phage should always be considered when PFGE is employed to determine clonal relationship.
In the present study, however, one of the six strains termed II-a, which lost Stx2 phage, as confirmed by PCR analysis, did not show any change in PFGE profile in comparison to the remaining 5 strains which contain both Stx1 and Stx2 phages (Tables 1 and 3). Furthermore, one of the five strains termed II-b also did not show any change in PFGE profile in comparison to the remaining 4 strains even though this strain also lost Stx2 phage, as confirmed by PCR analysis (Tables 1 and 3). This suggests that the loss of Stx2 phage is an important factor causing alteration in the PFGE profile but does not always reflect the alteration of the PFGE profile. In contrast to PFGE analysis, PCR-RFLP can easily detect the event of loss of the phage. However, the scope and applicability of PCR-RFLP will be limited if there are changes in nucleotide sequences at the primer binding regions resulting in false negativity in the PCR assay. Despite such limitations, the PCR-RFLP assay was successfully utilized to demonstrate clonality among STEC O157:H7 strains that were passaged through the bovine and human gastrointestinal tracts (4, 18).
It would be confusing for clinical microbiologists to distinguish between sporadic and outbreak-related strains if PFGE patterns were to change as a result of clonal turnover by either in vivo passage or in vitro cultivation (18). We recently demonstrated alteration of PFGE profiles of STEC strains caused by in vivo passages through the bovine intestine (4), while PCR-RFLP profiles remained unaltered (31). This study further demonstrates that RFLP patterns of STEC strains by PCR-RFLP assay were not affected due to in vitro clonal turnover caused by subculture and prolonged storage.
In conclusion, the PCR-RFLP assay on the basis of region V, a regulatory region of Stx phage, is a more practical and reliable method than PFGE for molecular epidemiological studies of STEC strains because of its ability to unambiguously determine clonality among Shiga toxin-producing strains. Therefore, PCR-RFLP may be used for the initial survey before PFGE analysis or as a supplementary molecular typing method in conjunction with PFGE while analyzing O157:H7 STEC strains.
Acknowledgments
We thank R. K. Nandy, National Institute of Cholera and Enteric Diseases, Kolkata, India, for critical reading of the manuscript.
Footnotes
Published ahead of print on 13 September 2006.
REFERENCES
- 1.Abdul-Raouf, U. M., L. R. Beuchat, and M. S. Ammar. 1993. Survival and growth of Escherichia coli O157:H7 on salad vegetables. Appl. Environ. Microbiol. 59:1999-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acheson, D. W. K., and G. T. Keusch. 1996. Which Shiga-toxin-producing types of E. coli are important? ASM News 62:302-306. [Google Scholar]
- 3.Akiba, M., T. Sameshima, and M. Nakazawa. 1999. The shift of genetic subtypes of Escherichia coli O157:H7 isolates from cattle. Epidemiol. Infect. 122:343-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiba, M., T. Sameshima, and M. Nakazawa. 2000. Clonal turnover of enterohemorrhagic Escherichia coli O157:H7 in experimentally infected cattle. FEMS Microbiol. Lett. 184:79-83. [DOI] [PubMed] [Google Scholar]
- 5.Barrett, T. J., H. Lior, J. H. Green, R. Khakhria, J. G. Wells, B. P. Bell, K. D. Greene, J. Lewis, and P. M. Griffin. 1994. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J. Clin. Microbiol. 32:3013-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beall, B., P. K. Cassiday, and G. N. Sanden. 1995. Analysis of Bordetella pertussis isolates from an epidemic by pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:3083-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besser, R. E., S. M. Lett, J. T. Weber, M. P. Doyle, T. J. Barrett, T. G. Wells, and P. M. Griffin. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269:2217-2220. [PubMed] [Google Scholar]
- 8.Besser, R. E., P. M. Griffin, and L. Slutsker. 1999. Escherichia coli O157:H7 gastroenteritis and the hemolytic uremic syndrome: an emerging infectious disease. Annu. Rev. Med. 50:355-367. [DOI] [PubMed] [Google Scholar]
- 9.Beutin, L., D. Geier, H. Steinruck, S. Zimmermann, and F. Scheutz. 1993. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J. Clin. Microbiol. 31:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grif, K., H. Karch, C. Schneider, F. D. Daschner, L. Beutin, T. Cheasty, H. Smith, B. Rowe, M. P. Dierich, and F. Allerberger. 1998. Comparative study of five different techniques for epidemiological typing of Escherichia coli O157. Diagn. Microbiol. Infect. Dis. 32:165-176. [DOI] [PubMed] [Google Scholar]
- 11.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 13.Iguchi, A., R. Osawa, J. Kawano, A. Shimizu, J. Terajima, and H. Watanabe. 2002. Effects of repeated subculturing and prolonged storage at room temperature of enterohemorrhagic Escherichia coli O157:H7 on pulsed-field gel electrophoresis profiles. J. Clin. Microbiol. 40:3079-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iguchi, A., R. Osawa, J. Kawano, A. Shimizu, J. Terajima, and H. Watanabe. 2003. Effects of lysogeny of Shiga toxin 2-encoding bacteriophages on pulsed-field gel electrophoresis fragment pattern of Escherichia coli K-12. Curr. Microbiol. 46:224-227. [DOI] [PubMed] [Google Scholar]
- 15.Iguchi, A., S. Iyoda, J. Terajima, H. Watanabe, and R. Osawa. 2006. Spontaneous recombination between homologous prophage regions causes large-scale inversions within the Escherichia coli O157:H7 chromosome. Gene 372:199-207. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, W. N., H. Lior, and G. S. Bezanson. 1983. Cytotoxic Escherichia coli O157:H7 associated with haemorrhagic colitis in Canada. Lancet i:76. [DOI] [PubMed] [Google Scholar]
- 17.Karch, H., T. Meyer, H. Rüssmann, and J. Heesemann. 1992. Frequent loss of Shiga-like toxin genes in clinical isolates of Escherichia coli upon subcultivation. Infect. Immun. 60:3464-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karch, H., H. Russmann, H. Schmidt, A. Schwarzkopf, and J. Heesemann. 1995. Long-term shedding and clonal turnover of enterohemorrhagic Escherichia coli O157 in diarrheal diseases. J. Clin. Microbiol. 33:1602-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karmali, M. A. 1989. Infection by verotoxin-producing Escherichia coli. Clin. Microbiol. Rev. 2:15-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan, A., S. Yamasaki, T. Sato, T. Ramamurthy, A. Pal, S. Datta, N. R. Chowdhury, S. C. Das, A. Sikdar, T. Tsukamoto, S. K. Bhattacharya, Y. Takeda, and G. B. Nair. 2002. Prevalence and genetic profiling of virulence determinants of non-O157 Shiga toxin-producing Escherichia coli isolated from cattle, beef and humans Calcutta, India. Emerg. Infect. Dis. 8:54-62. [PubMed] [Google Scholar]
- 21.Koort, J. M. K., S. Lukinmaa, M. Rantala, E. Unkila, and A. Siitonen. 2002. Technical improvement to prevent DNA degradation of enteric pathogens in pulsed-field gel electrophoresis. J. Clin. Microbiol. 40:3497-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeClerc, J. E., B. Li, W. L. Payne, and T. A. Cebula. 1996. High mutation frequencies among Escherichia coli and Salmonella pathogens. Science 274:1208-1211. [DOI] [PubMed] [Google Scholar]
- 23.Michino, H., K. Araki, S. Minami, S. Takaya, N. Sakai, M. Miyazaki, A. Ono, and H. Yanagawa. 1999. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 150:787-796. [DOI] [PubMed] [Google Scholar]
- 24.Murase, T., A. Nakamura, A. Matsushima, and S. Yamai. 1996. An epidemiological study of Salmonella enteritidis by pulsed-field gel electrophoresis (PFGE): several PFGE patterns observed in isolates from a food poisoning outbreak. Microbiol. Immunol. 40:873-875. [DOI] [PubMed] [Google Scholar]
- 25.Murase, T., S. Yamai, and H. Watanabe. 1999. Changes in pulsed-field gel electrophoresis patterns in clinical isolates of enterohemorrhagic Escherichia coli O157:H7 associated with loss of Shiga toxin genes. Curr. Microbiol. 38:48-50. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen, E. M., J. Engberg, and V. Fussing. 2001. Genotypic and serotypic stability of Campylobacter jejuni strains during in vitro and in vivo passage. Int. J. Med. Microbiol. 291:379-385. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien, A. D., and R. K. Holmes. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohnishi, M., J. Terajima, K. Kurokawa, K. Nakayama, T. Murata, K. Tamura, Y. Ogura, H. Watanabe, and T. Hayashi. 2002. Genomic diversity of enterohemorrhagic Escherichia coli O157 revealed by whole genome PCR scanning. Proc. Natl. Acad. Sci. USA 99:17043-17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parry, S. M., and R. L. Salmon. 1998. Sporadic STEC O157 infection: secondary household transmission in Wales. Emerg. Infect. Dis. 4:657-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shima, K., J. Terajima, T. Sato, K. Nishimura, K. Tamura, H. Watanabe, Y. Takeda, and S. Yamasaki. 2004. Development of a PCR-restriction fragment length polymorphism assay for the epidemiological analysis of Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 42:5205-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shima, K., N. Yoshii, M. Akiba, K. Nishimura, M. Nakazawa, and S. Yamasaki. 2006. Comparison of PCR-RFLP and PFGE for determining the clonality of enterohemorrhagic Escherichia coli strains. FEMS Microbiol. Lett. 257:124-131. [DOI] [PubMed] [Google Scholar]
- 32.Takeda, Y., H. Kurazono, and S. Yamasaki. 1993. Vero toxins (Shiga-like toxins) produced by enterohemorrhagic Escherichia coli (verocytotoxin-producing E. coli). Microbiol. Immunol. 37:591-599. [DOI] [PubMed] [Google Scholar]
- 33.Tarr, P. I., C. A. Gordon, and W. L. Chandler. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073-1086. [DOI] [PubMed] [Google Scholar]
- 34.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terajima, J., H. Izumiya, S. Iyoda, K. Tamura, and H. Watanabe. 2002. High genomic diversity of enterohemorrhagic Escherichia coli isolates in Japan and its applicability for the detection of diffuse outbreak. Jpn. J. Infect. Dis. 55:19-22. [PubMed] [Google Scholar]
- 36.Wang, G. D., and M. P. Doyle. 1998. Survival of enterohemorrhagic Escherichia coli O157:H7 in water. J. Food Prot. 61:662-667. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe, H., J. Terajima, H. Izumiya, and S. Iyoda. 2003. Molecular typing methods for STEC. Methods Mol. Med. 73:55-65. [DOI] [PubMed] [Google Scholar]
- 38.Yamasaki, S., and Y. Takeda. 1997. Enterohemorrhagic Escherichia coli O157:H7 episode in Japan with a perspective on Vero toxins (Shiga-like toxins). J. Toxicol. Toxin Rev. 16:229-240. [Google Scholar]