Abstract
We describe a rare case of a subcutaneous infection by both Phaeoacremonium venezuelense and Plectophomella sp. in a Brazilian male. Sequencing of a β-tubulin gene fragment allowed us to confirm the identification of the former. However, a similar procedure of sequencing rRNA gene fragments was not useful for the identification of the latter fungus.
CASE REPORT
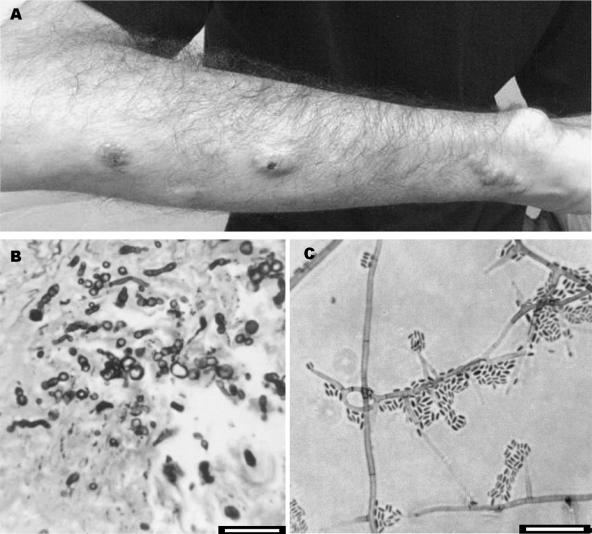
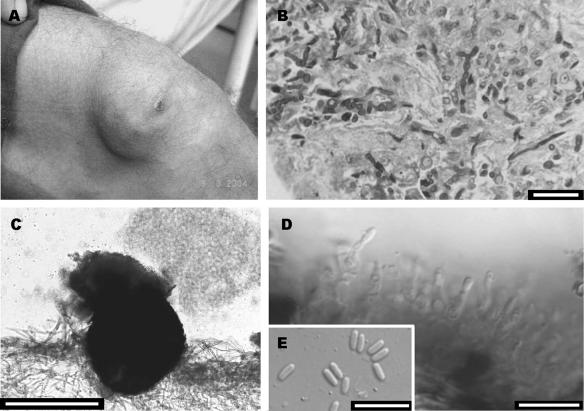
A 28-year-old Brazilian male was diagnosed with chronic myeloid leukemia in 2001. He underwent a bone marrow transplant in July 2002, but after 1 month he developed a graft-versus-host disease that compromised the skin and liver. After that, he started taking cyclosporine, mycophenolate mofetil, and prednisone. In 2004, he presented with several soft, erythematous nodules arranged in a linear distribution on the right arm (Fig. 1A) and two nodules forming a plaque lesion with fibrous consistency on the left knee (Fig. 2A). Three serial biopsies of both lesions were taken on different days for histopathological and microbiological study. The direct examination on KOH did not reveal any type of fungal structure. Grocott and periodic acid Schiff stains revealed the presence of dematiaceous hyphae in the two types of lesions (Fig. 1B and 2B, respectively). Cultures on routine media for bacteria and fungi showed the presence of a single fungus in the samples from the leg and another, different fungus in the samples from the arm. Infections were diagnosed as subcutaneous mycoses, and the fungi were sent to the Medical School at the Universitat Rovira i Virgili for identification. The lesions were surgically removed. Since the patient showed hepatic and renal dysfunctions and general deterioration, antifungal treatment was not initiated. The patient subsequently moved to another city and was lost to follow-up.
FIG. 1.
(A) Nodules on the right arm. (B) Grocott-Gomori methanamine silver stain showing pigmented hyphal elements. (C) Conidiogenous cells and conidia of Phaeoacremonium venezuelense. Bars, 10 μm.
FIG. 2.
(A) Nodule with a plaque lesion on the left knee. (B) Periodic acid Schiff stain showing hyphal elements. (C to E) Conidioma, conidiogenous cells, and conidia of a Plectophomella sp. Bars, 10 μm (B, D, and E) and 50 μm (C).
The two isolates were cultured on routine media for fungi. On the basis of its morphological features (i.e., darkly pigmented colonies and phialidic conidiogenous cells with conspicuous collarettes), the fungus isolated from the arm lesions was identified as a Phaeoacremonium sp. strain CBS 120024. On malt extract agar (Difco Laboratories, Detroit, Mich.), its colonies were camel to sunburn color, attained a diameter of 47 to 50 mm in 2 weeks at 25°C, and were able to grow at 40°C. Microscopically, it showed short and usually unbranched conidiophores, bearing an elongate ampulliform attenuated at the base or subcylindrical conidiogenous cells, often 9 to 17 μm long by 1 to 2.5 μm wide, from which fusiform-ellipsoidal hyaline conidia that were 3 to 6 μm long by 1 to 1.5 μm wide were produced (Fig. 1C). Conidiogenous cells with two apertures (polyphialides) were also present. Hyphae from aerial mycelium were verruculose, with warts not exceeding 2 μm wide. Following the morphological criteria given by Mostert et al. (7) to recognize Phaeoacremonium species, we were not able to identify our isolate at the species level, so we used the molecular method proposed by the same authors. A fragment of the β-tubulin (tub2) gene defined by primers BT2-F and BT2-R (2) was amplified, and the sequence obtained (479 bp) was compared with those in the BioloMICS database (http://www.cbs.knaw.nl/phaeoacremonium.htm). This sequence showed 99.41% similarity to a strain of Phaeoacremonium venezuelense (CBS 651.85; GenBank accession number AY579320).
The fungus isolated from the leg was preliminarily identified as Plectophomella sp. strain CBS 119963. In culture on oat meal agar (30 g oat flakes, 1 g MgSO4 · 7H2O, 1.5 g KH2PO4, 15 g agar, 1,000 ml tap water), it showed numerous stromatic conidiomata, which were black, globose to irregular, somewhat flattened, and composed of several merged cavities with relatively thick and tough walls composed of texture angularis. The inner cells of the wall were hyaline, and the outer cells were brown with dark incrustations. Pale white conidial slimy masses were released from barely differentiated ostioli, which were lined with protruding masses of sterile, hyaline subglobose cells (Fig. 2C). The conidiophores were one septate, 5 to 12.5 μm long, and 2 to 3 μm wide. The conidiogenous cells were phialidic, flask-shaped, 5 to 8 μm long, and 2 to 3 μm wide, with a minute collarette at the top. The conidiogenous cells sometimes showed an elongated neck bearing one to three percurrent proliferations. The conidia were ellipsoidal or (sub)cylindrical, hyaline, thin, smooth walled, and 3.5 to 7 μm long by 1 to 2 μm wide (Fig. 2D and E). To confirm the identification, the internal transcribed spacer (ITS) region defined by primers ITS5 and ITS4 was amplified. The sequence obtained (649 bp) was compared with those in the GenBank DNA database. However, in this case the procedure was not successful, since this sequence did not match any sequence deposited there to a significant degree of similarity. The closest sequence belonged to an isolate of Cenococcum geophilum (AY818600), an anamorph of the Dothideales (Ascomycota) whose morphological features are very different from those of our fungus.
This is a very interesting case of dual infection in a bone marrow transplant patient. Although infections by more than one species are not rare in immunosuppressed patients (5, 6, 10, 11) and even by two species of the same genus (3), it is not always easy to detect them. It is common in the clinical laboratory to pick up only one colony for identification if the colonies developed on the petri dishes are all similar. With this procedure, numerous mixed infections could have escaped detection. The same happens when multiple lesions with a similar appearance are present and samples are only taken from one. Fortunately, both were sampled in our case, perhaps because some were on the leg and others on the arm.
Phaeoacremonium venezuelense is a rare species recently erected (7) that comprises only five strains, including that of the present case. The previous ones were from Venezuela, isolated from a mycetoma (1); South Africa, isolated from Vitis vinifera; Canada, from tissue from ankle; the fourth strain has an unknown origin (7). It is also possible that, considering the fact that the morphological differences among the species of this genus are very subtle, previous strains would have been erroneously identified as the more common clinical species, P. parasiticum. This species can be differentiated from P. venezuelense by its brown colonies, longer and usually branched conidiophores, and prominent warts (up to 3 μm wide) on hyphae. According to Mostert et al. (7), P. venezuelense is morphologically characterized by beige to orange-brown colonies, verruculose mycelium with warts up to 1 μm wide, mostly short conidiophores, conidiogenous cells predominantly of type III (i.e., usually 15 to 23 μm long and subcylindrical, navicular, or awl shaped), fusiform-ellipsoidal conidia, and a maximum growth temperature of 40°C. Our isolate differs by the colony color, the morphology, the predominance of the type of conidiogenous cells, and the presence of polyphialides. This could be due to the influence of culture conditions, to the erroneous interpretation of some of Mostert's criteria, such as type of conidiogenous cells, or to the phenotypic variation of this species that is also admitted by Mostert et al. (7).
Although we identified the fungus isolated from the leg lesions as a species of Plectophomella, this identification is tentative and requires further confirmation. The closest ITS sequence to that of our fungus in BLAST searches was of Cenococcum species, but the percentage of similarity was low (approximately 40%). No sequences are currently available for the species of Plectophomella, including the type species P. visci. The morphological concept for the genus Plectophomella is rather broad, and it is likely that its species do not belong to a single lineage. Plectophomella species form eustromatic conidiomata, with phialidic conidiogenous cells placed directly on the inner wall or integrated in one to many septate conidiophores and small hyaline conidia (8). Our fungus differs in ecology (habitat) and morphology from the four species known in the genus described thus far. It morphologically resembles P. nypae, which also has short, one-septate conidiophores and conidia which seem similar in shape, although they are described as ellipsoidal or fusiform, 3 to 4.5 μm long, and 1.2 to 1.6 μm wide (4). Plectophomella nypae is only known from dead fronds of the marine alga Nypa fruticans, found on the coast of Brunei in the South China Sea. The other Plectophomella species produce much longer conidiophores (over 30 μm long), and all occur on land plants (8, 9). Thus far, Plectophomella has not been implicated in human infections.
The case described here illustrates the importance of culture and of the accurate identification of isolates when more than one species may be present, especially in immunosuppressed patients. Medical microbiologists should be aware of this possibility.
Nucleotide sequence accession numbers.
The sequences of a fragment of the β-tubulin gene of P. venezuelense and of the nuclear ribosomal ITS region of the Plectophomella isolate have been deposited in GenBank under accession numbers AM286785 and AM286786, respectively.
Footnotes
Published ahead of print on 27 September 2006.
REFERENCES
- 1.De Albornoz, M. B. 1974. Cephalosporium serrae, agente etiológico de micetomas. Mycopathol. Mycol. Appl. 54:485-498. [DOI] [PubMed] [Google Scholar]
- 2.Gilgado, F., J. Cano, J. Gené, and J. Guarro. 2005. Molecular phylogeny of the Pseudallescheria boydii species complex. Proposal of two new species. J. Clin. Microbiol. 43:4930-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guarro, J., M. Nucci, T. Akiti, and J. Gené. 2000. Mixed infection caused by two species of Fusarium in a human immunodeficiency virus-positive patient. J. Clin. Microbiol. 38:3460-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyde, K. D., and B. C. Sutton. 1992. Nypaella frondicola gen. et sp. nov., Plectophomella nypae sp. nov. (Coelomycetes) from intertidal fronds of Nypa fruticans. Mycol. Res. 96:210-214. [Google Scholar]
- 5.Lopes, J. O., E. S. de Mello, and C. Klock. 1995. Mixed intranasal infection caused by Fusarium solani and a zygomycete in a leukaemic patient. Mycoses 38:281-284. [DOI] [PubMed] [Google Scholar]
- 6.Marriot, D. E., K. H. Wong, E. Aznar, J. L. Hakness, D. A. Cooper, and D. Muir. 1997. Scytalidium dimidiatum and Lecythophora hoffmanii: unusual causes of fungi infection in a patient with AIDS. J. Clin. Microbiol. 35:2949-2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mostert, L., J. Z. Groenewald, R. C. Summerbell, V. Robert, D. A. Sutton, A. A. Padhye, and P. W. Crous. 2005. Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. J. Clin. Microbiol. 43:1752-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redfern, D. B., and B. C. Sutton. 1981. Canker and dieback of Ulmus glabra caused by Plectophomella concentrica, and its relationship to P. ulmi. Trans. Br. Mycol. Soc. 77:381-390. [Google Scholar]
- 9.Sutton, B. C. 1980. The coelomycetes. Commonwealth Mycological Institute, Kew, United Kingdom.
- 10.Vasiloudes, P., J. G. Morelli, and W. L. Weston. 1997. Painful skin papules caused by concomitant Acremonium and Fusarium in a neutropenic patient. J. Am. Acad. Dermatol. 37:1006-1008. [DOI] [PubMed] [Google Scholar]
- 11.Viallard, J. F., E. Monlun, D. Neau, C. Vital, M. Long-Boursier, and M. Lebras. 1995. Pneumonia due to Fonsecaea pedrosoi and cerebral abscesses due to Emericella nidulans in a bone marrow transplant recipient. Clin. Infect. Dis. 21:1346-1348. [DOI] [PubMed] [Google Scholar]