Abstract
Pigs can play an important role in the genetic reassortment of influenza viruses and as a reservoir for another lineage of influenza viruses that have the ability to reassort and be transmitted between species. In March and April 2006, novel H3N1 influenza A viruses were isolated from pigs with respiratory diseases at two different commercial swine farms in Korea. Genetic and phylogenetic analyses of the sequences of all eight viral RNA segments showed that the novel H3N1 swine influenza viruses were reassortants that acquired the hemagglutinin gene from an H3 human-like virus and other genes from swine influenza viruses that are currently circulating in Korea. Serologic and virologic tests in the infected farms suggested that pig-to-pig and farm-to-farm transmissions occurred. Clinical signs in pigs and experimentally infected mice suggest the potential to transmit the virus between swine and other mammalian hosts. To our knowledge, this is the first report of the isolation of the swine H3N1 subtype from domestic pigs under field conditions in Korea. Further surveillance will be needed to determine whether this novel subtype will continue to circulate in the swine population.
The influenza A virus is a highly infectious respiratory pathogen of birds and mammals, including humans and pigs. Normally, the virus is not transmissible between humans and birds because human tracheal epithelial cells lack the receptors needed for the attachment of avian influenza viruses. Pigs, on other hand, are susceptible to influenza viruses from both avian and mammalian origins because the tracheal epithelium of pigs possesses the virus receptors for both the α-2,3-N-acetylneuraminic acid-galactose linkages for avian influenza viruses and the α-2,6-N-acetylneuraminic acid-galactose linkages for human influenza viruses (15). Therefore, pigs can play an important role in the genetic reassortment of influenza viruses. Due to this dual susceptibility, pigs are postulated to be “mixing vessels” for influenza viruses (2, 3, 10). Furthermore, the zoonotic transmission of swine influenza viruses from pigs to humans has been well demonstrated (5, 10, 12, 21, 27, 30).
Currently, three subtypes (H1N1, H1N2, and H3N2) are commonly found in pigs throughout the world. In the United States, the classical H1N1 subtype was exclusively prevalent until 1998 (25). In 1998, H3N2 triple reassortants with genes derived from human (HA, NA, and PB1), swine (M, NS, and NP), and avian (PA and PB2) viruses were first isolated in the United States; since then, they have become endemic in swine populations (20, 36). These viruses underwent further reassortment to create additional H3N2 viruses that were isolated from pigs (36) as well as H1N2 viruses that were isolated from pigs (7, 19), turkeys (33), and wild ducks (26). This demonstrates that viruses containing this gene combination can cross the species barrier.
In Korea, three subtypes (H1N1, H1N2, and H3N2) of swine influenza viruses have been reported in the pig population. Phylogenetic analysis indicated that the Korean isolates were closely related to swine influenza viruses recently isolated from pigs in the United States (6, 16, 17, 32). We now describe the isolation and characterization of novel H3N1 swine influenza viruses from pigs in Korea. Genetic characterization showed that these viruses have a high level of homology to the swine H1N2 influenza viruses recently circulating among pigs in Korea, with the exception of the HA gene. The relatively low homology of the HA gene to swine H3N2 viruses isolated from pigs in Korea and the higher homology to the HA genes of human H3N2 viruses suggest that the H3N1 viruses are reassortants between swine and human-like influenza viruses.
MATERIALS AND METHODS
Clinical samples and virus isolation.
Two swine influenza viruses, Swine/Korea/PZ72-1/06 (Sw/Korea/PZ72-1/06) and Sw/Korea/CN22/06, were isolated in Madin-Darby canine kidney (MDCK) cells from nasal swabs of pigs showing a typical influenza-like illness. In Chunbuk province in March 2006, Sw/Korea/PZ72-1/06 was isolated from the nasal swabs of 8-week-old cross-bred pigs that showed respiratory disease signs including depression, coughing, sneezing, and loss of appetite. Sw/Korea/CN22/06 was isolated in April 2006 from the lung homogenates of a dead 9-week-old cross-bred pig from the Chungnam province that showed a typical influenza-like illness with a 30% mortality rate. The subtype of Sw/Korea/PZ72-1/06 and Sw/Korea/CN22/06 was determined to be H3N1 by two multiplex reverse transcription-PCR assays and sequencing as previously described (9).
Genomic sequencing and phylogenetic analysis.
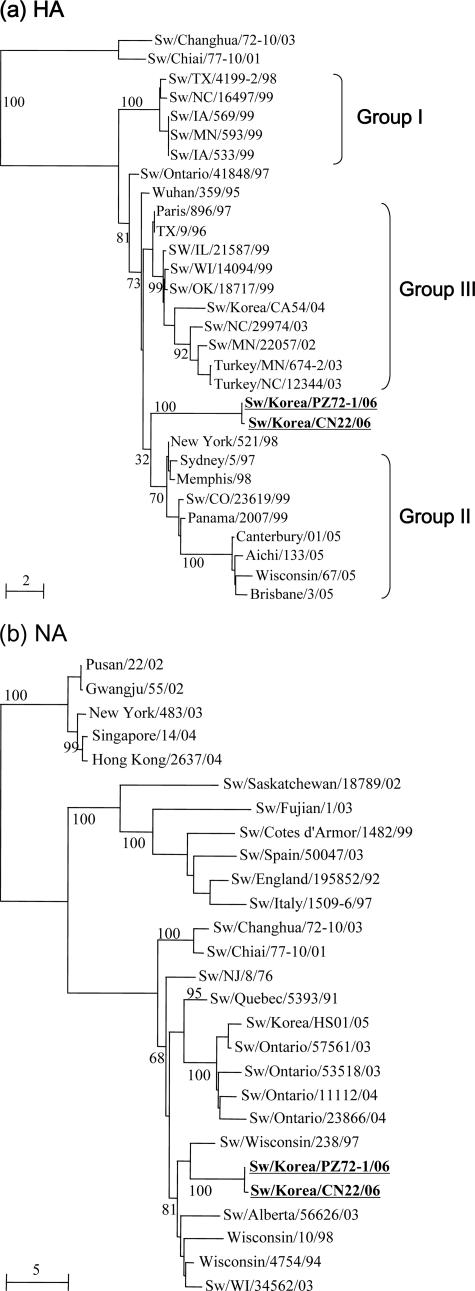
Viral RNA was extracted from cell culture isolates using a QIAamp Viral RNA Mini kit (QIAGEN, Valencia, CA). Reverse transcription-PCR was carried out under standard conditions using influenza-specific primers (9, 14). Nucleotide sequencing of the amplified products was carried out using a DNA sequencer (model 377; Applied Biosystems, Perkin-Elmer, Foster City, CA) and a Taq Dye Deoxy Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA). The sequences were resolved using the ABI PRISM collection program (Perkin-Elmer, Foster City, CA). The DNA sequences were compiled and edited using the Lasergene sequence analysis software package (DNASTAR, Madison, WI). Multiple sequence alignments were made using Clustal_X (1, 34). The rooted phylograms were prepared using the neighbor-joining algorithm and then plotted using NJ plot (28). The trees presented in Fig. 1 are based on the nucleotide sequence lengths of each gene segment (HA, 1,740 bp; NA, 1,410 bp; M, 975 bp, NP, 1,565 bp; NS, 890 bp; PA, 2,233 bp; PB1, 2,341 bp; PB2, 2,341 bp).
FIG. 1.
Phylogenetic trees of the nucleotide sequences for the HA (a) and NA (b) genes of the two H3N1 influenza viruses isolated from pigs in Korea compared with selected swine, human, and avian influenza virus strains. The nucleotide sequences were aligned using Clustal_X (1, 29), and the phylograms were generated by the neighbor-joining method using NJplot (24). The percent bootstrap values for each node are shown in each tree. The scale represents the number of substitutions per nucleotide. Standard postal abbreviations are used for state names in the United States.
Serologic test.
A hemagglutination inhibition (HI) assay was performed to determine the antigenic relationship between the H3N1 and H3N2 viruses. The swine antisera against the influenza viruses used in this study included Sw/MN/9088-2/98 (group I), Sw/CO/23619/99 (group II), and Sw/Korea/CA54/04 (group III, a recent Korea H3N2 isolate). A swine H1N2 Korean isolate (Sw/Korea/JI04/05) was also added for a serologic comparison.
Mouse experiments.
The extent of viral replication in mice (BALB/c) was measured after intranasal inoculation. Twenty-five mice were inoculated intranasally with 105.0 50% tissue culture infective doses (TCID50) of the respective influenza viruses. Control mice were sham infected with phosphate-buffered saline. Five mice of each group were sacrificed on days 3, 5, and 7 after inoculation, at which time the virus in the lung tissue was titrated in MDCK cells. The body weight of the remaining mice was measured daily on days 0 through 10 after inoculation.
Nucleotide sequence accession numbers.
The GenBank accession numbers assigned to the sequences determined in this study are as follows: DQ923506 to DQ923521 (sequence data will be provided upon request).
RESULTS
Sequence analysis.
Sequence analysis of the PCR products demonstrated that the two H3N1 isolates Sw/Korea/PZ72-1/06 and Sw/Korea/CN22/06 shared >99% nucleotide identity in each gene segment. This high genomic identity suggested that the same virus caused the two different outbreaks. The swine H3N1 virus was first reported in Taiwanese pig herds (35). However, the HA genes of the Korean viruses showed low nucleotide identity with the Taiwanese isolates Sw/Changhua/72-10/03 (79.3%) and Sw/Chiai/77-10/01 (81%). Furthermore, they showed only 91.5% nucleotide identity with the HA gene from a recent H3N2 swine influenza isolate in Korea, Sw/Korea/CA54/04. According to the Influenza Sequence Database (http://www.flu.lanl.gov), the HA and NA genes of Swine/Korea/PZ-72-1/06 had the highest level of sequence identity to those of New York/521/1998 (94.1%) and Sw/Wisconsin/238/97 (94.1%), respectively (Table 1). Table 2 shows the alignment of deduced amino acid sequences within the hemagglutinin 1 region of the HA genes of the H3N2 human, swine, and turkey and H3N1 swine influenza viruses. Although Sw/IL/21587/99, one of the swine isolates from the United States, shares 93% amino acid identity with Korean H3N1 isolates, they differed in only four amino acids within the receptor-binding (residues N137 and I226) and antigenic (residues R142 and Y155) sites (29). Most of the residues within the receptor-binding and antigenic sites of swine H3 viruses are relatively conserved, but they are quite variable compared with those of human and turkey H3 viruses (Table 2).
TABLE 1.
Sequence homology of each gene from Sw/Korea/ PZ72-1/06 compared with reference virus sequences available in GenBank
| Gene | % Nucleotide identity | Virus with the highest degree of sequence identity (GenBank accession no. or source)a | Subtype | Phylogenetic lineage |
|---|---|---|---|---|
| HA | 94.4 | New York/521/1998 (CY006499) | H3N2 | Human |
| NA | 94.1 | Swine/Wisconsin/238/97 (DQ280259) | H1N1 | Swine |
| M | 98.5 | Swine/Korea/JL04/05 (this study) | H1N2 | Swine |
| NP | 98.3 | Swine/Korea/JI04/05(this study) | H1N2 | Swine |
| NS | 98.3 | Swine/IN/14810/01 (AY060136) | H1N2 | Swine |
| PA | 97.2 | Swine/Korea/CY02/02 (AY129161) | H1N2 | Swine |
| PB1 | 97.8 | Swine/Korea/CY02/02 (AY129162) | H1N2 | Human |
| PB2 | 98.1 | Swine/Korea/JL04/05 (this study) | H1N2 | Swine |
The numbers in parentheses are GenBank accession numbers for the reference virus sequences.
TABLE 2.
Comparison of the amino acid sequences of the HA gene antigenic and receptor-binding sites in human, turkey, and swine H3 viruses
| Strain | Amino acid
|
||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antigenic sites
|
Receptor-binding sites
|
||||||||||||||||||||||||||||||||||||||||||
| 50 | 51 | 52 | 53 | 141 | 142 | 143 | 144 | 145 | 146 | 155 | 156 | 157 | 158 | 159 | 184 | 185 | 186 | 187 | 188 | 189 | 190 | 191 | 192 | 193 | 194 | 195 | 196 | 134 | 135 | 136 | 137 | 138 | 183 | 156 | 103 | 190 | 194 | 224 | 225 | 226 | 227 | 228 | |
| Majority | R | I | C | D | R | R | S | V | N | S | H | K | L | E | Y | P | S | T | D | S | D | Q | T | S | L | Y | V | Q | G | T | S | Y | A | W | K | H | D | L | R | G | I | S | S |
| New York/521/98 | • | • | • | • | • | • | • | I | • | K | • | Q | • | K | • | • | • | • | • | • | • | • | • | • | • | • | A | • | • | • | • | • | • | • | Q | • | • | • | • | • | V | • | • |
| Aichi/133/05 | G | • | • | • | • | • | • | N | • | • | T | H | • | K | F | • | G | • | • | N | • | • | I | • | • | • | A | • | • | • | • | S | • | • | H | • | • | • | • | D | V | P | • |
| Sw/Changhua/72-10/03 | • | • | • | N | • | G | • | G | • | • | Y | Q | S | • | N | • | • | • | • | R | E | • | • | N | • | • | • | R | • | G | • | • | • | • | Q | • | E | • | • | • | L | • | • |
| Turkey/MN/674-2/03 | • | • | • | N | • | G | • | • | K | • | • | E | • | G | • | • | • | • | • | R | • | • | • | • | • | • | • | • | • | • | • | S | • | • | E | • | • | • | • | • | • | • | • |
| Sw/TX/4199-2/98 | • | • | • | • | • | G | • | • | K | • | • | • | • | • | • | • | • | • | • | • | E | • | • | • | • | • | • | • | • | G | • | • | S | • | • | • | E | • | • | • | • | • | • |
| Sw/Korea/CA54/04 | • | • | • | • | • | G | • | • | • | • | • | • | • | • | • | • | • | • | • | • | G | • | • | • | • | • | • | • | • | • | • | • | S | • | • | • | G | • | • | • | V | • | • |
| Sw/IL/21587/99 | • | • | • | • | • | G | • | • | K | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | V | • | • |
| Sw/Korea/PZ72-1/06 | • | • | • | • | • | • | • | • | K | • | Y | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | N | • | • | • | • | • | • | • | • | • | • | • |
| Sw/Korea/CN22/06 | • | • | • | • | • | • | • | • | K | • | Y | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | N | • | • | • | • | • | • | • | • | • | • | • |
The NA genes of Korean H3N1 viruses have one amino acid deletion at position 41 on the stalk regions compared with the other swine N1 genes (data not shown). The remaining genes showed high nucleotide identity with Sw/Korea/JI04/05 and Sw/Korea/CY02/02 H1N2 Korean isolates (Table 1).
Phylogenetic analysis.
Phylogenetic analysis demonstrated that the HA genes of Korean swine H3N1 viruses were placed into a different cluster from that of other swine H3 viruses (36) (Fig. 1a). However, they share the same root (Wuhan/359/95) with swine H3N2 viruses of groups II and III found in the United States. The NA genes of both H3N1 viruses are placed into the U.S. swine cluster and are most closely related to Sw/Wisconsin/238/97, which is an old U.S. swine isolate (Fig. 1b). The remaining genes are most closely related to the recently isolated Korean H1N1 and H1N2 swine influenza viruses (data not shown). This suggests that the H3N1 viruses are reassortants between an unknown lineage of the H3N2 virus (HA gene) and swine influenza viruses (the other genes).
Serologic test.
To determine the extent of swine-to-swine transmission at infected farms, we collected sera from 30 pigs of each infected farm. Of the 60 swine sera, a total of 52 (86.6%) were highly reactive to the swine H3N1 isolates, demonstrating significant swine-to-swine transmission. Cross-reactivity between the H3N1 virus and H3N2 swine influenza viruses representing three genetic groups (36) was tested by HI assays. The results showed that the H3N1 virus reacted poorly with the sera of all three genetic groups of swine H3N2 viruses (Table 3). The antisera from the infected swine with the H3N1 virus showed high reactivity with Sw/Korea/PZ72-1/06 (homologous reaction) but weak reactivity with the other viruses, supporting the genetic data that the HA gene from these viruses originated from a novel source.
TABLE 3.
HI test results for Sw/Korea/PZ-72-1/06 and an additional selected swine influenza virus isolated in Korea
| Virus | Genotype | Titer of antiserum to virusb
|
||||
|---|---|---|---|---|---|---|
| Sw/Korea/HS05/05 | Sw/Korea/PZ72-1/06 | Sw/MN/9088-2/98 | Sw/Co/23619/99 | Sw/Korea/CA54/04 | ||
| Sw/Korea/JL04/05a | H1N2 | 640 | <20 | <20 | <20 | 20 |
| Sw/Korea/PZ-72-1/06 | H3N1 | 40 | 640 | 40 | 80 | 80 |
| Sw/MN/9088-2/98 | H3N2 | <20 | 40 | 640 | 80 | 80 |
| Sw/Co/23619/99 | H3N2 | <20 | 40 | 40 | 320 | 40 |
| Sw/Korea/CA54/04 | H3N2 | 20 | 160 | 40 | 80 | 640 |
Antisera collected from infected animals.
Titers in boldface type are the homologous reactions.
Mouse experiments.
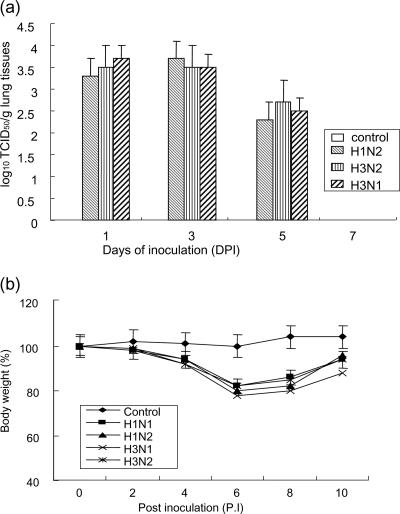
The relative virulence of the H3N1 subtype is not known, but the clinical histories of these isolates were associated with respiratory disease with a relatively high mortality rate. The relative virulence of swine influenza viruses was examined by using mice infected with Sw/Korea/PZ72-1/06 (H3N1), Sw/Korea/JI04/05 (H1N2), and Sw/Korea/CA54/04 (H3N2), which were isolated from swine farms in Korea. All viruses were recovered at high titers (3 to 4 log10 TCID50/g tissue) from the mouse lung homogenates 3 days after infection and disappeared at 7 days after infection. There was no significant difference in the viral titers between the groups (Fig. 2a). However, the mice infected with Sw/Korea/PZ72-1/06 showed a more severe loss of body weight than those infected with the other H1N2 and H3N2 viruses (Fig. 2b). All mice infected with swine influenza viruses showed clinical signs of infection, such as ruffled fur, inappetance, and labored breathing, within 4 days of infection. This demonstrates that viruses of a novel swine H3N1 subtype could be replicated in mice without adaptation and cause clinical diseases.
FIG. 2.
Mean viral titers of the lung homogenates (a) from five infected mice at different days after the experimental inoculation with negative control (phosphate-buffered saline) and H1N2, H3N2, and H3N1 swine influenza viruses. The mice were inoculated intranasally with 105.0 TCID50/head of swine influenza virus strains H1N2 (Sw/Korea/JI04/05), H3N2 (Sw/Korea/CA54/04), and H3N1 (Sw/Korea/PZ72-1/06). Five mice from each group were euthanized on 1, 3, 5, and 7 days postinfection (DPI) in order to titrate virus in the lung tissues. The remaining mice were observed for clinical signs and body weight was measured until 10 days postinfection (b).
DISCUSSION
We report here the first isolation and characterization of H3N1 swine influenza viruses from pigs with respiratory disease in Korea. The origin of the H3N1 subtype remains elusive, but phylogenetic analysis revealed that their internal genes are similar to those of other Korean swine influenza viruses. However, the HA and NA genes are not closely related to those of recently isolated Korean and U.S. swine influenza viruses (Fig. 1). Although a small number of Korean influenza viruses were used for comparison, these distinct HA (H3) and NA (N1) genes are unusual in the swine population in Korea. The swine H3N1 virus was first isolated in Taiwanese pig herds (35) and was recently isolated in U.S. pig herds (22). The HA gene of a recently isolated U.S. H3N1 swine influenza virus is similar to those of contemporary cluster III H3N2 viruses in the United States (22). However, phylogenetic and genetic analyses showed that these viruses were not the source of the Korean H3N1 viruses (Fig. 1) due to their low genetic identity and separated clusters in the HA genes. Furthermore, serologic tests showed that the H3N1 viruses reacted weakly with the swine H3 influenza virus groups found in the United States (36). This suggests that the H3N1 viruses in Korea are reassortants between human-like influenza viruses and recently circulating swine viruses in the Korean swine population. Further virologic surveillance of swine, avian, and human populations in Korea will be needed to understand the origin of these swine H3N1 viruses.
A certain constellation of the HA and NA surface molecules is essential for effective influenza virus replication. It is interesting that although H1N1 and H3N2 viruses circulate in human and swine populations throughout much of the world, H3N1 viruses are infrequently reported. This finding is in contrast to the relative abundance of H1N2 viruses (7, 11, 13, 19, 24), suggesting that the activities of the H3 and N1 proteins are not optimally balanced. It is worth noting that the N1 gene of the Korean H3N1 viruses has a single amino acid deletion in its stalk region. NA stalk deletions have previously been shown to alter NA activity (23), and it is tempting to speculate that the deletion may have been involved in the emergence of these H3N1 viruses.
With the limited clinical information available, it cannot be concluded that the swine H3N1 viruses were solely responsible for the severity of the disease observed in infected animals. Indeed, other bacteria were also found in some of the swine influenza virus-positive nasal swabs (data not shown). The swine influenza virus is recognized as an important contributor to the etiology of the porcine respiratory disease complex, infecting alone or in combination with other pathogens (8). Although a challenge study of the H3N1 virus was not carried out using pigs, mice infected with Sw/Korea/PZ72-1/06 and Sw/Korea/CN22/06 showed a more severe loss of body weight than those infected with the other H1N2 and H3N2 groups. This suggests that viruses of the novel swine H3N1 subtype could replicate in mammalian hosts without adaptation and cause clinical disease.
What is apparent is that the swine population is a reservoir of yet another lineage of influenza viruses that have the ability to reassort and to be transmitted between hosts. Given the evidence that pigs can support the reassortment of influenza viruses from humans and other species (3, 4, 18, 31, 37), it is prudent that we enhance surveillance for atypical influenza viruses in pigs as part of overall pandemic preparedness efforts. In addition, the potential for these H3N1 reassortant viruses to enter the human population should be considered.
Acknowledgments
This work was supported by grant no. RO1-2005-000-10585-0 from the Basic Research Program of the Korea Science and Engineering Foundation.
We thank Yeo-Jeong Choi and Won-June Choi for technical assistance.
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Aiyar, A. 2000. The use of CLUSTAL W and CLUSTAL X for multiple sequence alignment. Methods Mol. Biol. 132:221-241. [DOI] [PubMed] [Google Scholar]
- 2.Brown, I. H., P. Chakraverty, P. A. Harris, and D. J. Alexander. 1995. Disease outbreaks in pigs in Great Britain due to an influenza A virus of H1N2 subtype. Vet. Rec. 136:328-329. [DOI] [PubMed] [Google Scholar]
- 3.Brown, I. H., P. A. Harris, J. W. McCauley, and D. J. Alexander. 1998. Multiple genetic reassortment of avian and human influenza A viruses in European pigs, resulting in the emergence of an H1N2 virus of novel genotype. J. Gen. Virol. 79:2947-2955. [DOI] [PubMed] [Google Scholar]
- 4.Castrucci, M. R., I. Donatelli, L. Sidoli, G. Barigazzi, Y. Kawaoka, and R. G. Webster. 1993. Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology 193:503-506. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. 1988. Human infection with swine influenza virus—Wisconsin. Morb. Mortal. Wkly. Rep. 37:661-663. [PubMed] [Google Scholar]
- 6.Choi, C., S. K. Ha, and C. Chae. 2004. Detection and isolation of H1N1 influenza virus from pigs in Korea. Vet. Rec. 154:274-275. [DOI] [PubMed] [Google Scholar]
- 7.Choi, Y. K., S. M. Goyal, M. W. Farnham, and H. S. Joo. 2002. Phylogenetic analysis of H1N2 isolates of influenza A virus from pigs in the United States. Virus Res. 87:173-179. [DOI] [PubMed] [Google Scholar]
- 8.Choi, Y. K., S. M. Goyal, and H. S. Joo. 2003. Retrospective analysis of etiologic agents associated with respiratory diseases in pigs. Can. Vet. J. 44:735-737. [PMC free article] [PubMed] [Google Scholar]
- 9.Choi, Y. K., S. M. Goyal, S. W. Kang, M. W. Farnham, and H. S. Joo. 2002. Detection and subtyping of swine influenza H1N1, H1N2 and H3N2 viruses in clinical samples using two multiplex RT-PCR assays. J. Virol. Methods 102:53-59. [DOI] [PubMed] [Google Scholar]
- 10.Claas, E. C., Y. Kawaoka, J. C. de Jong, N. Masurel, and R. G. Webster. 1994. Infection of children with avian-human reassortant influenza virus from pigs in Europe. Virology 204:453-457. [DOI] [PubMed] [Google Scholar]
- 11.Gregory, V., M. Bennett, M. H. Orkhan, S. Al Hajjar, N. Varsano, E. Mendelson, M. Zambon, J. Ellis, A. Hay, and Y. P. Lin. 2002. Emergence of influenza A H1N2 reassortant viruses in the human population during 2001. Virology 300:1-7. [DOI] [PubMed] [Google Scholar]
- 12.Gregory, V., M. Bennett, Y. Thomas, L. Kaiser, W. Wunderli, H. Matter, A. Hay, and Y. P. Lin. 2003. Human infection by a swine influenza A (H1N1) virus in Switzerland. Arch. Virol. 148:793-802. [DOI] [PubMed] [Google Scholar]
- 13.Guo, Y. J., X. Y. Xu, and N. J. Cox. 1992. Human influenza A (H1N2) viruses isolated from China. J. Gen. Virol. 73:383-387. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann, E., J. Stech, Y. Guan, R. G. Webster, and D. R. Perez. 2001. Universal primer set for the full-length amplification of all influenza A viruses. Arch. Virol. 146:2275-2289. [DOI] [PubMed] [Google Scholar]
- 15.Ito, T., J. N. Couceiro, S. Kelm, L. G. Baum, S. Krauss, M. R. Castrucci, I. Donatelli, H. Kida, J. C. Paulson, R. G. Webster, and Y. Kawaoka. 1998. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 72:7367-7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung, K., and C. Chae. 2005. First outbreak of respiratory disease associated with swine influenza H1N2 virus in pigs in Korea. J. Vet. Diagn. Investig. 17:176-178. [DOI] [PubMed] [Google Scholar]
- 17.Jung, K., and C. Chae. 2004. Phylogenetic analysis of an H1N2 influenza A virus isolated from a pig in Korea. Arch. Virol. 149:1415-1422. [DOI] [PubMed] [Google Scholar]
- 18.Karasin, A. I., S. Carman, and C. W. Olsen. 2006. Identification of human H1N2 and human-swine reassortant H1N2 and H1N1 influenza A viruses among pigs in Ontario, Canada (2003 to 2005). J. Clin. Microbiol. 44:1123-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karasin, A. I., J. Landgraf, S. Swenson, G. Erickson, S. Goyal, M. Woodruff, G. Scherba, G. Anderson, and C. W. Olsen. 2002. Genetic characterization of H1N2 influenza A viruses isolated from pigs throughout the United States. J. Clin. Microbiol. 40:1073-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karasin, A. I., M. M. Schutten, L. A. Cooper, C. B. Smith, K. Subbarao, G. A. Anderson, S. Carman, and C. W. Olsen. 2000. Genetic characterization of H3N2 influenza viruses isolated from pigs in North America, 1977-1999: evidence for wholly human and reassortant virus genotypes. Virus Res. 68:71-85. [DOI] [PubMed] [Google Scholar]
- 21.Kimura, K., A. Adlakha, and P. M. Simon. 1998. Fatal case of swine influenza virus in an immunocompetent host. Mayo Clin. Proc. 73:243-245. [DOI] [PubMed] [Google Scholar]
- 22.Ma, W., M. Gramer, K. Rossow, and K.-J. Yoon. 2006. Isolation and genetic characterization of new reassortant H3N1 swine influenza virus from pigs in the Midwestern United States. J. Virol. 80:5092-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitnaul, L. J., M. N. Matrosovich, M. R. Castrucci, A. B. Tuzikov, N. V. Bovin, D. Kobasa, and Y. Kawaoka. 2000. Balanced hemagglutinin and neuraminidase activities are critical for efficient replication of influenza A virus. J. Virol. 74:6015-6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishikawa, F., and T. Sugiyama. 1983. Direct isolation of H1N2 recombinant virus from a throat swab of a patient simultaneously infected with H1N1 and H3N2 influenza A viruses. J. Clin. Microbiol. 18:425-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen, C. W., S. Carey, L. Hinshaw, and A. I. Karasin. 2000. Virologic and serologic surveillance for human, swine and avian influenza virus infections among pigs in the north-central United States. Arch. Virol. 145:1399-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen, C. W., A. Karasin, and G. Erickson. 2003. Characterization of a swine-like reassortant H1N2 influenza virus isolated from a wild duck in the United States. Virus Res. 93:115-121. [DOI] [PubMed] [Google Scholar]
- 27.Olson, J. G. 1977. Epizootic swine influenza with evidence of a low rate of human infection associated with occupational exposure to swine. Southeast Asian J. Trop. Med. Public Health 8:368-370. [PubMed] [Google Scholar]
- 28.Perriere, G., and M. Gouy. 1996. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78:364-369. [DOI] [PubMed] [Google Scholar]
- 29.Reid, A. H., T. G. Fanning, T. A. Janczewski, and J. K. Taubenberger. 2000. Characterization of the 1918 “Spanish” influenza virus neuraminidase gene. Proc. Natl. Acad. Sci. USA 97:6785-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnurrenberger, P. R., G. T. Woods, and R. J. Martin. 1970. Serologic evidence of human infection with swine influenza virus. Am. Rev. Respir. Dis. 102:356-361. [DOI] [PubMed] [Google Scholar]
- 31.Scholtissek, C., H. Burger, O. Kistner, and K. F. Shortridge. 1985. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology 147:287-294. [DOI] [PubMed] [Google Scholar]
- 32.Song, D. S., J. Y. Lee, J. S. Oh, K. S. Lyoo, K. J. Yoon, Y. H. Park, and B. K. Park. 2003. Isolation of H3N2 swine influenza virus in South Korea. J. Vet. Diagn. Investig. 15:30-34. [DOI] [PubMed] [Google Scholar]
- 33.Suarez, D. L., P. R. Woolcock, A. J. Bermudez, and D. A. Senne. 2002. Isolation from turkey breeder hens of a reassortant H1N2 influenza virus with swine, human, and avian lineage genes. Avian Dis. 46:111-121. [DOI] [PubMed] [Google Scholar]
- 34.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai, C. P., and M. J. Pan. 2003. New H1N2 and H3N1 influenza viruses in Taiwanese pig herds. Vet. Rec. 153:408. [PubMed] [Google Scholar]
- 36.Webby, R. J., S. L. Swenson, S. L. Krauss, P. J. Gerrish, S. M. Goyal, and R. G. Webster. 2000. Evolution of swine H3N2 influenza viruses in the United States. J. Virol. 74:8243-8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152-179. [DOI] [PMC free article] [PubMed] [Google Scholar]