Abstract
To investigate the impact of pregnancy on human herpesvirus 8 (HHV-8) reactivation in human immunodeficiency virus type 1 (HIV-1)-infected women, the HHV-8 DNA presence and load were analyzed in peripheral blood mononuclear cells (PBMCs) and cervicovaginal secretions (CVSs) from 15 pregnant women coinfected with HIV-1 and HHV-8. HHV-8 detection was analyzed in relation to anti-HHV-8 antibodies and HIV-1-related parameters. Nucleotide sequence analysis of an ORFK1 hypervariable region of the HHV-8 strains was performed. HHV-8 was detected in maternal PBMCs (5/15 women) from the second trimester and in CVSs (5/15 women) mainly from the third trimester. The HHV-8 load significantly increased late in pregnancy in both maternal compartments and was associated with a significant increase in HIV-1 shedding in the genital tract. Antilytic antibodies were significantly more common in HHV-8 DNA-positive women. An elevated HHV-8 load was found in the PBMCs of an infant born to a mother with large amounts of HHV-8 in both compartments at delivery. Different ORFK1 subtypes were found in maternal samples, whereas the same subtype was identified in the mother-child pair. These data suggest that pregnancy may induce HHV-8 replication in HIV-1-infected women. An augmented HHV-8 load may, in turn, influence mother-to-child transmission, since one of the HIV-1-infected mothers with HHV-8 reactivation transmitted her ORFK1 subtype to the infant, who showed a high level of HHV-8 viremia indicative of a primary infection. This finding documents for the first time the perinatal transmission of a specific HHV-8 subtype. Vertical transmission may thus play a role in HHV-8 spread also in areas of subendemicity among HIV-1-infected women.
Human herpesvirus 8 (HHV-8) is associated with Kaposi's sarcoma (KS), primary effusion lymphoma, and the plasma cell variant of Castleman's disease. HHV-8 has an unusual geographical distribution, being prevalent in African and Mediterranean countries and among Ashkenazi Jews, Arabs, and some Amerindian populations (14). Italy is an area of subendemicity, where the seroprevalence of HHV-8 mirrors the incidence of classic KS at the microgeographical level (8, 17, 42). In areas where the incidence of KS is sporadic, HHV-8 infection is acquired in adulthood and is mainly associated with sexual activity (30). In areas of endemicity and subendemicity, although sexual behavior may influence virus diffusion (1, 25, 36), HHV-8 infection is common in childhood and the rate of seroprevalence increases with age, suggesting that intrafamilial, horizontal transmission is the main modality of the spread of HHV-8 (7, 31, 36). Initial studies on vertical transmission showed that HHV-8 seroreactivity in newborns is mainly due to transplacental passage of maternal antibodies (6, 18). However, rare cases of KS in newborns were described, and HHV-8 DNA was also detected at birth in the peripheral blood mononuclear cells (PBMCs) of a very low percentage of infants from Zambia (3, 20, 29, 32). These findings indicate that in utero or intrapartum HHV-8 infection might, albeit rarely, occur in countries where HHV-8 is endemic. The rates of HHV-8 detection in cervicovaginal secretions (CVSs) was also higher among African women than among women from areas of nonendemicity or subendemicity (2, 5, 24, 41, 43), highlighting that the HHV-8 load in the female genital tract might influence vertical transmission. We planned to address the vertical transmission of HHV-8 from a different perspective. Reactivation of herpesvirus infections occurs during pregnancy (12, 23, 28, 33, 38), and the impact of pregnancy on HHV-8 replication has not yet been defined. The loss of containment of HHV-8 latency and a subsequent increase in the viral load may contribute to the development of HHV-8-associated disorders. Indeed, a few cases of KS and Castleman's disease diagnosed during pregnancy were reported in the literature (4, 22), and it was suggested that impaired immune control of HHV-8 replication as well as the imbalance of proangiogenic factors in pregnancy may account for this phenomenon. However, two cases of spontaneous KS remission during pregnancy were also described, along with the in vivo and in vitro antitumor activities of some human chorionic gonadotropin preparations (34). On the whole, these conflicting findings imply a role for HHV-8 replication during pregnancy and underscore the need for investigations to elucidate the interplay between viral replication, pregnancy, and HHV-8-related disorders.
The aim of this pilot study was to define the impact of pregnancy on HHV-8 replication in human immunodeficiency virus type 1 (HIV-1)-infected women, who represent a population at high risk for HHV-8 acquisition (5, 19) as well as reactivation during pregnancy. Indeed, successful pregnancy requires adjustments in the immune network regulation, such as modulation of the Th1/Th2 cytokine balance, and it is conceivable that the occurrence of these changes in immunocompromised patients might further impair immunosurveillance for intracellular pathogens and viral infections (35, 40). We therefore selected 15 pregnant women from southern Italy, an area with high HHV-8 seroprevalence, coinfected with HIV-1 and HHV-8 (5) and studied the presence and load of HHV-8 DNA in PBMCs and CVSs during the three trimesters of pregnancy in relation to anti-HHV8 antibodies and HIV-1-related parameters. When possible, PBMC samples from their children were also analyzed.
MATERIALS AND METHODS
Study population and samples.
The study participants were part of a cohort study of 58 HIV-1-infected pregnant women who were referred to the Clinic of Infectious Diseases, University of Bari, between September 1999 and November 2003. At the time of enrollment, 22 of the 58 subjects analyzed were found to be HHV-8 seroreactive. Informed consent was obtained from 15 HHV-8-seropositive pregnant women, who provided a blood sample and underwent vaginal washings at three time points corresponding to the three trimesters of gestation. Blood and CVS specimens were processed as described previously (5). CD4+-cell counts and the levels of HIV-1 viremia were measured at each time point. HIV-1 RNA levels were measured in CVS at two time points, before and during the third trimester. Table 1 reports data on the HIV-1 risk factors, delivery modalities, and treatment. Six women began highly active antiretroviral therapy (HAART) after the first trimester to reduce the risk of teratogenicity, seven received HAART before and during pregnancy, and two remained untreated throughout their pregnanacies. Samples collected before pregnancy were available for five women. One patient was included in the study at the second trimester, and another patient was lost after collection of the first PBMC and CVS samples. The last CVS sample was not collected in two patients because of genital bleeding; only one sample collected at delivery was available for another patient. Placenta samples from three women were also available.
TABLE 1.
Demographic and clinical characteristics of the 15 pregnant women coinfected with HIV-1 and HHV-8
| Characteristic | No. (%) |
|---|---|
| Age | |
| >30 yr | 8 (53) |
| <30 yr | 7 (47) |
| Mode of HIV-1 acquisition | |
| Heterosexual intercourse | 9 (60) |
| Injection drug use | 6 (40) |
| Mode of delivery | |
| Cesarean section | 13 (87) |
| Vaginal delivery | 2 (13) |
| CDC classificationa | |
| A | 5 (33) |
| B | 7 (47) |
| C | 3 (20) |
| Antiretroviral therapyb | |
| Schedule | |
| Whole pregnancy | 7 (47) |
| From the second trimester | 6 (40) |
| Untreated | 2 (13) |
| Type | |
| Two NRTIs, PI | 8 (62) |
| Two NRTIs, NNRTI | 5 (38) |
According to the definition of the Centers for Disease Control and Prevention (CDC), Atlanta, Ga., 1993.
Highly active antiretroviral therapy was composed of two nucleoside reverse transcriptase inhibitors (NRTIs) and a protease inhibitor (PI) or two nucleoside reverse transcriptase inhibitors and a nonnucleoside reverse transcriptase inhibitor (NNRTI).
Informed consent was obtained from the parents of 11 children. Blood samples taken within 48 h after birth were obtained from nine children, but samples from four children were not analyzed because of insufficient sample volumes for accurate HHV-8 molecular analyses. Samples were also collected at the time of pediatric evaluation; however, since after delivery and prophylactic treatment of their infants most women were referred to the pediatric centers of their original towns, most of the children were lost to follow-up. Follow-up samples were obtained at 1 to 2 months of age from three of the infants analyzed at birth. Sufficient amounts of blood were collected from two other children, at 2 and 7 months of age.
HHV-8 serological analyses.
Plasma samples were analyzed for the presence and titers of antibodies to a latency-associated nuclear antigen (LANA) and to a lytic phase-associated structural protein encoded by ORF65, as described previously (7, 8). Antibody titers were calculated as the reciprocal of the highest plasma dilution that gave positive results.
Quantitative assessment of HIV-1 RNA.
The numbers of HIV-1 RNA copies in plasma and cell-free CVSs were measured by using the standard and ultrasensitive procedures of the AMPLICOR HIV-1 MONITOR test (lowest detection limits, 400 and 50 copies/ml, respectively; Roche Diagnostic Systems, Branchburg, NJ). All results are reported as the log10 number of HIV-1 RNA copies per milliliter of plasma or CVS.
HHV-8 PCR and real-time PCR analyses.
DNA was extracted from at least 2 × 106 maternal PBMCs or cells obtained from CVSs and from at least 0.5 × 106 PBMCs from children. Amplifications were carried out by PCR and nested PCR, as described previously (5), by using 0.5 to 1 μg of DNA and primers specific for three nonoverlapping HHV-8 genomic regions. Two sets of primers (specific for the ORF26 and ORF25 regions) were described previously (5). The third set is specific for the ORFK8.1 region; the outer primers amplify a 420-bp region (nucleotides 76243 to 76662 on the BC-1 sequences; GenBank accession number U75698), and the inner set amplifies a 337-bp region (nucleotides 76297 to 76633). DNA samples, along with several negative controls randomly alternated with patient samples, were tested. Each DNA sample was tested in a blinded fashion at least twice with each set of primers, and the order of the samples and the negative controls was changed each time. DNA extracted from the CRO-AP/3 cell line (9), a kind gift from A. Carbone, was used as the positive control in all amplifications. HHV-8 DNA was quantified in maternal PBMC and CVS samples and in the infant's PBMC sample by a quantitative real-time PCR assay, as described previously (15), with ORF26-specific primers and probe.
Nucleotide sequence analyses.
A nested PCR product containing the hypervariable region 1 (VR1) of the ORFK1 gene (44) was obtained from the DNA samples of four PCR-positive women and an infant. DNA from the three remaining PCR-positive women was not available for this analysis. Reaction mixtures were prepared as described previously (5), except for the use of a high-fidelity DNA polymerase (PfuTurbo DNA polymerase; Stratagene, La Jolla, CA). The ORFK1-specific outer primers amplify a 396-bp region (nucleotides 51 to 446), and the nested primers amplify a 325-bp product (nucleotides 76 to 400). At least three independent PCR products for each patient were directly sequenced by using the BigDye Terminator cycle sequencing kit (version 1.1; Applied BioSystems) and subjected to capillary electrophoresis with an ABI Prism 310 genetic analyzer (Applied BioSystems). The nucleotide sequences were analyzed and aligned by using Lasergene (DNASTAR Inc., Madison, WI) and BioEdit (version 5.0.9; http://www.mbio.ncsu.edu/BioEdit/bioedit.html) sequence analysis software. Phylogenetic analyses were conducted with the PHYLIP phylogeny inference package (version 3.5c; J. Felsenstein, http://evolution.genetics.washington.edu/phylip.html). The unrooted phylogenetic trees were generated by the neighbor-joining method with the NEIGHBOR program by use of a 276-bp region that included VR1 region of the ORFK1 gene. To assess the reliability of the tree topology, 1,000 bootstrap replicates were generated by using the SEQBOOT program.
Statistical analyses.
Binomial comparisons were made by using Fisher's exact and t tests. Nonparametric tests (the Wilcoxon signed-rank test) were used to evaluate differences in HIV-1 RNA loads and in CD4+-cell counts among different groups of women in different trimesters of pregnancy. To include the data in the statistical analyses, HIV-1 RNA-negative samples were assigned an arbitrary value of 40 copies/ml, and plasma samples found to be negative for anti-HHV-8 antibodies were assigned an arbitrary antibody titer of 20. Antibody titers were log10 transformed to achieve normality, and the titers between groups were compared by means of an unpaired t test. All hypothesis tests were two tailed, and a P value of ≤0.05 indicated statistical significance.
Nucleotide sequence accession numbers.
The ORFK1 nucleotide sequences analyzed in this study have been submitted to GenBank and have been assigned the following accession numbers: AY772819 (M1ITA), AY772820 (F1ITA), AY772821 (M2ITA), AY772822 (M3ITA), AY772823 (M4ITA), and AY772824 (CRO-AP/3).
RESULTS
HHV-8 qualitative and quantitative DNA analyses.
The presence and load of HHV-8 DNA were investigated in 45 PBMC samples and 43 CVS samples from 15 HIV-1- and HHV-8-seropositive pregnant women (Table 2). PBMC and CVS samples collected before pregnancy and during the first trimester of gestation were found to be repeatedly PCR negative. HHV-8 was detected in 5 of 13 (38.5%) PBMC samples collected during the second trimester of pregnancy, with values ranging from 2 to 123 genome equivalents (GE)/105 PBMCs (median value, 15 GE/105 PBMCs) and in 2 of 14 (14.2%) samples obtained during the third trimester. These two samples showed elevated HHV-8 loads (883 and 890 GE/105 PBMCs). On the whole, HHV-8 was detected in the PBMCs from 5 of 15 pregnant women. The HHV-8 DNA detection rate was found to be significantly higher in samples drawn during the last two trimesters of pregnancy compared to the rates in samples drawn before or during the first trimester (P = 0.03, Fisher's exact test).
TABLE 2.
HHV-8 DNA detection in the 15 HIV-1-infected pregnant women
| Compartment | No. of pregnant women with PCR-positive samples/ total no. of women tested (%)
|
||||
|---|---|---|---|---|---|
| Before pregnancy | First trimester | Second trimester | Third trimester | Total | |
| PBMCa | 0/5 | 0/13 | 5/13 (38.5) | 2/14 (14.2) | 5/15 (33.3) |
| CVSb | 0/5 | 0/13 | 1/13 (7.7) | 5/12 (41.6) | 5/15 (33.3) |
PBMC, peripheral blood mononuclear cells.
CVS, cellular fraction of cervicovaginal secretions.
One of 13 (7.7%) cellular fractions of CVS samples collected during the second trimester showed detectable HHV-8 sequences (Table 2) and a low viral load (2 GE/105 cells). Among the 12 CVS samples obtained during the last trimester, 5 (41.6%) were found to be PCR positive (Table 2), with a median HHV-8 load of 352 GE/105 cells (range, 36 to 2,871 GE/105 cells). The HHV-8 detection rate was also significantly higher in samples collected from the genital compartment during the last two trimesters of gestation (P = 0.03, Fisher's exact test). Thus, 5 of 15 pregnant women harbored HHV-8 sequences in their CVS samples and more frequently so during the last trimester of pregnancy.
HHV-8 sequences were detected simultaneously in the genital and peripheral blood compartments in three women. The CVS samples of two patients with detectable HHV-8 loads in PBMC samples collected in the second and third trimesters were also PCR positive at delivery.
Samples of placental tissue from three women with HHV-8 DNA-positive specimens were found to be repeatedly PCR negative (data not shown).
On the whole, HHV-8 sequences were detected in 7 of the 15 pregnancies; the increased rate of viral detection and the increased viral load suggest that latent HHV-8 infection may reactivate in HIV-1-infected women during pregnancy.
Parameters linked to HHV-8 DNA detection.
HHV-8 DNA was found to be significantly more common (P = 0.04) in women more than 30 years of age (Table 3). The absence of antiviral therapy during the first trimester or throughout was also significantly correlated with HHV-8 detection (P = 0.04). Moreover, a HAART regimen that included a protease inhibitor was associated with the absence of HHV-8 detection (P = 0.02).
TABLE 3.
Parameters linked to HHV-8 DNA detection
| Characteristic | No. (%) of women
|
P valuea | Antibody GMTc
|
Antibody titer during the indicated trimester:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women with PCR-positive samples
|
Women with PCR-negative samples
|
|||||||||||
| Total | With PCR-positive samples | With PCR-negative samples | Women with PCR-positive samples | Women with PCR-negative samples | I | II | III | I | II | III | ||
| Age | ||||||||||||
| >30 yr | 8 (53) | 6 (75) | 2 (25) | 0.04 | ||||||||
| <30 yr | 7 (47) | 1 (14) | 6 (86) | |||||||||
| Antiretroviral therapy | ||||||||||||
| Schedule | ||||||||||||
| Whole pregnancy | 7 (53) | 1 (14) | 6 (86) | 0.04 | ||||||||
| From the second trimester or untreated | 8 (47) | 6 (75) | 2 (25) | |||||||||
| Type | ||||||||||||
| With protease inhibitor | 5 (33) | 5 (100) | 0.02 | |||||||||
| Without protease inhibitor or untreated | 10 (67) | 7 (70) | 3 (30) | |||||||||
| HHV-8 antibody detectionb | ||||||||||||
| ORF65 | 7 (40) | 6 (83) | 1 (17) | 0.01 | 252 (137-464)d | 50e | 348 | 230 | 200 | NDf | ND | ND |
| LANA | 15 (100) | 7 (47) | 8 (53) | 981 (477-2,018)g | 194 (113-333) | 1,212 | 1,007 | 800 | 297 | 200 | 135 | |
| At least one antigen | 15 (100) | 7 (47) | 8 (53) | |||||||||
All P values were estimated by Fisher's exact test; antibody titers were compared by means of unpaired t test after log10transformation to achieve normality.
HHV-8 seropositivity is reported as the presence of antibodies to a structural ORF65-encoded protein, a LANA, or to at least one of these two antigens.
Values in parentheses are the 95% CI.
P = 0.001 for the difference between the two groups.
Antibody titer for one woman. Statistical analysis was performed by assigning an arbitrary antibody titer of 20 to plasma samples found to be negative.
ND, not done.
P = 0.069 for the difference between the two groups.
All women were HHV-8 seropositive before pregnancy, and the antibody pattern did not change during pregnancy. However, while anti-LANA antibodies were detected in all women, the presence of anti-ORF65 antibodies was found to be significantly associated with HHV-8 detection during pregnancy (P = 0.01). Indeed, anti-ORF65 antibodies were detected in six of the seven women with PCR-positive samples but only in one of eight women with PCR-negative samples (Table 3). Moreover, the geometric mean titer (GMT) for anti-ORF65 antibodies was 252 (95% confidence interval [CI], 137 to 464) in women with PCR-positive samples, whereas an antibody titer of 50 was found in just one woman with undetectable HHV-8 sequences (P = 0.001) (Table 3). Anti-LANA antibody titers were found to be higher in women with PCR-positive samples (a GMT of 981 [95% CI, 477 to 2,018] versus a GMT of 194 [95% CI, 113 to 333] for women with PCR-negative samples), although the difference was not significant (P = 0.069). Evaluation of the trends of the antibody titers during pregnancy showed 42.5% and 34% decreases in the GMTs between the first and the third trimesters for anti-ORF65 and anti-LANA antibodies, respectively, in women with PCR-positive samples. Conversely, women with PCR-negative samples showed a most pronounced decline (55%) in the anti-LANA antibody GMT (Table 3).
Analysis of HIV-1-related immunovirological parameters in the peripheral blood.
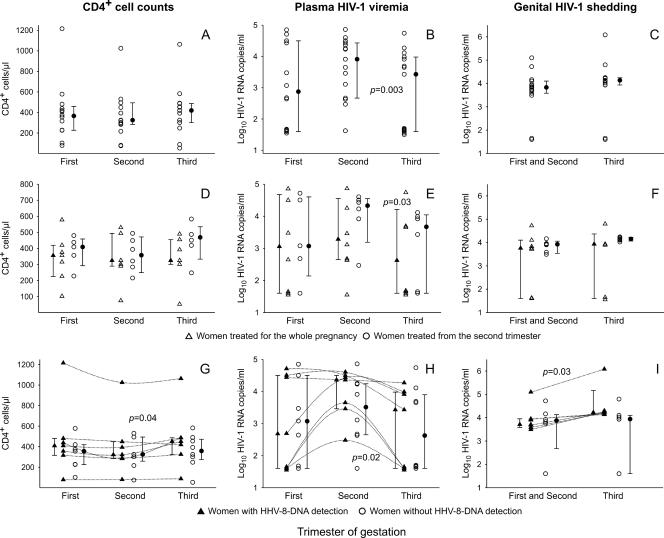
CD4+-cell counts remained stable in all women during the three trimesters (Fig. 1A), whereas a significant decrease in HIV-1 viremia was observed between the second and the third trimesters (P = 0.003, Wilcoxon signed-ranks test) (Fig. 1B). The decrease in HIV-1 viremia observed between the second and the third trimesters was found to be statistically significant whether or not the two untreated women were included in the analysis (data not shown).
FIG. 1.
HIV-1-associated immunovirological parameters in the 15 HHV-8-seropositive pregnant women. The distribution of CD4+-cell counts and the levels of plasma HIV-1 viremia and genital HIV-1 shedding, expressed as the log10 number of HIV-1 RNA copies per ml of plasma or cell-free fraction of cervicovaginal secretions in all pregnant women (A to C), according to the treatment schedule (D to F), and in women in whom HHV-8 DNA was or was not detected (G to I) are shown. The median, lower quartile (25th percentile), and upper quartile (75th percentile) values are represented by the corresponding symbols with bars. P values for significant differences are reported and were calculated by the Wilcoxon paired-matched signed-rank test. To show trends, values of the CD4+-cell counts and the levels of HIV-1 viremia of each woman with PCR-positive samples are joined.
Trends in HIV-1 viremia were found to be associated with the antiretroviral treatment schedule. A significant decrease in the number of HIV-1 RNA copies/ml was observed only in women treated from the second trimester (P = 0.03, Wilcoxon signed-ranks test) (Fig. 1E). These women also showed an increase in CD4+ lymphocytes between the second and the third trimesters (Fig. 1D), although this increase was not statistically significant (P = 0.06, Wilcoxon signed-ranks test).
Analysis of these parameters between women with and without PCR-positive samples revealed a simultaneous increase in CD4+-cell counts (P = 0.04, Wilcoxon signed-ranks test) and a decrease in the levels of plasma HIV-1 viremia between the second and the third trimesters, which were significant only in women with PCR-positive samples (P = 0.02, Wilcoxon signed-ranks test) (Fig. 1G and H).
Analysis of genital HIV-1 shedding.
No statistically significant variations in genital HIV-1 shedding were observed between the two gestational periods analyzed (Fig. 1C) in any of the women or when the women were subdivided according to the antiretroviral treatment schedule (Fig. 1F). Conversely, a significant increase in genital HIV-1 shedding was found only in women with detectable HHV-8 sequences (Fig. 1I) (P = 0.03, Wilcoxon paired-matched signed ranks).
HHV-8 detection by PCR analyses in children.
PBMC samples were available from five children born to mothers with PCR-positive samples and from two children born to mothers with PCR-negative samples. HHV-8 DNA was detected in the PBMCs from one infant born to a mother with PCR-positive samples. The results of HHV-8 quantitative analysis performed with samples from this woman were highly suggestive of an HHV-8 reactivation. Indeed, her PBMC samples showed an increase in the HHV-8 load from 123 to 890 GE/100,000 cells from the second to the third trimesters of gestation. Similarly, her CVS samples from the first and second trimesters showed undetectable HHV-8 levels, but the viral load increased to 660 GE/100,000 cells in the genital tract at delivery. The infant's PBMC sample was collected at 2 months of age and showed a very high HHV-8 load (4,363 GE/100,000 cells), consistent with a primary HHV-8 infection. Unfortunately, the blood sample obtained from the infant at birth was not of sufficient quantity to allow an accurate molecular analysis to be performed.
Nucleotide sequence analyses.
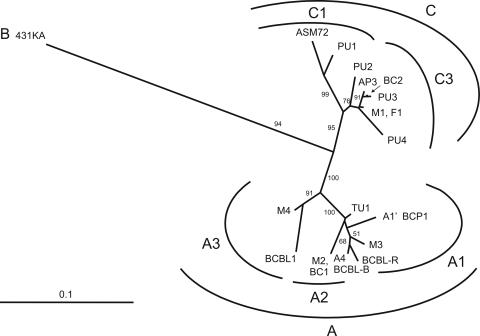
To characterize the HHV-8 subtype harbored in PCR-positive women, nucleotide sequence analysis of the PCR-amplified ORFK1 VR1 region was performed with HHV-8 DNA from 4 women. Material from the three remaining PCR-positive women was not available for this analysis. Figure 2 shows the alignment of a 276-bp coding region obtained from the four women (mothers M1 to M4) aligned to the CRO-AP/3 sequence and the predicted amino acid changes. The ORFK1 sequences amplified from the four women were different; the variability within this group ranged from 3.3 to 9.5% at the nucleotide sequence level and from 7.7 to 22.9% at the amino acid sequence level. All sequences were found to be different from that amplified from the CRO-AP/3 cells. Phylogenetic analyses (Fig. 3) showed the presence of four different ORFK1 subtypes. Subtype C3 was found in mother M1, and subtypes A2, A1, and A3 were found in mothers M2, M3, and M4, respectively. The newly characterized genotype harbored by the CRO-AP/3 cell line was found to belong to ORFK1 subtype C3.
FIG. 2.
Alignment of the hypervariable region 1 of the ORFK1 gene of the Italian HHV-8 strains amplified from the pregnant women. Only positions in which changes were observed in the pregnant women's sequences are listed and are aligned to the newly characterized CRO-AP/3 sequence (GenBank accession number AY772824). Numbering is according to the BC-1 sequence (GenBank accession number U75698). Amino acid changes in our set of sequences with respect to the sequence of CRO-AP/3 are also shown.
FIG. 3.
Phylogenetic analysis of a 276-nucleotide region of the ORFK1 gene of human herpesvirus 8 strains. The unrooted phylogenetic tree was constructed by the neighbor-joining method with the NEIGHBOR program of the PHYLIP package (version 3.5c) with a 276-bp region including the VR1 region of the ORFK1 gene (44). The 431KA sequence (prototype B) was used as the outgroup. The bar represents 0.1 nucleotide substitution per site. The bootstrap values (in percent) were calculated from 1,000 bootstrap replicates. The sequences used in the analysis included BCBL-R (prototype A1; GenBank accession number AF133038); BCP-1 (A1′, accession number AY787132); BC-1 (A2; GenBank accession number U75698), BCBL1 (A3; GenBank accession number U86667); BCBL-B (A4; GenBank accession number AF133039); ASM72 (C1; GenBank accession number AF133041); BC2 (C3; GenBank accession number: AF133042), PU1 to PU4 (GenBank accession numbers AF130286, AF130274, AF130270, and AF130271, respectively), AP3 (CRO-AP/3; GenBank accession number AY772824), and TU1ITA (GenBank accession number AY787131). Nucleotide sequences obtained from the analyzed women were denominated M1 (GenBank accession number: AY772819), M2 (GenBank accession number: AY772821), M3 (GenBank accession number: AY772822), and M4 (GenBank accession number AY772823), and they belong to the C3, A2, A1, and A3 subtypes, respectively. Nucleotide sequence analysis of the VR1 region amplified from the PBMC sample of a newborn (newborn F1; GenBank accession number AY772820) was found to be identical to the sequence of the mother (mother M1).
Nucleotide sequence analysis of the VR1 region amplified from the PBMC sample of the newborn (newborn F1) disclosed full identity with the mother's sequence (mother M1). We exclude the possibility of cross-contamination with the mother's sample, since the samples from all mothers and children were collected, extracted, and amplified at different times.
DISCUSSION
The data presented here show that HHV-8 infection may be reactivated during pregnancy in HIV-1-infected women. The rate of HHV-8 DNA detection in PBMCs of pregnant HIV-1- and HHV-8-infected women was found to be 33.3% (Table 2). This value is higher than that reported for nonpregnant HIV-1- and HHV-8-infected women of the same area, among whom HHV-8 DNA was detected in only 3.5 to 10% of PBMC samples (5, 41). Moreover, HHV-8 was detected in PBMC samples drawn during the second trimester of pregnancy from 5 of 15 women, and HHV-8 DNA detection was lost in samples collected during the last trimester from three of the five women (Table 2). The well-documented twofold increase in maternal leukocytes, as well as modifications in hormone and cytokine production, could account for the lack of HHV-8 detection at delivery, a phenomenon also described for other herpesviruses (see reference 12 and references therein). The lack of HHV-8 DNA detection during the last trimester might also suggest that a subset of patients could control HHV-8 reactivation in the peripheral compartment. On the other hand, the two women with persistent PCR-positive PBMC samples during the last trimester showed increased HHV-8 loads. It is conceivable that in this HIV-1-infected population, the increased HHV-8 load in PBMCs could have also been affected by the antiretroviral therapy (Table 3). Indeed, HHV-8 detection in PBMCs was significantly associated with a therapeutic regimen that did not include a protease inhibitor and with the absence of therapy throughout the first trimester. These results further confirm that a HAART regimen that includes a protease inhibitor administered during the entire pregnancy is, albeit indirectly, effective in decreasing HHV-8 to very low, undetectable levels in the peripheral blood (26, 27). In fact, inhibition of HIV-1 replication with HAART leads to the reconstitution of the immune system and regenerates effective immune responses against HHV-8. Moreover, protease inhibitors block the production of inflammatory cytokines, which, in turn, may result in the down-regulation of HHV-8 replication.
Viral reactivation in the peripheral blood compartment was also accompanied by a high HHV-8 detection rate (33.3%) in the genital tract (Table 2). This detection rate is more than 10-fold higher than that which we previously reported in nonpregnant HIV-1-infected women from the same geographic area (5) and exceeds that measured in other female populations from areas of nonendemicity (2, 43). In agreement with reports on the analysis of other herpesviruses in CVSs (2, 33, 38), HHV-8 was detected in the genital tract mainly at delivery. Local changes in maternal immune function might explain this phenomenon and, possibly, might also affect HIV-1 replication (21). In fact, the increased HHV-8 loads in the CVS samples were paralleled by an increase in the level of genital HIV-1 shedding during the third trimester (Fig. 1C). These findings indicate that the levels of these two viruses may be influenced by similar factors operating in specific body compartments, such as local changes in the cytokine/chemokine milieu or the presence of inflammation. Moreover, many herpesviruses were shown to directly influence HIV-1 replication, and a reciprocal regulatory interaction between HIV-1 and HHV-8 has been described previously (10, 39). Indeed, HIV-1 may induce HHV-8 lytic replication, whereas the HHV-8 protein responsible for the switch from latency to the lytic cycle, the ORF50-encoded RTA protein, might increase HIV-1 replication as well as susceptibility to HIV-1 infection and replication in nonpermissive cell lines. Therefore, along with the cytokine/chemokine milieu, local interactions between the two viruses could further contribute to their simultaneous increase in one body site and the parallel disappearance in the other one.
The preferential presence of antilytic antibodies in women with PCR-positive samples confirmed that the detection and titers of anti-ORF65 antibodies may be correlated with an ongoing productive HHV-8 replication (7, 11). This could, in turn, augment the pool of latently infected cells and thus justify the parallel higher anti-LANA antibody titers (Table 3). Antibody titers underwent a progressive decrease during pregnancy, compatible with dilution due to the physiological increase (30 to 35%) in the plasma volume, which is the most pronounced late in pregnancy. However, HHV-8 reactivation may lead to a more prolonged antigen stimulation and thus contribute to the maintenance of more stable anti-HHV-8 antibody levels, as was observed in women with PCR-positive samples (Table 3).
It was suggested that HHV-8 seropositivity might negatively influence the outcome of pregnancies by increasing the abortion rate or determining a low weight at birth (16, 37). Of the 15 children in our study, only 1 infant had a low birth weight. Moreover, no statistically significant differences were found by comparing the birth weights or the gestational ages of the newborns born to women with or without HHV-8 reactivation (data not shown), suggesting that in our study population, an increased viral load did not seem to have affected intrauterine growth or the timing of delivery.
Our findings suggest that analysis of HHV-8-associated parameters before and during pregnancy may be useful for the identification of HIV-1-infected women at higher risk for transmission. Indeed, while no case of vertical HIV-1 transmission was recorded (data not shown), a highly viremic, primary HHV-8 infection was documented in one infant (2 months old) born to a mother with HHV-8 reactivation. Transmission of the maternal isolate was confirmed by means of nucleotide sequence analysis of one of the most hypervariable regions of the viral genome, which identified the same ORFK1 subtype in the mother-child pair (Fig. 2 and 3). Although we could not determine whether infection occurred in utero or through postnatal exposure to the mother's saliva, intrapartum transmission seems to be unlikely since the delivery was by cesarean section. Moreover, the absence of HHV-8 sequences in the placental tissues analyzed could argue against in utero transmission, although further studies are needed to investigate this mode of transmission. It is also conceivable that HHV-8 reactivation may lead to increased oral shedding, facilitating perinatal transmission through infectious saliva (13).
In conclusion, although this study was limited to a relatively low number of pregnant women, it demonstrates that, like other herpesviruses, HHV-8 may be reactivated during pregnancy in HIV-1-infected women. The increased viral load may in turn account for a higher risk for perinatal HHV-8 transmission in this HIV-1-infected population. The evidence of an infant's primary infection with the mother's ORFK1 subtype further supports this hypothesis and documents for the first time the perinatal transmission of a specific HHV-8 subtype. As already reported in areas of endemicity, mother-to-child transmission may thus also play a role in the spread of HHV-8 among HIV-1-infected women in areas of subendemicity. These findings warrant further investigation of HIV-1-infected and uninfected pregnant women in areas of endemicity.
Acknowledgments
This work was supported by grants from Istituto Superiore di Sanità, Ministry of Health, Italy; the Italian Association for Cancer Research; the Italian Foundation for Cancer Research; and the University of Padova. M.B. was the recipient of a fellowship from Associazione Italiana per la Lotta contro le Leucemie, Linfomi e Mieloma.
We thank Lisa Smith for editing the manuscript, Pierantonio Gallo for artwork, and Antonella Vimercati for obstetrical care of the patients.
Footnotes
Published ahead of print on 30 August 2006.
REFERENCES
- 1.Baeten, J. M., B. H. Chohan, L. Lavreys, J. P. Rakwar, R. Ashley, B. A. Richardson, K. Mandaliya, J. J. Bwayo, and J. K. Kreiss. 2002. Correlates of human herpesvirus 8 seropositivity among heterosexual men in Kenya. AIDS 16:2073-2078. [DOI] [PubMed] [Google Scholar]
- 2.Boivin, G., C. Hankins, N. Lapointe, S. Walmsley, A. Gaudreau, P. Forest, F. Coutlee, et al. 2000. Human herpesvirus 8 infection of the genital tract of HIV-seropositive and HIV-seronegative women at risk of sexually transmitted diseases. AIDS 14:1073-1075. [DOI] [PubMed] [Google Scholar]
- 3.Brayfield, B. P., S. Phiri, C. Kankasa, J. Muyanga, H. Mantina, G. Kwenda, J. T. West, G. Bhat, D. B. Marx, W. Klaskala, C. D. Mitchell, and C. Wood. 2003. Postnatal human herpesvirus 8 and human immunodeficiency virus type 1 infection in mothers and infants from Zambia. J. Infect. Dis. 187:559-568. [DOI] [PubMed] [Google Scholar]
- 4.Bryant, A. E., M. Genc, R. M. Hurtado, and K. T. Chen. 2004. Pulmonary Kaposi's sarcoma in pregnancy. Am. J. Perinatol. 21:355-363. [DOI] [PubMed] [Google Scholar]
- 5.Calabrò, M. L., J. R. Fiore, A. Favero, A. Lepera, A. Saracino, G. Angarano, T. F. Schulz, and L. Chieco-Bianchi. 1999. Detection of human herpesvirus 8 in cervicovaginal secretions and seroprevalence in human immunodeficiency virus type 1-seropositive and -seronegative women. J. Infect. Dis. 179:1534-1537. [DOI] [PubMed] [Google Scholar]
- 6.Calabrò, M. L., P. Gasperini, M. Barbierato, L. Ometto, M. Zanchetta, A. De Rossi, and L. Chieco-Bianchi. 2000. A search for human herpesvirus 8 (HHV-8) in HIV-1 infected mothers and their infants does not suggest vertical transmission of HHV-8. Int. J. Cancer 85:296-297. [DOI] [PubMed] [Google Scholar]
- 7.Calabrò, M. L., P. Gasperini, J. R. Fiore, M. Barbierato, G. Angarano, and L. Chieco-Bianchi. 2001. Intrafamilial transmission of human herpesvirus 8. J. Natl. Cancer Inst. 93:154-156. [DOI] [PubMed] [Google Scholar]
- 8.Calabrò, M. L., J. Sheldon, A. Favero, G. R. Simpson, J. R. Fiore, E. Gomes, G. Angarano, L. Chieco-Bianchi, and T. F. Schulz. 1998. Seroprevalence of Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 in several regions of Italy. J. Hum. Virol. 1:207-213. [PubMed] [Google Scholar]
- 9.Carbone, A., A. M. Cilia, A. Gloghini, D. Capello, T. Perin, D. Bontempo, V. Canzonieri, U. Tirelli, R. Volpe, and G. Gaidano. 2000. Primary effusion lymphoma cell lines harbouring human herpesvirus type-8. Leuk. Lymphoma 36:447-456. [DOI] [PubMed] [Google Scholar]
- 10.Caselli, E., M. Galvan, F. Santoni, A. Rotola, A. Caruso, E. Cassai, and D. D. Luca. 2003. Human herpesvirus-8 (Kaposi's sarcoma-associated virus) ORF50 increases in vitro cell susceptibility to human immunodeficiency virus type 1 infection. J. Gen. Virol. 84:1123-1131. [DOI] [PubMed] [Google Scholar]
- 11.Cattelan, A. M., M. L. Calabrò, P. Gasperini, S. M. Aversa, M. Zanchetta, F. Meneghetti, A. De Rossi, and L. Chieco-Bianchi. 2001. Acquired immunodeficiency syndrome-related Kaposi's sarcoma regression after highly active antiretroviral therapy: biologic correlates of clinical outcome. J. Natl. Cancer Inst. Monogr. 28:44-49. [DOI] [PubMed] [Google Scholar]
- 12.Dahl, H., G. Fjaertoft, T. Norsted, F. Z. Wang, M. Mousavi-Jazi, and A. Linde. 1999. Reactivation of human herpesvirus 6 during pregnancy. J. Infect. Dis. 180:2035-2038. [DOI] [PubMed] [Google Scholar]
- 13.Dedicoat, M., R. Newton, K. R. Alkharsah, J. Sheldon, I. Szabados, B. Ndlovu, T. Page, D. Casabonne, C. F. Gilks, S. A. Cassol, D. Whitby, and T. F. Schulz. 2004. Mother-to-child transmission of human herpesvirus-8 in South Africa. J. Infect. Dis. 190:1068-1075. [DOI] [PubMed] [Google Scholar]
- 14.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwartz, and D. M. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67:175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasperini, P., M. Barbierato, C. Martinelli, P. Rigotti, F. Marchini, G. Masserizzi, F. Leoncini, L. Chieco-Bianchi, T. F. Schulz, and M. L. Calabrò. 2005. Use of a BJAB-derived cell line for isolation of human herpesvirus 8. J. Clin. Microbiol. 43:2866-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaye-Diallo, A., A. T. Toure, A. Gessain, A. Gueye-Ndiaye, A. N. Ndour, N. C. Toure-Kane, M. C. Dia, G. de The, and S. Mboup. 2001. Preliminary study of human herpesvirus type 8 infection in pregnant women in Dakar (Senegal). Bull. Soc. Pathol. Exot. 94:231-234. [PubMed] [Google Scholar]
- 17.Geddes, M., S. Franceschi, A. Barchielli, F. Falcini, S. Carli, G. Cocconi, E. Conti, P. Crosignani, L. Gafa, L. Giarelli, et al. 1994. Kaposi's sarcoma in Italy before and after the AIDS epidemic. Br. J. Cancer 69:333-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gessain, A., P. Mauclere, M. van Beveren, S. Plancoulaine, A. Ayouba, J. L. Essame-Oyono, P. M. Martin, and G. de The. 1999. Human herpesvirus 8 primary infection occurs during childhood in Cameroon, Central Africa. Int. J. Cancer 81:189-192. [DOI] [PubMed] [Google Scholar]
- 19.Goedert, J. J., D. H. Kedes, and D. Ganem. 1997. Antibodies to human herpesvirus 8 in women and infants born in Haiti and the USA. Lancet 349:1368. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez-Ortega, P., S. Hierro-Orozco, R. Sanchez-Cisneros, and L. F. Montano. 1989. Kaposi's sarcoma in a 6-day-old infant with human immunodeficiency virus. Arch. Dermatol. 125:432-433. [PubMed] [Google Scholar]
- 21.Henin, Y., L. Mandelbrot, R. Henrion, R. Pradinaud, J. P. Coulaud, and L. Montagnier. 1993. Virus excretion in the cervicovaginal secretions of pregnant and nonpregnant HIV-infected women. J. Acquir. Immune Defic. Syndr. 6:72-75. [PubMed] [Google Scholar]
- 22.Hernandez, J. L., J. Gomez-Roman, C. Ramos-Estebanez, D. Nan, J. Martin-Oviedo, J. A. Riancho, and J. Gonzalez-Macias. 2005. Human herpesvirus 8 and Epstein-Barr virus coinfection in localized Castleman disease during pregnancy. Haematologica 90(Suppl.):ECR35. [PubMed] [Google Scholar]
- 23.Hitti, J., D. H. Watts, S. K. Burchett, T. Schacker, S. Selke, Z. A. Brown, and L. Corey. 1997. Herpes simplex virus seropositivity and reactivation at delivery among pregnant women infected with human immunodeficiency virus-1. Am. J. Obstet. Gynecol. 177:450-454. [DOI] [PubMed] [Google Scholar]
- 24.Lampinen, T. M., S. Kulasingam, J. Min, M. Borok, L. Gwanzura, J. Lamb, K. Mahomed, G. B. Woelk, K. B. Strand, M. L. Bosch, D. C. Edelman, N. T. Constantine, D. Katzenstein, and M. A. Williams. 2000. Detection of Kaposi's sarcoma-associated herpesvirus in oral and genital secretions of Zimbabwean women. J. Infect. Dis. 181:1785-1790. [DOI] [PubMed] [Google Scholar]
- 25.Lavreys, L., B. Chohan, R. Ashley, B. A. Richardson, L. Corey, K. Mandaliya, J. O. Ndinya-Achola, and J. K. Kreiss. 2003. Human herpesvirus 8: seroprevalence and correlates in prostitutes in Mombasa, Kenya. J. Infect. Dis. 187:359-363. [DOI] [PubMed] [Google Scholar]
- 26.Leao, J. C., N. Kumar, K. A. McLean, S. R. Porter, C. M. Scully, A. V. Swan, and C. G. Teo. 2000. Effect of human immunodeficiency virus-1 protease inhibitors on the clearance of human herpesvirus 8 from blood of human immunodeficiency virus-1-infected patients. J. Med. Virol. 62:416-420. [DOI] [PubMed] [Google Scholar]
- 27.Lebbe, C., L. Blum, C. Pellet, G. Blanchard, O. Verola, P. Morel, O. Danne, and F. Calvo. 1998. Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi's sarcoma. AIDS 12:F45-F49. [DOI] [PubMed] [Google Scholar]
- 28.Lehtinen, M., P. Koskela, H. M. Ogmundsdottir, A. Bloigu, J. Dillner, M. Gudnadottir, T. Hakulinen, A. Kjartansdottir, M. Kvarnung, E. Pukkala, H. Tulinius, and T. Lehtinen. 2003. Maternal herpesvirus infections and risk of acute lymphoblastic leukemia in the offspring. Am. J. Epidemiol. 158:207-213. [DOI] [PubMed] [Google Scholar]
- 29.Mantina, H., C. Kankasa, W. Klaskala, B. Brayfield, J. Campbell, Q. Du, G. Bhat, F. Kasolo, C. Mitchell, and C. Wood. 2001. Vertical transmission of Kaposi's sarcoma-associated herpesvirus. Int. J. Cancer 94:749-752. [DOI] [PubMed] [Google Scholar]
- 30.Martin, J. N., D. E. Ganem, D. H. Osmond, K. A. Page-Shafer, D. Macrae, and D. H. Kedes. 1998. Sexual transmission and the natural history of human herpesvirus 8 infection. N. Engl. J. Med. 338:948-954. [DOI] [PubMed] [Google Scholar]
- 31.Mayama, S., L. E. Cuevas, J. Sheldon, O. H. Omar, D. H. Smith, P. Okong, B. Silvel, C. A. Hart, and T. F. Schulz. 1998. Prevalence and transmission of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int. J. Cancer 77:817-820. [DOI] [PubMed] [Google Scholar]
- 32.McCarty, K. A., and Z. Bungu. 1995. Kaposi's sarcoma in a two week old infant born to a mother with Kaposi's sarcoma/AIDS. Cent. Afr. J. Med. 41:330-331. [PubMed] [Google Scholar]
- 33.Ohashi, M., T. Yoshikawa, M. Ihira, K. Suzuki, S. Suga, S. Tada, Y. Udagawa, H. Sakui, K. Iida, Y. Saito, Y. Nisiyama, and Y. Asano. 2002. Reactivation of human herpesvirus 6 and 7 in pregnant women. J. Med. Virol. 67:354-358. [DOI] [PubMed] [Google Scholar]
- 34.Pfeffer, U., D. Bisacchi, M. Morini, R. Benelli, S. Minghelli, A. Vacca, D. M. Noonan, and A. Albini. 2002. Human chorionic gonadotropin inhibits Kaposi's sarcoma associated angiogenesis, matrix metalloprotease activity, and tumor growth. Endocrinology 143:3114-3121. [DOI] [PubMed] [Google Scholar]
- 35.Piccinni, M. P., C. Scaletti, E. Maggi, and S. Romagnani. 2000. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J. Neuroimmunol. 109:30-33. [DOI] [PubMed] [Google Scholar]
- 36.Plancoulaine, S., L. Abel, M. van Beveren, D. A. Tregouet, M. Joubert, P. Tortevoye, G. de The, and A. Gessain. 2000. Human herpesvirus 8 transmission from mother to child and between siblings in an endemic population. Lancet 356:1062-1065. [DOI] [PubMed] [Google Scholar]
- 37.Sarmati, L., C. Ticconi, R. Santangelo, M. Montano, G. Rezza, and M. Andreoni. 2003. Does the risk of abortion increase in women with high human herpesvirus-8 antibody titers? J. Infect. Dis. 188:173-174. [DOI] [PubMed] [Google Scholar]
- 38.Shen, C. Y., S. F. Chang, M. F. Chao, S. L. Yang, G. M. Lin, W. W. Chang, C. W. Wu, M. S. Yen, H. T. Ng, J. C. Thomas, et al. 1993. Cytomegalovirus recurrence in seropositive pregnant women attending obstetric clinics. J. Med. Virol. 41:24-29. [DOI] [PubMed] [Google Scholar]
- 39.Varthakavi, V., R. M. Smith, H. Deng, R. Sun, and P. Spearman. 2002. Human immunodeficiency virus type-1 activates lytic cycle replication of Kaposi's sarcoma-associated herpesvirus through induction of KSHV Rta. Virology 297:270-280. [DOI] [PubMed] [Google Scholar]
- 40.Veenstra van Nieuwenhoven, A. L., A. Bouman, H. Moes, M. J. Heineman, L. F. de Leij, J. Santema, and M. M. Faas. 2002. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil. Steril. 77:1032-1037. [DOI] [PubMed] [Google Scholar]
- 41.Viviano, E., F. Vitale, F. Ajello, A. M. Perna, M. R. Villafrate, F. Bonura, M. Arico, G. Mazzola, and N. Romano. 1997. Human herpesvirus type 8 DNA sequences in biological samples of HIV-positive and negative individuals in Sicily. AIDS 11:607-612. [DOI] [PubMed] [Google Scholar]
- 42.Whitby, D., M. Luppi, P. Barozzi, C. Boshoff, R. A. Weiss, and G. Torelli. 1998. Human herpesvirus 8 seroprevalence in blood donors and lymphoma patients from different regions of Italy. J. Natl. Cancer Inst. 90:395-397. [DOI] [PubMed] [Google Scholar]
- 43.Whitby, D., N. A. Smith, S. Matthews, S. O'Shea, C. A. Sabin, R. Kulasegaram, C. Boshoff, R. A. Weiss, A. de Ruiter, and J. M. Best. 1999. Human herpesvirus 8: seroepidemiology among women and detection in the genital tract of seropositive women. J. Infect. Dis. 179:234-236. [DOI] [PubMed] [Google Scholar]
- 44.Zong, J. C., D. M. Ciufo, D. J. Alcendor, X. Wan, J. Nicholas, P. J. Browning, P. L. Rady, S. K. Tyring, J. M. Orenstein, C. S. Rabkin, I. J. Su, K. F. Powell, M. Croxson, K. E. Foreman, B. J. Nickoloff, S. Alkan, and G. S. Hayward. 1999. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J. Virol. 73:4156-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]