Abstract
A pyrosequencing protocol was developed as a rapid and reliable method to identify the mutations of the dhfr and dhps genes of Plasmodium falciparum that are associated with antifolate resistance. The accuracy and specificity of this method were tested using six laboratory-cultured P. falciparum isolates harboring known single nucleotide polymorphisms (SNPs) in the genes dhfr (codons 50, 51, 59, 108, and 164) and dhps (codons 436, 437, 540, 581, and 613). The lowest threshold for detection of all the SNPs tested by pyrosequencing was the equivalent of two to four parasite genomes. Also, this method was highly specific for P. falciparum, as it did not amplify any DNA products from the other species of human malaria parasites. We also mixed wild-type and mutant-type parasite DNAs in various proportions to determine how pyrosequencing, restriction fragment length polymorphism (RFLP), and direct conventional sequencing (for dhfr) compared with each other in detecting different SNPs in the mixture. In general, pyrosequencing and RFLP showed comparable sensitivities in detecting most of the SNPs in dhfr except for the 164L mutation, which required at least twice the amount of DNA for pyroseqencing as for RFLP. For detecting SNPs in dhps, pyrosequencing was slightly more sensitive than RFLP and direct sequencing. Overall, pyrosequencing was faster and less expensive than either RFLP or direct sequencing. Thus, pyrosequencing is a practical alternative method that can be used in a high-throughput format for molecular surveillance of antimalarial-drug resistance.
Drug-resistant Plasmodium falciparum is a serious public health threat in countries where malaria is endemic. In the event that the genetic basis for the drug resistance is known, genetic markers are potentially useful surrogates for monitoring the emergence and dispersion of drug resistance, especially in population-based studies (5, 22). The prevalences of single nucleotide polymorphisms (SNPs) in the P. falciparum genes for dihydropteroate synthetase (dhps) and dihydrofolate reductase (dhfr) have been associated with resistance to sulfadoxine and pyrimethamine, respectively (3, 6, 7, 10, 11, 14, 15, 21, 23, 28). Conventional molecular methods used to evaluate sulfadoxine and pyrimethamine drug resistance focus on identifying individual mutations in the genes dhfr (codons 50, 51, 59, 108, and 164) and dhps (codons 436, 437 540, 581, and 613) (13, 17, 24, 25). These techniques include conventional DNA sequencing, allele-specific PCR, PCR-restriction fragment length polymorphism (RFLP) analysis, dot blot/probe hybridization techniques, real-time PCR, the SNaPshot primer extension method, and sequence-specific oligonucleotide probe-enzyme-linked immunosorbent assay (1, 4, 12, 13, 30). Each of these techniques offers its own advantages and limitations. Among the techniques, RFLP is one of the most commonly used, but it is laborious and expensive (18, 26, 29). Therefore, a cost-effective and high-throughput genotyping method would be ideal for large-scale population-based studies.
In this study, we investigated pyrosequencing as a high-throughput method for genotyping SNPs associated with antifolate resistance in dhfr and dhps. Pyrosequencing allows direct sequencing by the synthesis of short fragments of DNA by a novel enzymatic-cascade system (2). The enzymatic reactions are catalyzed by ATP sulfurylase and luciferase. The inorganic pyrophosphates that are released after deoxynucleotide incorporation are monitored, and free nucleotides are degraded by apyrase, thus allowing iterative nucleotide addition. Scores are determined by computer-automated comparisons of predicted SNP patterns with raw data. Sequences generally do not require manual interpretation, which provides consistency in scoring and the interpretation of results (2, 8, 20).
We have developed a pyrosequencing protocol to detect the mutations in the dhfr and dhps genes of P. falciparum in our laboratory. In the present study, we evaluated the method's sensitivity and specificity in detecting mutations from mixtures of known concentrations of DNAs from laboratory-cultured parasite isolates with different genotypes. We focused the analysis on point mutations in dhfr codons 51, 59, 108, and 164 and dhps codons 436, 437, 540, 581, and 613. We also validated our results by comparing them with conventional sequencing and PCR-RFLP methods. These data show that pyrosequencing is a sensitive and reliable method to detect mutations associated with drug resistance.
MATERIALS AND METHODS
Parasite culture and extraction of DNA.
Six laboratory-cultured P. falciparum strains (3D7, FCR3, HB3, V1/S, K1, and W2) that have been shown to harbor different mutations in dhfr and dhps were used to test the specificity and detection limit of the pyrosequencing technique used in this study. These parasites were cultured in vitro using standard protocols (27). The parasite cultures were synchronized by 5% sorbitol treatment, and the cultures were harvested during the ring stage. Parasitemias for each strain were calculated after the microscopic examination of Giemsa-stained thin blood films. Known quantities of parasites were stored in a liquid nitrogen tank (diluted to between 59,343 and 89,760 parasites/μl). Extraction of DNA from the parasites was performed with a QIAamp DNA Mini Kit (QIAGEN, Valencia, CA). DNA was isolated from a total of 8 × 106 parasites for each strain and suspended in 200 μl of water; therefore, 1 μl of sample contained the DNA equivalent of 40,000 parasites. The DNA was aliquoted and stored at −20°C. Since none of the cultured parasite lines had a mutation at codon 540 of dhps, DNA from a field isolate from Peru (donated by C. Plowe) was used as a control to test the mutation at this codon.
Sensitivity and specificity analysis.
From the stock DNAs (40,000 parasites/μl), we made serial 10-fold dilutions up to a final concentration equivalent of 2 parasite genomes per microliter. These diluted DNAs were used to test the least amount of DNA required for the pyrosequencing assay. DNAs from Plasmodium vivax SV4, Plasmodium malariae Uganda I, and Plasmodium ovale Nigeria I were also extracted for testing the species specificities of PCR primers.
PCR for pyrosequencing.
The PCR and sequencing primers for the dhfr and dhps genes were synthesized at the CDC Biotechnology Core Facility. The primer sequences are shown in Table 1. The primary and nested PCRs were generated in 50-μl reaction volumes, which contained 1 μl DNA, 0.5 μM of forward and reverse PCR primers, 16 μM deoxynucleotide triphosphates, 1× buffer (Applied Biosystems, Foster City, CA), 0.75 mM MgCl2 (dhfr) or 1.0 mM MgCl2 (dhps), and 2.5 units Taq DNA polymerase (Promega, Madison, WI). The primary PCR cycling conditions were 95°C for 5 min; 45 cycles with denaturation at 92°C for 30 s, annealing at 45°C for 45 s or 30 s (dhps), and elongation at 72°C or 65°C (dhps) for 45 s; 1 cycle at 72°C for 15 min; and a final hold at 4°C. The thermal-cycling conditions for the nested reactions for dhfr codons 50, 51, and 59 and dhps codons 436, 437, 540, and 581 were 95°C for 5 min; 25 cycles with denaturation at 92°C for 30 s, annealing at 45°C for 45 s, and elongation at 65°C for 45 s; 1 cycle at 72°C for 15 min; and a final hold at 4°C. The thermal-cycling conditions for the nested reactions for dhfr codons 108 and 164 and dhps codon 613 were 95°C for 5 min; 25 cycles with denaturation at 92°C for 30 s, annealing at 42°C for 30 s, and elongation at 65°C for 45 s; 1 cycle at 72°C for 15 min; and a final hold at 4°C. PCR was performed using an Icycler Thermal cycler (Bio-Rad, Hercules, CA).
TABLE 1.
PCR primers for pyrosequencing
| Primer name | Sequence (5′→3′)a | Purpose |
|---|---|---|
| prim1F | ATGATGGAACAAGTCTGCGAC | Primary PCR dhfr |
| prim1R | ACATTTTATTATTCGTTTTCT | Primary PCR dhfr |
| prim2F | GGGGTATTAAATGTTAATTATGATTCT | Primary PCR dhps |
| prim2R | GGGGACCTGAAAAGAAATACATAA | Primary PCR dhps |
| sec1F | GCGACGTTTTCGATATTTATGC | Nested PCR, dhfr codons 50, 51, 59 |
| sec1R | B-GGCATATCATTTACATTATCCACAGTTT | Nested PCR, dhfr codons 50, 51, 59 |
| sec2F | B-CTAATTCTAAAAAATTACAAAATGT | Nested PCR, dhfr codons 108, 164 |
| sec2R | CACATTCATATGTACTATTT | Nested PCR, dhfr codons 108, 164 |
| sec3F | B-TGTTCAAAGAATGTTTTGAATGA | Nested PCR, dhps codons 436, 437 |
| sec3R | CCATTCTTTTTGAAATAATTGTAAT | Nested PCR, dhps codons 436, 437 |
| sec4F | GTTCTAATGCATAAAAGAGG | Nested PCR, dhps codons 540, 581 |
| sec4R | B-TAAGAGTTTAATAGATTGATCATGTTTCTTC | Nested PCR, dhps codons 540, 581 |
| sec5F | B-TGTATATGATGAGTATCCAC | Nested PCR, dhps codons 613 |
| sec5R | GTGTGATTTGTCCACAATAT | Nested PCR, dhps codons 613 |
| 50/51F | GGTCTAGGAAATAAAGGAGT | Pyrosequencing primer, dhfr codons 50/51 |
| 59F | CCCTAGATATGAAATATTTT | Pyrosequencing primer, dhfr codon 59 |
| 108R | TGGAATGCTTTCCCAG | Pyrosequencing primer, dhfr codon 108 |
| 164R | ATTCTTGATAAACAACGGAA | Pyrosequencing primer, dhfr codon 164 |
| 436/437R | GGATTAGGTATAACAAAAGG | Pyrosequencing primer, dhps codons 436/437 |
| 540R | ATCCACATACAATGGAT | Pyrosequencing primer, dhps codon 540 |
| 581R | TTGATATTGGATTAGGATTT | Pyrosequencing primer, dhps codon 581 |
| 613R | CATTTTGATCATTCATGC | Pyrosequencing primer, dhps codon 613 |
B, biotinylated.
Pyrosequencing reactions.
Single-stranded biotinylated PCR products were prepared for sequencing using the Pyrosequencing Vacuum Prep Tool (Biotage AB, Uppsala, Sweden). Three microliters of Streptavidin Sepharose HP beads (Amersham Biosciences, Uppsala, Sweden) was added to 40 μl binding buffer (10 mM Tris-HCl, pH 7.6, 2 M NaCl, 1 mM EDTA, 0.1% Tween 20) and mixed with 20 μl PCR product and 20 μl water for 10 min at room temperature using an Orbit Digital Shaker (Labnet International, Woodbridge, NJ). The beads containing the immobilized templates were captured on the filter probes after the vacuum was applied and then washed with 70% ethanol for 5 s, denaturation solution (0.2 M NaOH) for 10 s, and washing buffer (10 mM Tris-acetate, pH 7.6) for 5 s. The vacuum was then released, and the beads were released into a PSQ 96 Plate Low (Biotage AB) containing 45 μl annealing buffer (20 mM Tris-acetate, 2 mM MgAc2, pH 7.6) and 0.5 μM sequencing primer.
Pyrosequencing reactions were performed according to the manufacturer's instructions using the PSQ 96 SNP Reagent Kit (Biotage AB), which contained the enzyme, substrate, and nucleotides. The assays were performed on the PSQ 96MA (Biotage AB) using the nucleotide dispensation orders shown in Table 2. The sample genotype was determined using the SNP Software (Biotage AB).
TABLE 2.
Dispensation orders for pyrosequencing
| Codon(s) | Primer(s) | Sequence analyzed | Dispensation order |
|---|---|---|---|
| 50/51 | 50/51F | ATTACCATGGAAAT/CGTAA/TTTCCCTAG | GATACATGATCAGTATGC |
| 59 | 59F | T/CGTGCAGTTACAACAT | ATCAGTGCAGTAC |
| 108 | 108R | G/T/CTTGTTCTT | AGCTCGTC |
| 164 | 164R | CCTCCTAT/AAATAAAACATTTATA | GCTCTAATAATAAGC |
| 540 | 540R | A/GAACTAACAAATTATGA | TAGAGCTACA |
| 581 | 581R | GC/GGAAGAAACATGATCAA | TGCGCAGACA |
| 436/437 | 436/437R | AC/GCAGA/CGGATT | TAGCTAGACTGA |
| 613 | 613R | AATGGGC/A/TAATAAA | GATGCTAGT |
Testing of artificially mixed parasite strains.
In order to determine how well the pyrosequencing assay would perform for identifying parasite samples from mixed infections, we mixed DNAs from selected parasites in different ratios and used these mixtures for PCR amplifications. 3D7 (wild type) and V1/S (mutant) were mixed for detecting dhfr gene mutations. HB3 (wild type) was mixed with K1 (mutations at codons 437 and 581) or W2 (mutations at codons 436, 437, and 613) to determine the dhps gene mutations. Since we did not have any laboratory strain with the Glu-540 mutation, we did not test the codon 540 mutation in the mixture experiments. Two different dilutions of DNA (40 parasites/μl and 400 parasites/μl) were used for each strain in the DNA mixture experiments. The ratios of mixed parasite lines, 3D7 and V1/S and HB3 and K1 or W2, were as follows: 1:20, 1:10, 1:6.6, 1:5, 1:3.3, 1:2.5, 1:1 (5%, 10%, 15%, 20%, 30%, 40%, and 50%), and also mixed in reverse order. The mixed parasite DNAs were PCR amplified, and the same primary PCR-amplified fragments were used in the nested secondary amplification for pyrosequencing, RFLP, and direct sequencing.
PCR-RFLP.
PCR-RFLP was performed using previously published methods (9, 14) with minor modifications. Amplified DNA from the nested PCR was subjected to restriction enzyme digestion. Briefly, 10 μl of PCR product was incubated with mutation-specific restriction enzymes according to the manufacturer's instructions (New England Biolabs, Beverly, MA, or Promega, Madison, WI). The restriction enzymes EcoRI, BsrGI, BsrI, and PsiI were used to detect the mutations of dhfr codons 51, 59, 108, and 164, respectively. The enzymes HindIII for codon 436, AvaII for codon 437, MwoI for codon 581, and XmnI for codon 613 were used for the digestion of dhps gene amplicons. The digested products were visualized by electrophoresis on 2% UltraPure Agarose-1000 (Invitrogen, Carlsbad, CA).
DNA sequencing.
To confirm the pyrosequencing results of each single strain and to compare the results with pyrosequencing and RFLP from the mixed infections, all primary PCR products of dhfr were sequenced with an ABI 3100 genetic analyzer (Applied Biosystems, Foster City, CA).
Cost estimates.
We calculated the approximate cost for genotyping mutations in dhfr (codons 50, 51, 59, 108,and 164) and dhps (codons 436, 437, 540, 581, and 613) by pyrosequencing, RFLP, and direct-sequencing methods. In estimating the cost, we included the actual cost of the agents that we used, except for the cost of primers, since they were available to us from our core facility. The equipment and labor costs were not included. For pyrosequencing, we included the cost involved with DNA isolation: PCRs, substrate, enzymes (polymerase, sulfurylase, luciferase, and apyrase), and reaction plates. In estimating the cost of conventional sequencing, we included expenses associated with DNA isolation, PCRs, cleanup columns, sequencing dye terminator reactions, buffer, polymer, plates, lids, and Hidi formamide (Applied Biosystems). RFLP cost estimates were based on expenses associated with DNA isolation, PCRs, agarose, DNA molecular weight standards, and restriction enzymes. These estimates were for a single experiment and did not include any repetitions when an experiment failed.
RESULTS
Sensitivity of pyrosequencing.
The sensitivity of pyrosequencing was determined by subjecting various concentrations of parasite DNAs (a DNA equivalent ranging from 40,000 parasites to 2 parasites) from six different laboratory isolates of P. falciparum for PCR amplifications. The results indicated that the pyrosequencing method was sensitive enough to detect two parasite genome equivalents of DNA for most of the dhfr mutations (except strain V1/S) and two to four parasite genome equivalents of DNA for dhps mutations in a 50-μl PCR mixture (Table 3). DNAs from at least four parasite genomes were required for detecting dhfr mutations from the V1/S strain. DNAs from two to four parasites were needed for detecting SNPs of dhps from W2, V1/S, FCR3, HB3, and K1. A minimum of four parasite equivalents of DNA was needed for genotyping the 3D7 strain (Table 3). Genotyping as determined by this method was accurate with respect to the identification of individual allelic forms of all six parasite isolates tested.
TABLE 3.
Sensitivity and specificity of pyrosequencing in detecting SNPs of a single strain
| Strain | Amino acid at codon (dhfr genotypes):
|
Sensitivityb
|
Amino acid at codon (dhps genotypes):
|
Sensitivity
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 51 | 59 | 108 | 164 | 4Pa | 2P | 436 | 437 | 540 | 581 | 613 | 4P | 2P | |
| 3D7 | C | N | C | S | I | + | + | S | G | K | A | A | + | − |
| V1/S | C | I | R | N | L | + | +/− | F | G | K | A | T | + | +/− |
| FCR3 | C | N | C | T | I | + | + | S | A | K | A | A | + | +/− |
| HB3 | C | N | C | N | I | + | + | S | A | K | A | A | + | +/− |
| K1 | C | N | N | N | I | + | + | S | G | K | G | A | + | +/− |
| W2 | C | I | R | N | I | + | + | F | G | K | A | S | + | + |
| Peru | NA | S | G | E | G | A | NA | NA | ||||||
| P. vivax SV4 | − | − | − | − | ||||||||||
| P. malariae Uganda I | − | − | − | − | ||||||||||
| P. ovale Nigeria I | − | − | − | − | ||||||||||
Parasite genome equivalents of DNA.
Positive (+) or negative (−) PCR results are indicated. +/−, the results were variable in repeat experiments; NA, not tested. The DNA sequences of all of the laboratory strains were reconfirmed by direct sequencing. The results were consistent between the different codons within the same gene. We tested each strain at DNA concentrations equivalent to 40,000, 4,000, 400, 40, 4, and 2 parasites. Genotyping results for dhfr and dhps were positive for all P. falciparum strains from 40,000 to 40 parasites and negative for P. vivax SV4, P. malariae Uganda I, and P. ovale Nigeria I at all six concentrations (data not shown).
Specificity of pyrosequencing.
The specificity of pyrosequencing was tested using DNAs from all species of four human plasmodium parasites, including six P. falciparum strains selected for this study. The pyrosequencing assays clearly identified all genotypes of P. falciparum DNA samples and were concordant with the known genotypes of the cultured parasite strains (Table 3). The results were highly specific for individual alleles. No erroneous typing of SNPs was found for any clone. In addition, no nonspecific amplification was found with P. vivax SV4, P. malariae Uganda I, and P. ovale Nigeria I (Table 3).
Detection of mixed parasite strains.
Since field isolates of parasites may contain both drug-sensitive and drug-resistant parasites, we wanted to investigate how the pyrosequencing assay would perform in detecting mixtures of DNAs. In addition, we also wanted to know how pyrosequencing compared with RFLP and direct sequencing in detecting mixed parasite strains. Known amounts of wild-type (3D7) and mutant (V1/S) parasites were mixed in different proportions, as explained in Materials and Methods (Tables 4, 5, 6, and 7). The genotype reading was automatic based on the pyrogram (Fig. 1A and B). In mixtures containing a high proportion of wild-type DNA compared to mutant DNA, as much as 10 to 15% mutant DNA was needed to detect the dhfr 51, 59, and 108 mutations using both 40 parasites/μl and 400 parasites/μl of DNA stocks (Tables 4 and 5). A similar sensitivity was observed when the mutant DNA was in higher proportion than the wild type. Interestingly, to detect the 164L mutation, at least 30% mutant DNA was needed for pyrosequencing, while only 15% was needed for RFLP when the wild-type DNA was at a higher ratio to mutant DNA in the mixture. In contrast, when mutant DNA was at a higher ratio, 10% wild-type DNA was sufficient for detection of the wild-type codon, indicating that the sensitivity of this assay to detect the 164L mutation is influenced by the relative proportions of wild-type DNA and mutant DNA in the mixture. For direct-sequencing analysis, most of the mutations could be detected at 15% (except 164L, which required 30%) with a low concentration of DNA (40 parasites/μl) and at 20 to 30% with higher concentrations of DNA (400 parasites/μl). In general, a minimum of 30% wild-type DNA was required for detecting wild-type codons by direct sequencing of mixed samples in both low and high DNA concentrations tested (Tables 4 and 5).
TABLE 4.
Comparison of different methods for detecting dhfr SNPs in mixed populations of P. falciparum parasites with different genotypes (DNA stock equal to 40 parasites/μl for each strain)a
| Codon | Parasite proportion (%)b
|
Pyroic
|
RFLP
|
DSd
|
||||
|---|---|---|---|---|---|---|---|---|
| 3D7 | V1/S | Amino acid code | No. of expts with positive reactions | Amino acid code | No. of expts with positive reactions | Amino acid code | No. of expts with positive reactions | |
| 51 | 100 | 0 | N | 3/3 | N | 3/3 | N | 3/3 |
| 95 | 5 | N | 3/3 | N | 3/3 | N | 3/3 | |
| 90 | 10 | N | 2/3 | N | 3/3 | N | 3/3 | |
| N/I | 1/3 | |||||||
| 85 | 15 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 80 | 20 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 70 | 30 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 60 | 40 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 50 | 50 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 40 | 60 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 30 | 70 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 20 | 80 | N/I | 3/3 | N/I | 2/3 | I | 3/3 | |
| I | 1/3 | |||||||
| 15 | 85 | N/I | 3/3 | N/I | 2/3 | I | 3/3 | |
| I | 1/3 | |||||||
| 10 | 90 | N/I | 2/3 | N/I | 2/3 | I | 3/3 | |
| I | 1/3 | I | 1/3 | |||||
| 5 | 95 | N/I | 1/3 | I | 3/3 | I | 3/3 | |
| I | 2/3 | |||||||
| 0 | 00 | I | 3/3 | I | 3/3 | I | 3/3 | |
| 59 | 100 | 0 | C | 3/3 | C | 3/3 | C | 3/3 |
| 95 | 5 | C | 3/3 | C | 3/3 | C | 3/3 | |
| 90 | 10 | C | 2/3 | C/R | 3/3 | C | 3/3 | |
| C/R | 1/3 | |||||||
| 85 | 15 | C/R | 2/3 | C/R | 3/3 | C/R | 3/3 | |
| 80 | 20 | C/R | 3/3 | C/R | 3/3 | C/R | 3/3 | |
| 70 | 30 | C/R | 3/3 | C/R | 3/3 | C/R | 3/3 | |
| 60 | 40 | C/R | 3/3 | C/R | 3/3 | C/R | 3/3 | |
| 50 | 50 | C/R | 3/3 | C/R | 3/3 | C/R | 3/3 | |
| 40 | 60 | C/R | 3/3 | C/R | 3/3 | C/R | 3/3 | |
| 30 | 70 | C/R | 3/3 | C/R | 3/3 | C/R | 3/3 | |
| 20 | 80 | C/R | 3/3 | C/R | 2/3 | R | 3/3 | |
| R | 1/3 | |||||||
| 15 | 85 | C/R | 2/3 | C/R | 2/3 | R | 3/3 | |
| R | 1/3 | |||||||
| 10 | 90 | C/R | 1/3 | C/R | 2/3 | R | 3/3 | |
| R | 2/3 | R | 1/3 | |||||
| 5 | 95 | R | 3/3 | R | 3/3 | R | 3/3 | |
| 0 | 00 | R | 3/3 | R | 3/3 | R | 3/3 | |
| 108 | 100 | 0 | S | 3/3 | S | 3/3 | S | 3/3 |
| 95 | 5 | S | 3/3 | S | 3/3 | S | 3/3 | |
| 90 | 10 | S | 2/3 | S | 3/3 | S | 3/3 | |
| S/N | 1/3 | |||||||
| 85 | 15 | S/N | 2/3 | S/N | 3/3 | S/N | 3/3 | |
| 80 | 20 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 70 | 30 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 60 | 40 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 50 | 50 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 40 | 60 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 30 | 70 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 20 | 80 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 15 | 85 | S/N | 2/3 | S/N | 3/3 | N | 3/3 | |
| N | 1/3 | |||||||
| 10 | 90 | S/N | 1/3 | S/N | 2/3 | N | 3/3 | |
| N | 2/3 | N | 1/3 | |||||
| 5 | 95 | N | 3/3 | S/N | 1/3 | N | 3/3 | |
| N | 2/3 | |||||||
| 0 | 00 | N | 3/3 | N | 3/3 | N | 3/3 | |
| 164 | 100 | 0 | I | 3/3 | I | 3/3 | I | 3/3 |
| 95 | 5 | I | 3/3 | I | 3/3 | I | 3/3 | |
| 90 | 10 | I | 3/3 | I | 3/3 | I | 3/3 | |
| 85 | 15 | I | 3/3 | I/L | 3/3 | I | 3/3 | |
| 80 | 20 | I | 3/3 | I/L | 3/3 | I | 3/3 | |
| 70 | 30 | I/L | 3/3 | I/L | 3/3 | I/L | 3/3 | |
| 60 | 40 | I/L | 3/3 | I/L | 3/3 | I/L | 3/3 | |
| 50 | 50 | I/L | 3/3 | I/L | 3/3 | I/L | 3/3 | |
| 40 | 60 | I/L | 3/3 | I/L | 3/3 | I/L | 3/3 | |
| 30 | 70 | I/L | 3/3 | I/L | 3/3 | I/L | 3/3 | |
| 20 | 80 | I/L | 3/3 | I/L | 3/3 | L | 3/3 | |
| 15 | 85 | I/L | 2/3 | I/L | 3/3 | L | 3/3 | |
| L | 1/3 | |||||||
| 10 | 90 | I/L | 2/3 | L | 3/3 | L | 3/3 | |
| L | 1/3 | |||||||
| 5 | 95 | L | 3/3 | L | 3/3 | L | 3/3 | |
| 0 | 00 | L | 3/3 | L | 3/3 | L | 3/3 | |
Each concentration was tested in triplicate.
DNA proportion of wild-type parasites (3D7) and mutant parasites (V1/S).
Pyro, Pyrosequencing.
DS, direct sequencing.
TABLE 5.
Comparison of different methods for detecting dhfr SNPs in mixed population of P. falciparum parasites with different genotypes (DNA stock equal to 400 parasites/μl for each strain)a
| Codon | Parasite proportion (%)
|
Pyro
|
RFLP
|
DS
|
||||
|---|---|---|---|---|---|---|---|---|
| 3D7 | V1/S | Amino acid code | No. of expts with positive reactions | Amino acid code | No. of expts with positive reactions | Amino acid code | No. of expts with positive reactions | |
| 51 | 100 | 0 | N | 3/3 | N | 3/3 | N | 3/3 |
| 95 | 5 | N | 3/3 | N | 3/3 | N | 3/3 | |
| 90 | 10 | N/I | 3/3 | N/I | 3/3 | N | 3/3 | |
| 85 | 15 | N/I | 3/3 | N/I | 3/3 | N | 3/3 | |
| 80 | 20 | N/I | 3/3 | N/I | 3/3 | N | 1/3 | |
| N/I | 2/3 | |||||||
| 70 | 30 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 60 | 40 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 50 | 50 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 40 | 60 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 30 | 70 | N/I | 3/3 | N/I | 3/3 | N/I | 3/3 | |
| 20 | 80 | N/I | 3/3 | N/I | 3/3 | I | 3/3 | |
| 15 | 85 | N/I | 3/3 | N/I | 3/3 | I | 3/3 | |
| 10 | 90 | N/I | 3/3 | N/I | 3/3 | I | 3/3 | |
| 5 | 95 | I | 3/3 | I | 3/3 | I | 3/3 | |
| 0 | 100 | I | 3/3 | I | 3/3 | I | 3/3 | |
| 59 | 100 | 0 | C | 3/3 | C | 3/3 | C | 3/3 |
| 95 | 5 | C | 3/3 | C | 3/3 | C | 3/3 | |
| 90 | 10 | C/R | 3/3 | C/R | 3/3 | C | 3/3 | |
| 85 | 15 | C/R | 3/3 | C/R | 3/3 | C | 3/3 | |
| 80 | 20 | C/R | 3/3 | C/R | 3/3 | C | 2/3 | |
| C/R | 1/3 | |||||||
| 70 | 30 | C/R | 3/3 | C/R | 3/3 | C/R | 3/3 | |
| 60 | 40 | C/R | 3/3 | C/R | 3/3 | C/R | 3/3 | |
| 50 | 50 | C/R | 3/3 | C/R | 3/3 | C/R | 3/3 | |
| 40 | 60 | C/R | 3/3 | C/R | 3/3 | C/R | 3/3 | |
| 30 | 70 | C/R | 3/3 | C/R | 3/3 | C/R | 2/3 | |
| R | 1/3 | |||||||
| 20 | 80 | C/R | 3/3 | C/R | 3/3 | C/R | 1/3 | |
| R | 2/3 | |||||||
| 15 | 85 | C/R | 3/3 | C/R | 3/3 | R | 3/3 | |
| 10 | 90 | C/R | 3/3 | C/R | 3/3 | R | 3/3 | |
| 5 | 95 | R | 3/3 | R | 3/3 | R | 3/3 | |
| 0 | 100 | R | 3/3 | R | 3/3 | R | 3/3 | |
| 108 | 100 | 0 | S | 3/3 | S | 3/3 | S | 3/3 |
| 95 | 5 | S | 3/3 | S | 3/3 | S | 3/3 | |
| 90 | 10 | S | 3/3 | S | 3/3 | S | 3/3 | |
| 85 | 15 | S | 1/3 | S/N | 3/3 | S | 3/3 | |
| S/N | 2/3 | |||||||
| 80 | 20 | S/N | 3/3 | S/N | 3/3 | S | 1/3 | |
| S/N | 2/3 | |||||||
| 70 | 30 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 60 | 40 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 50 | 50 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 40 | 60 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 30 | 70 | S/N | 3/3 | S/N | 3/3 | S/N | 3/3 | |
| 20 | 80 | S/N | 3/3 | S/N | 3/3 | N | 3/3 | |
| 15 | 85 | S/N | 3/3 | S/N | 3/3 | N | 3/3 | |
| 10 | 90 | S/N | 2/3 | S/N | 3/3 | N | 3/3 | |
| N | 1/3 | |||||||
| 5 | 95 | N | 3/3 | S/N | 3/3 | N | 3/3 | |
| 0 | 100 | N | 3/3 | N | 3/3 | N | 3/3 | |
| 164 | 100 | 0 | I | 3/3 | I | 3/3 | I | 3/3 |
| 95 | 5 | I | 3/3 | I | 3/3 | I | 3/3 | |
| 90 | 10 | I | 3/3 | I | 3/3 | I | 3/3 | |
| 85 | 15 | I | 3/3 | I/L | 3/3 | I | 3/3 | |
| 80 | 20 | I | 3/3 | I/L | 3/3 | I | 3/3 | |
| 70 | 30 | I/L | 3/3 | I/L | 3/3 | I/L | 3/3 | |
| 60 | 40 | I/L | 3/3 | I/L | 3/3 | I/L | 3/3 | |
| 50 | 50 | I/L | 3/3 | I/L | 3/3 | I/L | 3/3 | |
| 40 | 60 | I/L | 3/3 | I/L | 3/3 | I/L | 3/3 | |
| 30 | 70 | I/L | 3/3 | I/L | 3/3 | I/L | 3/3 | |
| 20 | 80 | I/L | 3/3 | I/L | 3/3 | L | 3/3 | |
| 15 | 85 | I/L | 3/3 | I/L | 2/3 | L | 3/3 | |
| L | 1/3 | |||||||
| 10 | 90 | I/L | 3/3 | I/L | 1/3 | L | 3/3 | |
| L | 2/3 | |||||||
| 5 | 95 | L | 3/3 | L | 3/3 | L | 3/3 | |
| 0 | 100 | L | 3/3 | L | 3/3 | L | 3/3 | |
See Table 4 for abbreviations and definitions.
TABLE 6.
Comparison of different methods for detecting dhps SNPs in mixed population of P. falciparum parasites with different genotypes (DNA stock equal to 40 parasites/μl for each strain)a
| Codon | Parasite proportion (%)
|
Pyro
|
RFLP
|
||||
|---|---|---|---|---|---|---|---|
| HB3 | K1 | W2 | Amino acid code | No. of expts with positive reactions | Amino acid code | No. of expts with positive reactions | |
| 437 | 100 | 0 | A | 3 | A | 3 | |
| 95 | 5 | A | 3 | A | 3 | ||
| 90 | 10 | A/G | 3 | A | 3 | ||
| 85 | 15 | A/G | 3 | A | 3 | ||
| 80 | 20 | A/G | 3 | A/G | 3 | ||
| 70 | 30 | A/G | 3 | A/G | 3 | ||
| 60 | 40 | A/G | 3 | A/G | 3 | ||
| 50 | 50 | A/G | 3 | A/G | 3 | ||
| 40 | 60 | A/G | 3 | A/G | 3 | ||
| 30 | 70 | A/G | 3 | A/G | 3 | ||
| 20 | 80 | A/G | 3 | A/G | 3 | ||
| 15 | 85 | G | 3 | A/G | 1 | ||
| G | 2 | ||||||
| 10 | 90 | G | 3 | A/G | 1 | ||
| G | 2 | ||||||
| 5 | 95 | G | 3 | G | 3 | ||
| 0 | 100 | G | 3 | G | 3 | ||
| 581 | 100 | 0 | A | 3 | A | 3 | |
| 95 | 5 | A | 3 | A | 1 | ||
| A/G | 2 | ||||||
| 90 | 10 | A/G | 3 | A/G | 3 | ||
| 85 | 15 | A/G | 3 | A/G | 3 | ||
| 80 | 20 | A/G | 3 | A/G | 3 | ||
| 70 | 30 | A/G | 3 | A/G | 3 | ||
| 60 | 40 | A/G | 3 | A/G | 3 | ||
| 50 | 50 | A/G | 3 | A/G | 3 | ||
| 40 | 60 | A/G | 3 | A/G | 3 | ||
| 30 | 70 | A/G | 3 | A/G | 3 | ||
| 20 | 80 | A/G | 3 | A/G | 3 | ||
| 15 | 85 | A/G | 3 | A/G | 1 | ||
| G | 2 | ||||||
| 10 | 90 | A/G | 3 | A/G | 1 | ||
| G | 2 | ||||||
| 5 | 95 | G | 3 | G | 3 | ||
| 0 | 100 | G | 3 | G | 3 | ||
| 436 | 100 | 0 | S | 3 | S | 3 | |
| 95 | 5 | S | 1 | S | 3 | ||
| S/F | 2 | ||||||
| 90 | 10 | S/F | 3 | S | 3 | ||
| 85 | 15 | S/F | 3 | S/F | 3 | ||
| 80 | 20 | S/F | 3 | S/F | 3 | ||
| 70 | 30 | S/F | 3 | S/F | 3 | ||
| 60 | 40 | S/F | 3 | S/F | 3 | ||
| 50 | 50 | S/F | 3 | S/F | 3 | ||
| 40 | 60 | S/F | 3 | S/F | 3 | ||
| 30 | 70 | S/F | 3 | S/F | 3 | ||
| 20 | 80 | S/F | 3 | S/F | 3 | ||
| 15 | 85 | S/F | 3 | S/F | 3 | ||
| 10 | 90 | S/F | 3 | F | 3 | ||
| 5 | 95 | F | 3 | F | 3 | ||
| 0 | 100 | F | 3 | F | 3 | ||
| 613 | 100 | 0 | A | 3 | A | 3 | |
| 95 | 5 | A | 1 | A | 3 | ||
| A/S | 2 | ||||||
| 90 | 10 | A/S | 3 | A | 3 | ||
| 85 | 15 | A/S | 3 | A/S | 3 | ||
| 80 | 20 | A/S | 3 | A/S | 3 | ||
| 70 | 30 | A/S | 3 | A/S | 3 | ||
| 60 | 40 | A/S | 3 | A/S | 3 | ||
| 50 | 50 | A/S | 3 | A/S | 3 | ||
| 40 | 60 | A/S | 3 | A/S | 3 | ||
| 30 | 70 | A/S | 3 | A/S | 3 | ||
| 20 | 80 | A/S | 3 | A/S | 3 | ||
| 15 | 85 | A/S | 3 | A/S | 3 | ||
| 10 | 90 | S | 3 | S | 3 | ||
| 5 | 95 | S | 3 | S | 3 | ||
| 0 | 100 | S | 3 | S | 3 | ||
Each concentration was tested in triplicate. See Table 4 for abbreviations and definitions.
TABLE 7.
Comparison of different methods for detecting dhps SNPs in mixed population of P. falciparum parasites with different genotypes (DNA stock equal to 400 parasites/μl for each strain)a
| Codon | Parasite proportion (%)
|
Pyro
|
RFLP
|
||||
|---|---|---|---|---|---|---|---|
| HB3 | K1 | W2 | Amino acid code | No. of expts with positive reactions | Amino acid code | No. of expts with positive reactions | |
| 437 | 100 | 0 | A | 3 | A | 3 | |
| 95 | 5 | A | 2 | A | 3 | ||
| A/G | 1 | ||||||
| 90 | 10 | A/G | 3 | A | 3 | ||
| 85 | 15 | A/G | 3 | A/G | 3 | ||
| 80 | 20 | A/G | 3 | A/G | 3 | ||
| 70 | 30 | A/G | 3 | A/G | 3 | ||
| 60 | 40 | A/G | 3 | A/G | 3 | ||
| 50 | 50 | A/G | 3 | A/G | 3 | ||
| 40 | 60 | A/G | 3 | A/G | 3 | ||
| 30 | 70 | A/G | 3 | A/G | 3 | ||
| 20 | 80 | A/G | 3 | A/G | 3 | ||
| 15 | 85 | A/G | 3 | A/G | 3 | ||
| 10 | 90 | A/G | 3 | A/G | 3 | ||
| 5 | 95 | G | 3 | A/G | 3 | ||
| 581 | 100 | 0 | A | 3 | A | 3 | |
| 95 | 5 | A | 3 | A/G | 3 | ||
| 90 | 10 | A/G | 3 | A/G | 3 | ||
| 85 | 15 | A/G | 3 | A/G | 3 | ||
| 80 | 20 | A/G | 3 | A/G | 3 | ||
| 70 | 30 | A/G | 3 | A/G | 3 | ||
| 60 | 40 | A/G | 3 | A/G | 3 | ||
| 50 | 50 | A/G | 3 | A/G | 3 | ||
| 40 | 60 | A/G | 3 | A/G | 3 | ||
| 30 | 70 | A/G | 3 | A/G | 3 | ||
| 20 | 80 | A/G | 3 | A/G | 3 | ||
| 15 | 85 | A/G | 3 | A/G | 2 | ||
| G | 1 | ||||||
| 10 | 90 | A/G | 3 | G | 3 | ||
| 5 | 95 | G | 3 | G | 3 | ||
| 0 | 100 | G | 3 | G | 3 | ||
| 436 | 100 | 0 | S | 3 | S | 3 | |
| 95 | 5 | S/F | 3 | S | 3 | ||
| 90 | 10 | S/F | 3 | S | 3 | ||
| 85 | 15 | S/F | 3 | S/F | 3 | ||
| 80 | 20 | S/F | 3 | S/F | 3 | ||
| 70 | 30 | S/F | 3 | S/F | 3 | ||
| 60 | 40 | S/F | 3 | S/F | 3 | ||
| 50 | 50 | S/F | 3 | S/F | 3 | ||
| 40 | 60 | S/F | 3 | S/F | 3 | ||
| 30 | 70 | S/F | 3 | S/F | 3 | ||
| 20 | 80 | S/F | 2 | S/F | 3 | ||
| F | 1 | ||||||
| 15 | 85 | F | 3 | S/F | 3 | ||
| 10 | 90 | F | 3 | F | 3 | ||
| 5 | 95 | F | 3 | F | 3 | ||
| 0 | 100 | F | 3 | F | 3 | ||
| 613 | 100 | 0 | A | 3 | A | 3 | |
| 95 | 5 | A/S | 3 | A | 3 | ||
| 90 | 10 | A/S | 3 | A | 3 | ||
| 85 | 15 | A/S | 3 | A/S | 3 | ||
| 80 | 20 | A/S | 3 | A/S | 3 | ||
| 70 | 30 | A/S | 3 | A/S | 3 | ||
| 60 | 40 | A/S | 3 | A/S | 3 | ||
| 50 | 50 | A/S | 3 | A/S | 3 | ||
| 40 | 60 | A/S | 3 | A/S | 3 | ||
| 30 | 70 | A/S | 3 | A/S | 3 | ||
| 20 | 80 | A/S | 3 | A/S | 3 | ||
| 15 | 85 | A/S | 3 | A/S | 3 | ||
| 10 | 90 | S | 3 | A/S | 3 | ||
| 5 | 95 | S | 3 | A/S | 3 | ||
| 0 | 100 | S | 3 | S | 3 | ||
Each concentration was tested in triplicate. See Table 4 for abbreviations and definitions.
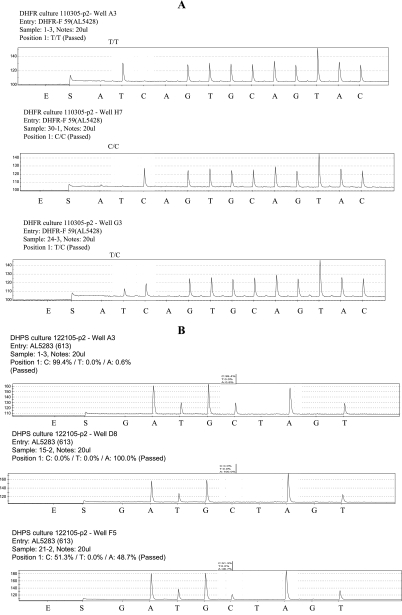
FIG. 1.
Pyrograms generated from a PSQ 96MA showing peak profiles for dhfr at codon 59 (A) and dhps at codon 613 (B), given here to illustrate how the results were reported. SNP sites are marked by open areas on the pyrogram. (A) T/T, wild type; C/C, mutant allele; T/C, mixed alleles. (B) Three allelic types at the same position could also be reported. The percentages of SNPs were used for double checking the scores of alleles, in addition to the SNP calling pattern.
In detecting the dhps mutations, pyrosequencing required as little as 10% mutant DNA to detect the 437 and 581 mutations consistently using mixtures of HB3 wild-type and K1 mutant DNAs (Table 6), and as little as 5% mutant DNA was sufficient to detect mutations in codons 436 and 613 in an HB3 and W2 mutant mixture (Table 7). Ten to 15% wild-type DNA was needed to detect a wild-type codon in the mixtures. Compared to pyrosequencing, RFLP required slightly larger amounts of mutant DNA, ranging from 15 to 20%, to detect most codons (except codon 581, which required only 5%). In detecting wild-type codons in the mixed DNA, RFLP and pyrosequencing showed only small differences in the minimum amounts of DNA required for detection (5 to 15% for RFLP and 10 to 15% for pyrosequencing).
Cost analysis.
The cost of reagents and supplies for each method was also calculated in our study, as explained in Materials and Methods. This was an approximate estimation and would vary depending upon the commercial source of reagents used. The cost estimate for pyrosequencing of these two genes was $11.40 per sample ($2.28 per SNP); it was $18.30 per sample ($3.66 per SNP) for conventional sequencing and $32.90 per sample ($6.58 per SNP) for RFLP. In addition, restriction digestion with BsrGI for codon 59 and AvaII for codon 437 often required repetition due to incomplete digestion, thereby increasing the cost of the RFLP method (this additional cost is not considered in the above estimate).
DISCUSSION
In this report, we describe the development of a pyrosequencing method for the detection of SNPs associated with antifolate resistance in the dhfr and dhps genes of P. falciparum. Pyrosequencing was sensitive enough to detect two to four parasite genome equivalents of DNA from six different laboratory isolates of P. falciparum and was highly specific in differentiating P. falciparum from other human malaria parasites. Therefore, it can be reliably used in areas where P. falciparum coexists with other species of human malaria parasites. Overall, pyrosequencing was a highly reliable method for genotyping, and it was also faster and less expensive than the commonly used RFLP method and direct sequencing.
Irrespective of the original stock concentrations of DNA template used for PCR amplification, the pyrosequencing and RFLP methods showed similar levels of sensitivity in detecting the mutant forms of dhfr SNPs, with the exception of codon 164 in mixed samples. Pyrosequencing required the presence of at least 30% mutant DNA in mixed samples to detect the 164L mutation, while RFLP was sensitive enough to detect this mutation in as little as 15% of a mixture. The reason for the low sensitivity of pyrosequencing to detect mutation at codon 164 in mixed samples is unclear. It could be due to the constraints imposed by the residues surrounding the mutation or the sequencing primer used in the assay. In detecting SNPs of the dhps gene, especially mutants, pyrosequencing was slightly more sensitive than RFLP. It is important to point out here that the same primary PCR-amplified products were used as templates for nested PCRs involving pyrosequencing, RFLP, and direct sequencing.
Conventional sequencing is more costly and time-consuming than pyrosequencing for large-scale genotyping studies. The present study also demonstrated that the conventional sequencing method was less sensitive than pyrosequencing in detecting mixed infections. Often, direct sequencing required 30% mutant or wild-type DNA in mixed samples to detect SNPs. In a previous study, it was found that detection of the dhps 540 mutation by a conventional sequencing method required at least 20 to 30% mutant DNA in the mixed samples (26). Another study, using dhps of the rodent parasite Plasmodium chabaudi, contradicted these results. It reported that conventional sequencing could detect even lower concentrations of DNA, and this was attributed to its low background fluorescence (16). However, our results here showed that conventional sequencing still required a higher ratio of target DNA to detect the minor allele, even with low background fluorescence.
Although RFLP and pyrosequencing showed similar levels of sensitivity for detecting many mutations, the main disadvantage with RFLP was the loss of specificity when PCR-amplified fragments did not give a consistent digestion pattern. We often had to repeat RFLP experiments due to incomplete digestion of target DNA by the restriction enzymes to confirm the results. In our experience, when incomplete digestion was seen with enzyme BsrGI for codon 59 and AvaII for codon 437, the mixed alleles could not easily be verified. This misclassification of a single infection as a mixed infection by RFLP would be even more problematic when applied to field samples.
The analysis with pyrosequencing also indicated that this technique gave different levels of sensitivity in detecting different SNPs in mixed samples. We speculate that this is due to the target sequences, which are A+T rich, since pyrosequencing is done at low temperatures where secondary structure might have a higher impact. When we used parasite DNAs from single isolates, the pyrosequencing and conventional sequencing methods showed similar sensitivity levels. However, pyrosequencing appeared to be more sensitive than conventional sequencing in identifying genotypes in mixed samples. In pyrosequencing, genotyping is determined based on the individual peak heights of each nucleotide dispensation. Conventional sequencing overlaps the fluorescent signal for every possible nucleotide at that specific size. It is probably due to this difference that it may be easier to call genotypes by pyrosequencing, even in the presence of a minor allele, than by conventional sequencing.
Pyrosequencing also has other advantages in detecting SNPs for drug resistance. Large-scale pyrosequencing has recently been compared to the TaqMan technique and found to yield comparable results, suggesting that pyrosequencing could be adopted for high-throughput genotyping (19). Pyrosequencing can be performed with direct-PCR or nested-PCR products, as long as one of the PCR primers is biotinylated. Since pyrosequencing gives a nucleotide sequence for short DNA fragments, it is easier to identify a cluster of mutations within close proximity using a single reaction (e.g., codons 50/51 and 436/437 in dhfr and dhps, respectively). In addition, pyrosequencing can also detect any potential new mutation that had not been previously described within that region, as well as easily detecting multiple allelic types, as in the case of codon 108: AGC (S) to AAC (N) or ACC (T).
The procedure of pyrosequencing is straightforward, and personnel can be easily trained to perform the technique. The reagents used for pyrosequencing were relatively inexpensive compared to those for RFLP and conventional sequencing. RFLP was at least threefold more expensive than pyrosequencing in reagent costs alone. Repetition of RFLP experiments further escalated the cost of reagents for the procedure.
Pyrosequencing was also faster than RFLP and direct sequencing. After the PCR step, samples in the 96-well plates can be finished on a PSQ 96MA machine within 9 to 20 min by our protocols. The autocalling feature in the pyrosequencing software makes the final calling of genotypes not only simple, but also objective, while RFLP relies on manual reading of gel pictures, which becomes cumbersome when the restriction enzyme digestion is incomplete. In summary, the present study has shown that pyrosequencing is a reliable and less expensive high-throughput method that could be used as an alternative method for genotyping mutations associated with drug resistance in molecular surveillance studies of malaria.
Acknowledgments
Financial support from the CDC Antimicrobial Resistance Working Group is appreciated.
We thank Robert Wohlhueter and Brian Holloway of the Biotechnology Core Facility, CCID, CDC, for their suggestions and support. We are grateful to Christopher Plowe for donation of a control DNA sample used in this study.
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Abdel-Muhsin, A. M., L. C. Ranford-Cartwright, A. R. Medani, S. Ahmed, S. Suleiman, B. Khan, P. Hunt, D. Walliker, and H. A. Babiker. 2002. Detection of mutations in the Plasmodium falciparum dihydrofolate reductase (dhfr) gene by dot-blot hybridization. Am. J. Trop. Med. Hyg. 67:24-27. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadian, A., M. Ehn, and S. Hober. 2006. Pyrosequencing: history, biochemistry and future. Clin. Chim. Acta 363:83-94. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed, A., D. Bararia, S. Vinayak, M. Yameen, S. Biswas, V. Dev, A. Kumar, M. A. Ansari, and Y. D. Sharma. 2004. Plasmodium falciparum isolates in India exhibit a progressive increase in mutations associated with sulfadoxine-pyrimethamine resistance. Antimicrob. Agents Chemother. 48:879-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alifrangis, M., S. Enosse, R. Pearce, C. Drakeley, C. Roper, I. F. Khalil, W. M. Nkya, A. M. Ronn, T. G. Theander, and I. C. Bygbjerg. 2005. A simple, high-throughput method to detect Plasmodium falciparum single nucleotide polymorphisms in the dihydrofolate reductase, dihydropteroate synthase, and P. falciparum chloroquine resistance transporter genes using polymerase chain reaction and enzyme-linked immunosorbent assay-based technology. Am. J. Trop. Med. Hyg. 72:155-162. [PubMed] [Google Scholar]
- 5.Anderson, T. J., and C. Roper. 2005. The origins and spread of antimalarial drug resistance: lessons for policy makers. Acta Trop. 94:269-280. [DOI] [PubMed] [Google Scholar]
- 6.Basco, L. K., R. Tahar, A. Keundjian, and P. Ringwald. 2000. Sequence variations in the genes encoding dihydropteroate synthase and dihydrofolate reductase and clinical response to sulfadoxine-pyrimethamine in patients with acute uncomplicated falciparum malaria. J. Infect. Dis. 182:624-628. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, D. R., P. Wang, M. Read, W. M. Watkins, P. F. Sims, and J. E. Hyde. 1994. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase:dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulfadoxine. Eur. J. Biochem. 224:397-405. [DOI] [PubMed] [Google Scholar]
- 8.Clarke, S. C. 2005. Pyrosequencing: nucleotide sequencing technology with bacterial genotyping applications. Exp. Rev. Mol. Diagn. 5:947-953. [DOI] [PubMed] [Google Scholar]
- 9.Contreras, C. E., J. F. Cortese, A. Caraballo, and C. V. Plowe. 2002. Genetics of drug-resistant Plasmodium falciparum malaria in the Venezuelan state of Bolivar. Am. J. Trop. Med. Hyg. 67:400-405. [DOI] [PubMed] [Google Scholar]
- 10.Cowman, A. F., M. J. Morry, B. A. Biggs, G. A. Cross, and S. J. Foote. 1988. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 85:9109-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis, J., M. T. Duraisingh, J. K. Trigg, H. Mbwana, D. C. Warhurst, and C. F. Curtis. 1996. Direct evidence that asparagine at position 108 of the Plasmodium falciparum dihydrofolate reductase is involved in resistance to antifolate drugs in Tanzania. Trans. R. Soc. Trop. Med. Hyg. 90:678-680. [DOI] [PubMed] [Google Scholar]
- 12.Duraisingh, M. T., J. Curtis, and D. C. Warhurst. 1998. Plasmodium falciparum: detection of polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes by PCR and restriction digestion. Exp. Parasitol. 89:1-8. [DOI] [PubMed] [Google Scholar]
- 13.Durand, R. E. J., J. Sayeh, J.-F. Delabre, A. Marmorat-Khuong, J. P. Di Piazza, and J. Le Bras. 2000. Use of molecular beacons to detect an antifolate resistance-associated mutation in Plasmodium falciparum. Antimicrob. Agents Chemother. 44:3461-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberl, K. J., T. Jelinek, A. O. Aida, G. Peyerl-Hoffmann, C. Heuschkel, A. O. el Valy, and E. M. Christophel. 2001. Prevalence of polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes of Plasmodium falciparum isolates from southern Mauritania. Trop. Med. Int. Health 6:756-760. [DOI] [PubMed] [Google Scholar]
- 15.Gregson, A., and C. V. Plowe. 2005. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol. Rev. 57:117-145. [DOI] [PubMed] [Google Scholar]
- 16.Hunt, P., R. Fawcett, R. Carter, and D. Walliker. 2005. Estimating SNP proportions in populations of malaria parasites by sequencing: validation and applications. Mol. Biochem. Parasitol. 143:173-182. [DOI] [PubMed] [Google Scholar]
- 17.Kublin, J. G., F. K. Dzinjalamala, D. D. Kamwendo, E. M. Malkin, J. F. Cortese, L. M. Martino, R. A. Mukadam, S. J. Rogerson, A. G. Lescano, M. E. Molyneux, P. A. Winstanley, P. Chimpeni, T. E. Taylor, and C. V. Plowe. 2002. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J. Infect. Dis. 185:380-388. [DOI] [PubMed] [Google Scholar]
- 18.Nair, S., A. Brockman, L. Paiphun, F. Nosten, and T. J. Anderson. 2002. Rapid genotyping of loci involved in antifolate drug resistance in Plasmodium falciparum by primer extension. Int. J. Parasitol. 32:852-858. [DOI] [PubMed] [Google Scholar]
- 19.Nordfors, L., J. Marten., G. Sandberg, C. Lavebratt, S. Sengul, M. Schalling, and P. Arner. 2002. Large-scale genotyping of single nucleotide polymorphisms by PyrosequencingTM and validation against the 5′ nuclease (Taqman®) assay. Hum. Mutat. 19:395-401. [DOI] [PubMed] [Google Scholar]
- 20.Pati, N., V. Schowinsky, O. Kokanovic, V. Magnuson, and S. Ghosh. 2005. A comparison between SNaPshot, pyrosequencing, and biplex invader SNP genotyping methods: accuracy, cost, and throughput. J. Biochem. Biophys. Methods 60:1-12. [DOI] [PubMed] [Google Scholar]
- 21.Peterson, D. S., D. Walliker, and T. E. Wellems. 1988. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc. Natl. Acad. Sci. USA 85:9114-9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plowe, C. V. 2003. Monitoring antimalarial drug resistance: making the most of the tools at hand. J. Exp. Biol. 206:3745-3752. [DOI] [PubMed] [Google Scholar]
- 23.Plowe, C. V., A. Djimde, M. Bouare, O. Doumbo, and T. E. Wellems. 1995. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am. J. Trop. Med. Hyg. 52:565-568. [DOI] [PubMed] [Google Scholar]
- 24.Plowe, C. V., J. F. Cortese, A. Djimde, O. C. Nwanyanwu, W. M. Watkins, P. A. Winstanley, J. G. Estrada-Franco, R. E. Mollinedo, J. C. Avila, J. L. Cespedes, D. Carter, and O. K. Doumbo. 1997. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J. Infect. Dis. 176:1590-1596. [DOI] [PubMed] [Google Scholar]
- 25.Reeder, J. C., K. H. Rieckmann, B. Genton, K. Lorry, B. Wines, and A. F. Cowman. 1996. Point mutations in the dihydrofolate reductase and dihydropteroate synthetase genes and in vitro susceptibility to pyrimethamine and cycloguanil of Plasmodium falciparum isolates from Papua New Guinea. Am. J. Trop. Med. Hyg. 55:209-213. [DOI] [PubMed] [Google Scholar]
- 26.Shaio, M.-F., P. Wang, C.-S. Lee, P. F. D. Sims, and J. E. Hyde. 1998. Development and comparison of quantitative assays for the dihydropteroate synthetase codon 540 mutation associated with sulfadoxine resistance in Plasmodium falciparum. Parasitology 116:203-210. [DOI] [PubMed] [Google Scholar]
- 27.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 28.Wang, P., M. Read, P. F. Sims, and J. E. Hyde. 1997. Sulfadoxine resistance in the human malaria parasite Plasmodium falciparum is determined by mutations in dihydropteroate synthetase and an additional factor associated with folate utilization. Mol. Microbiol. 23:979-986. [DOI] [PubMed] [Google Scholar]
- 29.Wilson, P. E., A. P. Alker, and S. R. Meshnick. 2005. Real-time PCR methods for monitoring antimalarial drug resistance. Trends Parasitol. 21:278-283. [DOI] [PubMed] [Google Scholar]
- 30.Zolg, J. W., G. X. Chen, and J. R. Plitt. 1990. Detection of pyrimethamine resistance in Plasmodium falciparum by mutation-specific polymerase chain reaction. Mol. Biochem. Parasitol. 39:257-265. [DOI] [PubMed] [Google Scholar]