Abstract
Amebic infections involving the central nervous system are rare and difficult to diagnose. Magnetic resonance imaging (MRI) at timed intervals may be helpful, where scans reveal enhancing lesions and increased signal. We report a unique case of granulomatous amebic encephalitis that was proven pathologically with progressive radiological findings on MRI.
CASE REPORT
A 51-year-old white male who had received a renal transplant 3 months earlier presented with a 3-day history of left-sided facial twitching. The symptoms would last 30 s, occurring every 30 min. Over the next week, he developed left arm and leg numbness and weakness and, with a presumptive diagnosis of stroke, was subsequently admitted to the hospital. The patient's other symptoms included gastritis and fatigue. He also had developed skin ulcerations on his legs 3 weeks prior to admission.
The patient's past medical history was significant for type 1 diabetes mellitus, from which he suffered for 24 years. He had developed end-stage renal disease and received his first renal transplant in 1995. In 2000, he had lost his transplant due to chronic rejection and had been put back on dialysis for the next 4 years. Three months prior to admission, he had undergone his second renal transplant. His medications included prednisone (20 mg daily), mycophenolate, and tacrolimus. His other past medical history included coronary artery disease, with a coronary artery bypass graft in 2001. He had a history of hypertension, peripheral vascular disease, and diabetic neuropathy.
The patient was a former general contractor. He considered himself physically active and frequently played indoor volleyball on sand courts. He also spent time gardening and fishing. One month prior to admission, he had gone on a fishing trip to a lake in northern Utah, although he denied direct water exposure.
The neurological examination revealed an intact mental status. Cranial nerve abnormalities included mildly anisocoric pupils, with the left pupil measuring 4 mm and the right pupil measuring 3 mm. A mild left lower facial weakness and flattened left nasolabial fold were also observed. Although his motor tone was normal, there was some decreased bulk in the distal motor groups. A left pronator drift was present. Testing showed that the remainder of his strength was intact. The patient had slowed rapid alternating movements of his left hand and dysmetria of the left upper extremity. He had trace patellar reflexes and absent ankle reflexes. Plantar responses were equivocal bilaterally, and there was no clonus observed. Romberg's sign was present. Sensory examination revealed decreased pinprick and temperature sensation in his hands and feet.
The physical exam was also notable for multiple macular-papular erythematous lesions, predominantly on his anterior legs (Fig. 1). Otherwise, the examination results were normal.
FIG. 1.
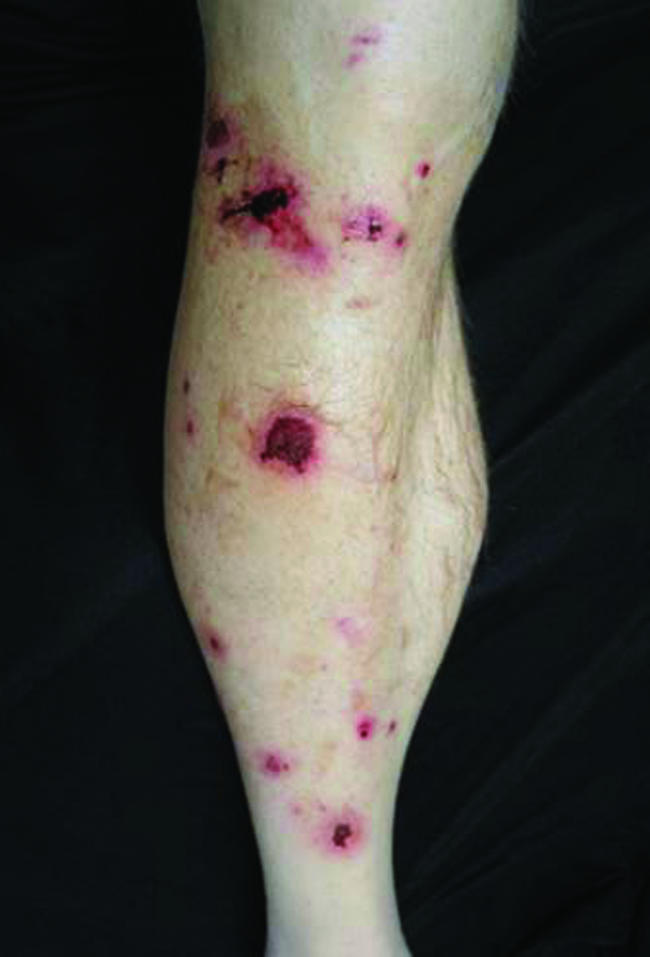
Lower leg lesions.
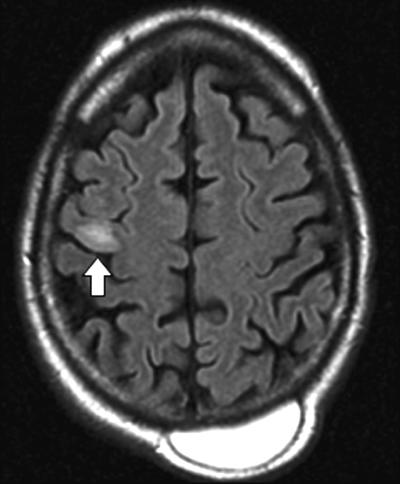
MRI of the brain, performed without contrast on admission, revealed a slight area of restricted diffusion on diffusion-weighted imaging on the right frontal lobe and increased signal on the fluid-attenuated inversion recovery (FLAIR) sequence (Fig. 2). The patient was admitted for possible subacute ischemic stroke causing seizures secondary to cortical irritation. He was given intravenous phenytoin for suspected seizures and cefazolin for the ulcerations on his lower extremities.
FIG. 2.
Fluid attenuated inversion recovery (FLAIR) sequence on initial presentation (hospital day 1). Note the increased signal in the right frontal cortex (see arrow). There is also an incidental posterior hematoma.
Despite the phenytoin, the patient subsequently developed further partial motor seizure activity involving his left arm. A follow-up MRI with contrast demonstrated enlargement and enhancement of the hyperintense lesion. Conditions such as cerebritis, aggressive demyelinating disease, and vascular lesions were considered. By then, the patient had developed rigors and fevers. Given his immunocompromised condition, therapy with broad-spectrum antibiotics, including intravenous vancomycin, trimethoprim-sulfamethoxazole, fluconazole, and meropenem, was begun. Although infection with free-living amebae was also included in the differential diagnosis, specific antiamebic treatment was not started prior to the brain biopsy (discussed below).
His initial cerebrospinal fluid (CSF) profile was as follows: leukocyte count, 0 cells/mm3; erythrocyte count, 1 cell/mm3; glucose concentration, 112 mg/dl; and total protein concentration, 30 mg/dl. CSF Gram staining and culture results were negative. Herpes simplex virus-specific PCR yielded negative results, as did India ink and cryptococcal-antigen testing. Serum samples obtained at the time of admission were significant for a mild anemia (hemoglobin concentration, 11.2 g/dl). The leukocyte count was normal at 5,950 cells/mm3, with 87% granulocytes, 7% lymphocytes, 4.7% monocytes, and 0.8% eosinophils. His serum creatinine concentration was slightly elevated at 1.6 mg/dl, although a recent baseline had been 1.5 mg/dl. Levels of electrolytes and transaminases were within normal limits.
Serial MRI examinations demonstrated further enlargement signal abnormality with enhancement. Consent for biopsy of the enhancing brain lesion was obtained, and the biopsy was performed on hospital day 8. The skin lesions on his anterior shins were also biopsied. Liposomal amphotericin B, pentamidine, rifampin, azithromycin, flucytosine, sulfadiazine, and metronidazole were subsequently initiated and continued for the remainder of his hospitalization. By hospital day 14, MRI revealed continued progression of disease, now involving the right posterior frontal and anterior parietal lobe with interval extension of heterogeneous T2 signal and of subtle heterogeneous patchy enhancement. There was also interval development of leptomeningeal enhancement.
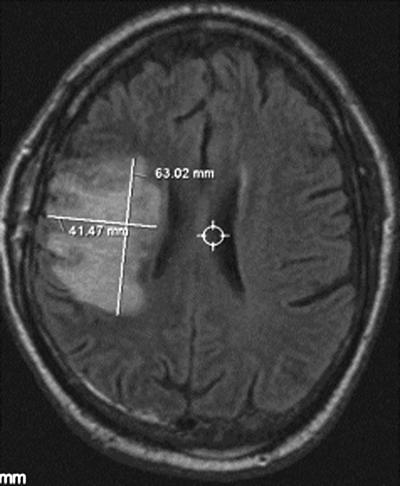
Despite therapy, the patient exhibited a deteriorating mental status and left hemiparesis of the arm and leg. On hospital day 21, after 10 days of antimicrobial therapy, multiple stereotactic biopsy specimens of the right frontal lobe were obtained. An MRI revealed an increased signal on the FLAIR sequence (Fig. 3) and increase in size and enhancement. After discussion with the patient and his family, a palliative right posterior frontal and parietal evacuation of the brain abscess was performed on hospital day 30. Despite resection and continuing antimicrobial therapy, the patient declined further. He and his family elected to receive hospice care at home, where he succumbed to infection and died 3 weeks later.
FIG. 3.
FLAIR sequence performed on hospital day 21. Note the increased area of hyperintensity.
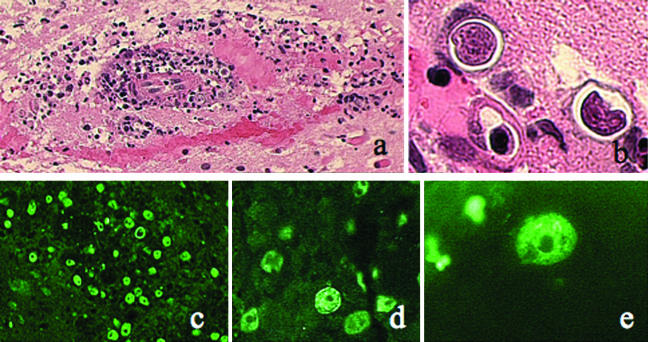
Histological examination of the multiple stereotactic biopsy specimens revealed extensive destruction of brain tissue by a granulomatous process, with several areas of necrosis. There were numerous inflammatory cells and multinucleated giant cells with granuloma formation. Scattered throughout the tissue were amebic trophozoites with a nucleus and a prominent round nucleolus. Degenerating amebas were also found within the cytoplasm of many of the giant cells. Amebic cysts with double walls were visible on hematoxylin and eosin stain (Fig. 4). The cyst walls were positive by periodic acid-Schiff and Gomori methenamine-silver staining.
FIG. 4.
Brain biopsy specimen. Panels a and b show histopathology revealed by hematoxylin and eosin stain. (a) Numerous inflammatory cells in brain parenchyma and around vessels. Magnification, ×100. (b) Small organisms within thin-walled cysts. Magnification, ×1,000. Panels c to e show the results of immunofluorescence analysis using rabbit anti-Acanthamoeba sera. (c) Magnification, ×100. (d) Magnification, ×400. (e) Magnification. ×1,000.
Immunofluorescence analysis using rabbit anti-Acanthamoeba and -B. mandrillaris sera revealed that the infecting agent belonged to the genus Acanthamoeba, for the amebas in the brain tissue reacted extensively with the anti-Acanthamoeba serum (Fig. 4) and showed no reactivity with the anti-B. mandrillaris serum (1, 10). There was no evidence of bacteria or acid-fast organisms in the tissue.
Macerated brain tissue was also inoculated on to nonnutrient agar plates covered with Escherichia coli and incubated at 37°C. After 24 h of incubation, many trophozoites with morphology consistent with Acanthamoeba species were observed. The amebas were subsequently established in liquid medium without any bacteria and designated as CDC:V548. Total DNA was extracted from the CDC:V548 cultures that were confluent using the DNeasy kit (QIAGEN, Inc., Valencia, CA). Following DNA extraction, PCR was used to amplify a portion of the Acanthamoeba nuclear small-subunit rRNA gene (ribosomal DNA [18S rDNA]) using Acanthamoeba genus-specific primers. This primer set amplifies a region of the 18S rDNA that permits genotypic identification of an Acanthamoeba isolate following primary sequence analysis and comparison (3). Three microliters of purified PCR product (QIAquick PCR purification kit; QIAGEN, Inc., Valencia, CA) was used directly in subsequent sequencing reactions.
DNA sequencing of the 18S rDNA was done with an ABI 310 automated fluorescent sequencing system utilizing BigDye sequencing kit version 1.1 (Applied Biosystems, Foster City, CA). Sequencing was done using a set of conserved primers and methods that have been described previously (3, 11, 17). Sequencing reactions were purified by ethyl alcohol precipitation, and purified products were resuspended in 25 μl of template suppression buffer (TSR buffer; Applied Biosystems, Inc., Foster City, CA). The fluorescent data were analyzed using Sequence Analysis Software 3.4 for the ABI 310 system (Applied Biosystems, Inc, Foster City, CA). The CDC:V548 sequence that was obtained was aligned by eye to other Acanthamoeba species sequences in our 18S rDNA database using the sequence alignment program XESEE (4).
PCR amplification of CDC:V548 using Acanthamoeba genus-specific 18S rDNA primers JDP1 and JDP2 produced a single band approximately of 463 base pairs (bp). Comparison of the primary sequence from CDC:V548 with the Acanthamoeba 18S rDNA database shows that CDC:V548 was 99.5% similar in a primary sequence alignment (416 of 418 nucleotides identical in the aligned region, with PCR primer regions excluded for this calculation) to Acanthamoeba species V006, which is the type strain for Acanthamoeba genotype T1 (GenBank accession no. U07400). Acanthamoeba genotype T1 is a distinct 18S rDNA genotype, differing from its most closely related genotype (T4) by a dissimilarity percentage range of 9.7 to 10.8%.
The skin biopsy specimens obtained prior to the start of antibiotic therapy were consistent with a neutrophilic abscess with no amebas observed. Gram staining revealed gram-positive bacteria largely in clusters but also in chains. Culture results yielded only methicillin-sensitive Staphylococcus aureus.
Amebic infections of the central nervous system are typically classified into two types: primary amebic encephalitis caused by Naegleria fowleri and granulomatous amebic encephalitis (GAE) produced by both Acanthamoeba spp. and Balamuthia mandrillaris. Primary amebic encephalitis typically occurs in healthy patients who come in contact with fresh water contaminated with N. fowleri. This is usually an acute, lethal central nervous infection. The organism enters the nasal mucosa and can spread through the olfactory tracts, crossing the cribiform plate into the subarachnoid space and producing hemorrhagic necrosis of brain tissue (12).
Granulomatous amebic encephalitis occurs as an opportunistic infection in immunocompromised hosts, although cases due to Balamuthia have been reported in healthy patients (1, 5-7). Acanthamoeba is ubiquitous and can be found in soil and water, both in the home and in outdoor environments. Balamuthia is more likely to be from soil. Entry sites for infection include skin, as well as upper respiratory and urogenital tracts (14). Cases of Balamuthia have been reported with patients having skin lesions for months to years prior to developing encephalitis (12). The amebas are disseminated via blood to the brain, resulting in granulomatous encephalitis. The course is subacute to chronic, generally with a fatal result.
Neurological presentations of GAE include confusion, headache, fever, meningismal signs, seizures, increased intracranial pressure signs, and a higher frequency of focal neurologic signs and somnolence (1). In our case, the patient did present with seizures and left-sided neurological findings. Interestingly, the skin ulcerations began 3 weeks prior to his admission. While he denied contact with fresh water, our patient went on a fishing trip one week prior to the onset of his lower extremity lesions. Although no amebas were found on microscopic analysis of the skin biopsy, the lesions were the likely entry site. Initial histopathologic analysis of skin biopsy specimens will frequently misidentify cysts and trophozoites, and stains and cultures remain negative for organisms (16).
In cases of GAE, the CSF profile typically reveals mildly low to normal glucose concentrations. Pleocytosis with predominant lymphocytosis is also commonly seen, as are high protein levels. A previous report of GAE detailed a markedly depressed CSF glucose concentration (7). In our patient, the CSF demonstrated a slightly elevated glucose level, which was likely due to his diabetes mellitus.
On imaging, computerized tomography (CT) may reveal multiple low-density areas of variable sizes in the cerebral cortex with mild mass effect. MRI will reveal areas of high signal on T2 weighted images that enhance either in a heterogeneous or ring-like pattern (7). The sectors of infection will create a granulomatous inflammatory reaction causing hemorrhagic necrosis. The location of brain involvement may vary with the type of amebic infection. Acanthamoeba species have been reported to involve the posterior structures of the brain (13) while Balamuthia has been described to typically involve the cortex (7).
The diagnosis of GAE may be determined by immunofluorescence and hematoxyline-eosin staining of the ameba. Unlike N. fowleri, which has three stages, including a flagellated form, Acanthamoeba and Balamuthia have only two stages, cysts and trophozoites, both of which may be observed (12). Trophozoites are the infective form; these enter the body and disseminate. The parasite will then encyst under difficult environmental conditions, such as food source depletion, inflammatory response, and even the presence of antimicrobials. In GAE, trophozoites and cysts often surround areas of necrosis, where the ameba have ingested cells. Acanthamoeba can be isolated from infected tissues in vitro using a nonnutrient agar and a suitable bacterial food source such as E. coli or Enterobacter aerogenes (12). Culture may be more difficult when the ameba has encysted. Distinguishing GAE from other infections in immunocompromised patients can prove challenging. In a case report of GAE, serologic testing and stereotactic brain biopsy were found not to be diagnostic (2). Early diagnosis is preferable, and better standardized methods for assessing antimicrobial susceptibility are needed (9).
Quick identification is important as N. fowleri is highly sensitive to amphotericin B. Acanthamoeba and Balamuthia are more difficult to treat and typically involve a multidrug approach. Various regimens including pentamidine, ketoconazole, trimethoprim-sulfamethoxazole, and flucytosine have been used on the basis that many of these drugs are amebastatic and not amebicidal. Treatment of disseminated amebic infections is contingent on early diagnosis prior to CNS involvement (8, 15). In the case of two immunocompetent patients with GAE caused by Balamuthia, antimicrobial therapy with flucytosine, pentamidine, fluconazole, sulfadiazine, and a macrolide was successful (5). Cerebral abscesses may be surgically drained, and recent reports have indicated that corticosteroids should not be used in cases of cerebral edema due to the tendency of worsening Acanthamoeba infection (8).
Currently, there are 15 different Acanthamoeba genotypes that have been identified by 18S rDNA sequence analysis. These genotypes are discriminated from one another by a minimum of 5% sequence divergence (3). Some genotypes appear to be associated with specific clinical manifestations (3, 11, 17). For example, over 97% of Acanthamoeba dermatitis cases are associated with genotype T4, a very common Acanthamoeba genotype. T4 isolates have also been recovered from other infections, such as skin and lung infections. Acanthamoeba genotypes T7, T8, and T9 are environmental isolates that have not been shown to be associated with any infections caused by Acanthamoeba. Genotypes T1, T10, and T12 are rare genotypes that have been isolated exclusively from GAE infections caused by Acanthamoeba (11). The natural distribution of these genotypes is unknown, since they have not been found in environmental isolates. The isolate examined in this study, CDC:V548, is classified as an Acanthamoeba species genotype T1 due to high sequence similarity (99.5%) with Acanthamoeba species V006, the type isolate for genotype T1. It is important to note that this high level of similarity was observed in a region of the gene that has very high levels of variability across all isolates of Acanthamoeba. This further supports our identification of this isolate as Acanthamoeba genotype T1. This conclusion is consistent with other cases of GAE that have resulted in the identification of T1 Acanthamoeba isolates. In our case, no Acanthamoeba organisms were isolated from the cutaneous lesions, although the lesions are hypothesized to be the likely entry point of the infection.
In conclusion, opportunistic infections should be considered for any immunocompromised patients demonstrating a progressive pattern of neurological deficits. In such cases, the use of MRI imaging combined with biopsy can be extremely useful in establishing a diagnosis. Our case is the first report detailing GAE in a patient who was initially thought to have a stroke. Given the high morbidity of GAE, practicing physicians should have a high index of suspicion when treating potential cases in immunosuppressed patients.
Nucleotide sequence accession number.
The CDC:V548 18S rDNA partial gene sequence obtained in this study has been deposited in GenBank under the accession number DQ339096.
Acknowledgments
P.A.F., G.C.B., and D.J.K. were supported by grant EY09073 from the National Eye Institute, National Institutes of Health, Bethesda, MD.
Footnotes
Published ahead of print on 20 September 2006.
REFERENCES
- 1.Bakardjiev, A., P. H. Azimi, N. Ashouri, D. P. Ascher, D. Janner, F. L. Schuster, G. S. Visvesvara, and C. Glaser. 2003. Amebic encephalitis caused by Balamuthia mandrillaris: report of four cases. Pediatr. Infect. Dis. J. 22:447-452. [DOI] [PubMed] [Google Scholar]
- 2.Bloch, K. C., and F. L. Schuster. 2005. Inability to make a premortem diagnosis of Acanthamoeba species infection in a patient with fatal granulomatous amebic encephalitis. J. Clin. Microbiol. 43:3003-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booton, G. C., G. S. Visvesvara, T. J. Byers, D. J. Kelly, and P. A. Fuerst. 2005. Identification and distribution of Acanthamoeba species genotypes associated with nonkeratitis infections. J. Clin. Microbiol. 43:1689-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabot, E. L., and A. T. Beckenbach. 1989. Simultaneous editing of multiple nucleic and protein sequences with ESEE. Comput. Appl. Biosci. 5:233-234. [DOI] [PubMed] [Google Scholar]
- 5.Deetz, T. R., M. H. Sawyer, G. Billman, F. L. Schuster, and G. S. Visvesvara. 2003. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin. Infect. Dis. 37:1304-1312. [DOI] [PubMed] [Google Scholar]
- 6.Denney, C. F., V. J. Iragui, L. D. Uber-Zak, N. C. Karpinski, E. J. Ziegler, G. S. Visvesvara, and S. L. Reed. 1997. Amebic meningoencephalitis caused by Balamuthia mandrillaris. Clin. Infect. Dis. 25:1354-1358. [DOI] [PubMed] [Google Scholar]
- 7.Katz, J. D., A. H. Ropper, L. Adelman, M. Worthington, and P. Wade. 2000. A case of Balamuthia mandrillaris meningoencephalitis. Arch. Neurol. 57:1210-1212. [DOI] [PubMed] [Google Scholar]
- 8.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez, A. J. 1980. Is Acanthamoeba encephalitis an opportunistic infection? Neurology 30:567-574. [DOI] [PubMed] [Google Scholar]
- 10.Martinez, M. S., G. Gonzalez-Mediero, P. Santiago, A. Rodriguez de Lope, J. Diz, C. Conde, and G. S. Visvesvara. 2000. Granulomatous amebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J. Clin. Microbiol. 38:3892-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder, J. M., G. C. Booton, J. Hay, I. A. Niszl, D. V. Seal, M. B. Markus, P. A. Fuerst, and T. J. Byers. 2001. Use of subgenic 18s ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 39:1903-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster, F. L., and G. S. Visvesvara. 2004. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 34:1001-1024. [DOI] [PubMed] [Google Scholar]
- 13.Sell, J. J., F. W. Rupp, and W. W. Orrison. 1997. Granulomatous amebic encephalitis caused by Acanthamoeba. Neuroradiology 39:434-436. [DOI] [PubMed] [Google Scholar]
- 14.Shirabe, T., Y. Monobe, and G. S. Visvesvara. 2002. Case report: an autopsy case of amebic meningoencephalitis. The first Japanese case caused by Balamuthia mandrillaris. Neuropathology 22:213-217. [DOI] [PubMed] [Google Scholar]
- 15.Slater, C. A., J. Z. Sickel, G. S. Visvesvara, R. C. Pabico, and A. A. Gaspari. 1994. Successful treatment of disseminated Acanthamoeba infection in an immunocompromised patient. N. Engl. J. Med. 331:85-87. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg, J. P., R. L. Galindo, E. S. Kraus, and K. G. Ghanem. 2002. Disseminated acanthamebiasis in a renal transplant recipient with osteomyelitis and cutaneous lesions: case report and literature review. Clin. Infect. Dis. 35:e43-e49. [Online.] [DOI] [PubMed] [Google Scholar]
- 17.Stothard, D. R., J. M. Schroeder-Diedrich, M. H. Awwad, R. J. Gast, D. R. Ledec, S. Rodriguez-Zaragoza, C. L. Dean, P. A. Fuerst, and T. J. Byers. 1998. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 45:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]