Abstract
Multidrug-resistant tuberculous meningitis is fatal without rapid diagnosis and use of second-line therapy. It is more common in human immunodeficiency virus (HIV)-positive patients. Beijing genotype strains of Mycobacterium tuberculosis are associated with drug resistance, particularly multidrug resistance, and their prevalence is increasing worldwide. The prevalence of Beijing genotype strains among Mycobacterium tuberculosis isolates from the cerebrospinal fluid of HIV-positive (n = 35) and HIV-negative (n = 187) patients in Ho Chi Minh City was determined. The Beijing genotype was significantly associated with HIV status (odds ratio [OR] = 2.95 [95% confidence interval {CI}, 1.38 to 6.44]; P = 0.016), resistance to any drug (OR = 3.34 [95% CI, 1.87 to 5.95]; P < 0.001) and multidrug resistance (Fisher's exact test; P = 0.001). The association of the Beijing genotype with drug resistance was independent of HIV status. This is the first report of Beijing genotype association with HIV status, which may be an association unique to tuberculous meningitis.
Tuberculous meningitis (TBM) represents the most severe form of tuberculosis (TB). Mortality is 30% in cases of infection with a fully sensitive organism, and coinfection with human immunodeficiency virus (HIV) is associated with increased mortality and morbidity (21). The diagnosis and effective treatment of multidrug-resistant (MDR) TBM is not currently possible in many areas with high or increasing HIV prevalence.
The increased susceptibility of HIV-infected patients to tuberculosis, particularly extrapulmonary tuberculosis, is well established. The consequence is an increased incidence of TBM in areas with high or rising HIV prevalence rates (7, 19, 20). HIV-infected patients with tuberculosis in Vietnam have higher rates of drug resistance, MDR TB, treatment failure (odds ratio [OR] = 2.35) (16) and mortality (OR = 30) (16) than those without HIV infection. A similar pattern has been demonstrated in HIV-infected TBM patients, with higher rates of MDR TB (OR = 12.35) (22) and mortality (OR = 4.24) (22).
The Beijing genotype has been associated with drug resistance, MDR TB, and young age (and therefore active transmission) in several settings (2). To date, no studies have shown associations with HIV status (for a review, see reference 2). It has been speculated that the genotype is hypervirulent and an “escape mutant” from Mycobacterium bovis BCG vaccination (27). Several studies have attempted to characterize the immune interactions and transmission patterns of Beijing-type strains, but the reasons for its successful global spread remain poorly understood. It appears to demonstrate induction of Th2 polarized immunity in tissue culture models (10) and elicits a nonprotective immune response and greater mortality in BALB/c mice (9). Tsenova et al. showed that infection of rabbits with Beijing genotype isolates was associated with greater dissemination from the lung and led to higher bacillary loads in the cerebrospinal fluid (CSF) (24). The altered pathogenesis of the strain was attributed to a polyketide synthase-derived phenolic glycolipid that is not produced by some Mycobacterium tuberculosis genotypes due to a 7-bp deletion in the pks gene.
The Beijing genotype is apparently the second most prevalent genotype worldwide (4). The genotype was first described in 1995 by van Soolingen et al. when 86% of isolates from Beijing, China, were identified as a single spoligotype. It became the focus of attention for M. tuberculosis virulence studies following the identification of a major outbreak of MDR TB in New York City as due to the Beijing genotype, subsequently designated the W strain (12). There is some evidence that it originated in Central Asia and that it subsequently spread from China (11), where it has been the predominant genotype for at least 50 years (15). The prevalence of Beijing genotypes generally decreases in countries further west than China and Russia. It is currently around 50% in East Asia and 13% globally (2). Other geographical areas have seen increases in the prevalence of Beijing genotypes following its initial introduction (5, 14). Investigation of the genotype has revealed further distinct deleted regions (25) that may contribute to the unusual but as yet poorly characterized phenotype.
In Vietnam, Beijing genotypes represent approximately 40% of M. tuberculosis strains and are apparently endemic and stable in the community. An unusual feature of M. tuberculosis in Vietnam is the presence of a second highly prevalent spoligotype (octal code, 777-777-774-413-771), ST319, or the Vietnam genotype (1). This genotype lacks spacers 13, 26, 27, 29 to 33, and 34; has a single IS6110 copy; and represents around 20% of the isolates from southern Vietnam.
The present study investigated the prevalence of these two genotypes and their relationship to HIV status and drug resistance in Vietnamese adults with TBM.
MATERIALS AND METHODS
Patients and samples.
M. tuberculosis isolates from the CSF of adults (>14 years old) with TBM were collected between 2001 and 2003. The majority of the samples in the present study (n = 170/222) were collected as part of a randomized, controlled trial of adjunctive dexamethasone for treatment of TBM, reported in detail elsewhere (23). Additional isolates from adults uninfected with HIV (n = 52) were collected as part of a larger typing study on the bacterial genetics of extrapulmonary TB, also to be reported in detail elsewhere (unpublished data). Isolates were collected at two hospitals in Ho Chi Minh City (HCMC), southern Vietnam: Pham Ngoc Thach Hospital for Tuberculosis and Lung Disease and the Hospital for Tropical Diseases. All patients included in the present study gave informed consent to HIV testing, and trial protocols for all clinical studies were reviewed by the ethics committees of both hospitals, the Health Services of Ho Chi Minh City, and the Oxford University Tropical Research Ethics Committee.
Drug susceptibility testing.
Isolates were tested at Pham Ngoc Thach Hospital by standard World Health Organization 1% proportion methods for susceptibility to isoniazid (0.2 μg/ml), rifampin (40 μg/ml), ethambutol (2 μg/ml), and streptomycin (4 μg/ml).
Spoligotyping.
Spoligotyping of isolates was performed according to the standard protocol (6). Briefly, DNA was extracted by the cetyltrimethylammonium bromide method and diluted to a working solution of 15 ng/ml. PCR products were amplified with the primers Dra (biotin labeled) and DRb, hybridized to the spoligotype membrane (Isogen Bioscience B.V., Maarsen, The Netherlands), incubated with streptavidin-peroxidase, and detected with the ECL system (Amersham, United Kingdom). The results were analyzed on Bionumerics software (Applied Maths, Belgium). Beijing genotype here is used to designate all “Beijing-type” variants, i.e., any isolate missing spacers 1 to 34, with at least three spacers from 35 to 43.
Analysis.
Univariate logistic regression was used to estimate the OR for association of variables with the Beijing or Vietnam genotype. A forward stepwise logistic regression model (P of <0.05 to enter; P of >0.055 to remove) was used to identify variables associated with the Beijing genotype on multivariate analysis. The variables examined in the model were HIV status, age, gender, and drug resistance (to any first-line drug). It was not possible to include MDR in the model because no MDR isolates were found in the non-Beijing genotype patient group. Association between the Beijing genotype and the patient's outcome (death/survival at 9 months) was examined in a stepwise logistic regression model for outcome, using the same covariates. Statistical analysis was performed using STATA SE9 software (STATA Corporation, Texas).
RESULTS
Spoligotypes were available for isolates from 35 HIV-positive patients (Fig. 1) and 187 HIV-negative patients (Fig. 2). Among the HIV-positive group, 4 (11%) patients presented with grade 1 TBM, 20 (57%) with grade 2, and 11 (31%) with grade 3. The median age was 26 (range, 18 to 54) years. Twenty-four patients (69%) died during treatment or follow-up (to 1 year). The median time to death was 58 (range, 1 to 267) days. No patients received antiretroviral treatment, as it was not available in the Vietnamese health system at the time of the study. Detailed discussion of the clinical presentation of these patients is presented elsewhere (21, 23).
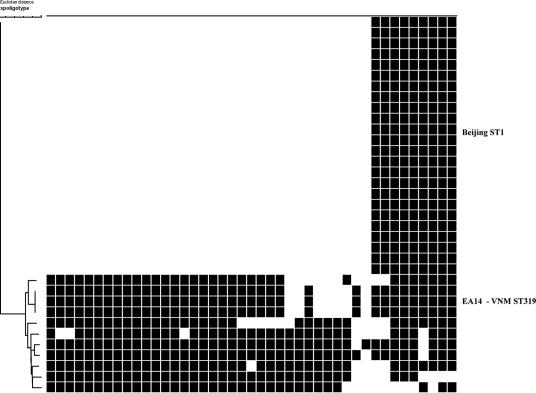
FIG. 1.
Spoligotypes of M. tuberculosis isolates from the CSF of 35 HIV-positive Vietnamese adults with tuberculous meningitis. Spacers 1 to 43 are represented from left to right. A dendrogram created with Bionumerics software (neighbor joining; Euclidean distance) is presented on the left. Major genotypes are identified on the right.
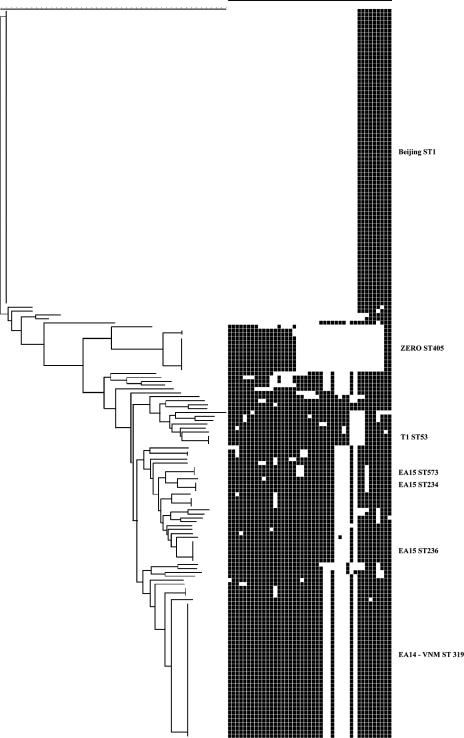
FIG. 2.
Spoligotypes of M. tuberculosis isolates from the CSF of 187 HIV-negative Vietnamese adults with tuberculous meningitis. Spacers 1 to 43 are represented from left to right. A dendrogram created with Bionumerics software (neighbor joining; Euclidean distance) is presented on the left. Major genotypes are identified on the right.
Sixty-nine percent (n = 24/35) of isolates from HIV-positive patients were Beijing genotype, while only 42% (n = 79/187) of isolates from the HIV-negative group were Beijing type. Conversely, 9% (n = 3/35) of isolates from HIV-positive and 19% (n = 35/187) of isolates from HIV-negative patients were Vietnam genotype. This difference was statistically significant (χ2 = 8.292; P = 0.016).
However, when Beijing genotypes were excluded from the analysis, the prevalences of the Vietnam spoligotype were similar in the two groups (27% in HIV-positive patients and 32% in HIV-negative patients), and there was no association with HIV status (χ = 0.1211; P = 0.728).
The remaining eight isolates in the HIV-positive group were all orphan types: 1 LAM clade, 1 Haarlem clade, and 6 miscellaneous genotypes. The remaining 73 isolates in the HIV-negative group contained 12 LAM clade isolates, 41 EA clade genotypes, 4 Haarlem isolates, and 3 T53 genotypes. Forty-five percent (33/73) of these isolates clustered, with the largest cluster being a LAM genotype. There were 28 orphan genotypes, including 1 isolate apparently containing all 43 spacers.
Susceptibility patterns to first-line drugs are presented in Table 1. In the HIV-positive group, 15% (n = 4/26) of the Beijing strain isolates were MDR TB, while only 6% (n = 5/87) of the Beijing-type strains in the HIV-negative group were MDR TB. No MDR or ethambutol-resistant isolates were seen among the non-Beijing isolates in either group.
TABLE 1.
Resistance patterns of M. tuberculosis isolates from 35 HIV-positive and 187 HIV-negative Vietnamese adults with TBM
| Resistance pattern | HIV positive (n = 35)
|
HIV negative (n = 187)
|
||
|---|---|---|---|---|
| Beijing [n (%)] | Non-Beijing [n (%)] | Beijing [n (%)] | Non-Beijing [n (%)] | |
| Fully sensitive | 14 (53.8) | 4 (44.4) | 46 (52.9) | 80 (80.0) |
| Streptomycin | 0 | 0 | 17 (19.5) | 7 (7.0) |
| Isoniazid | 0 | 0 | 2 (2.3) | 9 (9.0) |
| Rifampin | 0 | 0 | 0 | 1 (1.0) |
| Isoniazid + streptomycin | 8 (30.8) | 5 (55.6) | 17 (19.5) | 2 (2.0) |
| Isoniazid + rifampin + streptomycin | 3 (11.5) | 0 | 3 (3.4) | 0 |
| Isoniazid + rifampin + streptomycin + ethambutol | 1 (3.8) | 0 | 1 (1.1) | 0 |
| Isoniazid + streptomcin + ethambutol | 0 | 0 | 0 | 1 (1.0) |
| Isoniazid + rifampin + streptomycin | 0 | 0 | 1 (1.1) | 0 |
| Total | 26 (100) | 9 (100) | 87 (100) | 100 (100) |
There was a significant association of the Beijing genotype with resistance to any drug, with 47% of Beijing isolates and only 23% of non-Beijing isolates being resistant to any first-line drug (OR = 3.34 [95% confidence interval {CI}, 1.87 to 5.95]; P < 0.001). This association was independent of HIV status (adjusted OR = 3.196 [95% CI = 1.764 to 5.69]; P < 0.001), but the association was stronger in the HIV-negative group (P = 0.003). The Beijing genotype was also strongly associated with MDR TB (Fisher's exact test; P < 0.001). As there was no MDR in the non-Beijing group, it was not possible to model this variable in logistic regression.
On univariate analysis, the Beijing genotype was significantly associated with young age (P = 0.0046): the median age of patients with non-Beijing genotype isolates was 34 (range, 14 to 77) years, while the median age for patients with Beijing genotype isolates was 28 (range, 16 to 78) years. The association of the Beijing genotype with young age did not reach statistical significance independently of HIV status (P = 0.053), which may be due to the small numbers of HIV-positive isolates in this study; a larger study would be needed to confirm the nature of this association. The Beijing genotype showed no association with gender (P = 0.285).
In multivariate analysis, the Beijing genotype was associated with resistance to any first-line drug (P < 0.001) and HIV-positive status (P = 0.045), but not with age (P = 0.60). The Vietnam genotype showed no association with drug resistance (Fisher's exact test; P = 0.25) or age (P = 0.880). There was also no association of MDR with age (P = 0.828), although the number of patients was too small to confirm or refute an association.
The Beijing genotype was not a predictor of death after 9 months of treatment in a stepwise logistic regression model, unlike HIV (OR = 9.09 [95% CI, 3.81 to 21.68]; P < 0.001) and older age (OR = 1.02 [95% CI, 1.00 to 1.05]; P = 0.009). Surprisingly, drug resistance and MDR were not associated with mortality in this model, but this may be due to the correlation with HIV status and the small numbers of MDR TB patients.
DISCUSSION
This is the first report of Beijing genotype association with HIV. Ten studies, including one in HCMC, have found no association between HIV status and the Beijing genotype in pulmonary tuberculosis. Twenty previous studies from geographically diverse locations, including Europe, North America, South America, Africa, and Asia, investigating the site of disease in relation to genotype have shown no association between extrapulmonary TB and the Beijing genotype (reviewed in reference 2). Our own large study of HIV-negative adults from HCMC showed no association of the Beijing genotype with TBM, with 42% (n = 80/187) and 37% (n = 88/237) Beijing genotype among TBM and pulmonary cases, respectively (P = 0.23). It is therefore possible that this association is unique to TBM, or to Vietnam, or that it exists in HIV-negative tuberculosis but is too small to reach significance in this study. Despite the small number of isolates from HIV-positive patients in this study, the association was relatively strong (OR = 2.98 [95% CI, 1.38 to 6.44]). It is not clear if this was due to strain-related differences in immunopathogenesis, which may be amplified by HIV coinfection, or to epidemiological factors. The Beijing genotype is associated with treatment failure and relapse but not with HIV among pulmonary TB patients in Vietnam, irrespective of primary drug resistance, age, or sex (OR = 3.2 [95% CI, 1.4 to 7.1]) (8). The reasons for treatment failure and relapse independent of drug resistance are not known, but it appears that infections with Beijing genotype strains may be more persistent. Such persistence in sputum despite being under treatment could lead to a longer duration of infectiousness and greater transmission potential. The increased susceptibility of HIV-positive patients to disseminated TB and TBM is well documented (3). The independent association of the Beijing strain with both HIV and MDR TB represents a potential for further increasing the incidence of MDR TBM in settings with high or rising HIV prevalence and high rates of endemic TB. HIV prevalence in HCMC is rising, and although still largely concentrated among intravenous-drug users and commercial sex workers, is starting to spread beyond these communities. In 2003, the national prevalence was estimated at 1.2%, but one-fourth of all infections were concentrated in HCMC (26). Surveillance of all patients with TB in HCMC has shown an increase in HIV prevalence from 0.5% in 1995 to 4% in 2000 (17).
Outcomes for MDR TBM are extremely poor (13, 22), particularly in settings where rapid susceptibility testing and second-line therapies are not routinely available.
The association of the Beijing strain with drug resistance and MDR has been documented in other settings, though this has not been a consistent finding. It has been postulated that in much of Asia, nonassociation is probably due to the endemic nature of the Beijing genotype, whereas in areas where the proportion of Beijing strains is increasing and therefore “epidemic,” associations with drug resistance are found (2). The association with drug resistance is particularly strong in Vietnam (1), unlike most of Asia.
It is possible that this pattern is due to the active transmission of these strains among young age groups, who are generally vulnerable to the development of drug resistance. However, an intriguing possibility is that this strain is “hypermutable” and therefore able to adapt more rapidly to drug pressure (18).
Associations of the Beijing strain with young age did not reach significance independently of HIV status, a reflection of the fact that the HIV epidemic in Vietnam was concentrated among mostly young intravenous-drug users at the time of the study. However, the Beijing genotype has been associated with young age (P < 0.001) (1) in Vietnam; it is possible that a larger study would confirm a similar association in those with TBM. An association between genotype and young age suggests active transmission of a strain. It is not clear, however, if this is due to an increased susceptibility to the strain in the population or to increased transmissibility of the strain.
The second predominant strain in Vietnam, ST319, was not associated with any variable in the model: age, HIV, or drug resistance. This strain represents an “ancient” strain type under classification schemes, whereas the Bejing genotype is classified as “modern.” It is likely that this genotype was circulating in Vietnam prior to the introduction of the Beijing genotype and that East Asian (EAI) clade genotypes represent the endemic M. tuberculosis strains, circulating in all age groups, while the Beijing “epidemic” strain has been relatively recently introduced or achieved selective advantage and is being actively transmitted among the younger population group.
In summary, we have demonstrated a strong association between the Beijing genotype, drug resistance, and HIV infection in a large cohort of Vietnamese adults with TBM. The outcome of TBM in individuals coinfected with HIV is poor regardless of the drug susceptibility of the infecting organism (21). However, in this and other settings (2), HIV coinfection is associated with drug-resistant infections, which has important implications for the clinical outcome for these patients (22). Our findings suggest that the Beijing strain of M. tuberculosis may be more capable than other strains of causing disseminated disease in HIV-infected adults and that Beijing strains are more likely to be drug resistant. Although the poor outcome of TB in HIV-coinfected patients is multifactorial, we suggest that infection with Beijing genotype M. tuberculosis may be further bad news for this population of patients. Further studies examining the transmission of Beijing genotype bacteria within the HIV-infected population are warranted, together with the investigation of the abilities of different strains to disseminate from the lung and cause extrapulmonary disease.
Acknowledgments
This research was funded by the Wellcome Trust.
We thank the staff of Pham Ngoc Thach Hospital for Tuberculosis and Lung Disease and the Hospital for Tropical Diseases microbiology laboratories for help with culture and drug susceptibility testing.
Footnotes
Published ahead of print on 13 September 2006.
REFERENCES
- 1.Anh, D. D., M. W. Borgdorff, L. N. Van, N. T. Lan, T. van Gorkom, K. Kremer, and D. van Soolingen. 2000. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg. Infect. Dis. 6:302-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2006. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg. Infect. Dis. 12:736-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berenguer, J., S. Moreno, F. Laguna, T. Vicente, M. Adrados, A. Ortega, J. Gonzalez-LaHoz, and E. Bouza. 1992. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 326:668-672. [DOI] [PubMed] [Google Scholar]
- 4.Brudey, K., J. R. Driscoll, L. Rigouts, W. M. Prodinger, A. Gori, S. A. Al-Hajoj, C. Allix, L. Aristimuno, J. Arora, V. Baumanis, L. Binder, P. Cafrune, A. Cataldi, S. Cheong, R. Diel, C. Ellermeier, J. T. Evans, M. Fauville-Dufaux, S. Ferdinand, D. Garcia de Viedma, C. Garzelli, L. Gazzola, H. M. Gomes, M. C. Gutierrez, P. M. Hawkey, P. D. van Helden, G. V. Kadival, B. N. Kreiswirth, K. Kremer, M. Kubin, S. P. Kulkarni, B. Liens, T. Lillebaek, H. M. Ly, C. Martin, C. Martin, I. Mokrousov, O. Narvskaia, Y. F. Ngeow, L. Naumann, S. Niemann, I. Parwati, M. Z. Rahim, V. Rasolofo-Razanamparany, T. Rasolonavalona, M. L. Rossetti, S. Rusch-Gerdes, A. Sajduda, S. Samper, I. Shemyakin, U. B. Singh, A. Somoskovi, R. Skuce, D. van Soolingen, E. M. Streicher, P. N. Suffys, E. Tortoli, T. Tracevska, V. Vincent, T. C. Victor, R. Warren, S. F. Yap, K. Zaman, F. Portaels, N. Rastogi, and C. Sola. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caminero, J. A., M. J. Pena, M. I. Campos-Herrero, J. C. Rodriguez, I. Garcia, P. Cabrera, C. Lafoz, S. Samper, H. Takiff, O. Afonso, J. M. Pavon, M. J. Torres, D. van Soolingen, D. A. Enarson, and C. Martin. 2001. Epidemiological evidence of the spread of a Mycobacterium tuberculosis strain of the Beijing genotype on Gran Canaria Island. Am. J. Respir. Crit. Care Med. 164:1165-1170. [DOI] [PubMed] [Google Scholar]
- 6.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karstaedt, A. S., S. Valtchanova, R. Barriere, and H. H. Crewe-Brown. 1998. Tuberculous meningitis in South African urban adults. QJM 91:743-747. [DOI] [PubMed] [Google Scholar]
- 8.Lan, N. T., H. T. Lien, B. Tung Le, M. W. Borgdorff, K. Kremer, and D. van Soolingen. 2003. Mycobacterium tuberculosis Beijing genotype and risk for treatment failure and relapse, Vietnam. Emerg. Infect. Dis. 9:1633-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez, B., D. Aguilar, H. Orozco, M. Burger, C. Espitia, V. Ritacco, L. Barrera, K. Kremer, R. Hernandez-Pando, K. Huygen, and D. van Soolingen. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 133:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manca, C., M. B. Reed, S. Freeman, B. Mathema, B. Kreiswirth, C. E. Barry III, and G. Kaplan. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 72:5511-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokrousov, I., H. M. Ly, T. Otten, N. N. Lan, B. Vyshnevskyi, S. Hoffner, and O. Narvskaya. 2005. Origin and primary dispersal of the Mycobacterium tuberculosis Beijing genotype: clues from human phylogeography. Genome Res. 15:1357-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munsiff, S. S., B. Nivin, G. Sacajiu, B. Mathema, P. Bifani, and B. N. Kreiswirth. 2003. Persistence of a highly resistant strain of tuberculosis in New York City during 1990-1999. J. Infect. Dis. 188:356-363. [DOI] [PubMed] [Google Scholar]
- 13.Padayatchi, N., S. Bamber, H. Dawood, and R. Bobat. 2006. Multidrug-resistant tuberculous meningitis in children in Durban, South Africa. Pediatr. Infect. Dis. J. 25:147-150. [DOI] [PubMed] [Google Scholar]
- 14.Pena, M. J., J. A. Caminero, M. I. Campos-Herrero, J. C. Rodriguez-Gallego, M. I. Garcia-Laorden, P. Cabrera, M. J. Torres, B. Lafarga, F. Rodriguez de Castro, S. Samper, F. Canas, D. A. Enarson, and C. Martin. 2003. Epidemiology of tuberculosis on Gran Canaria: a 4 year population study using traditional and molecular approaches. Thorax 58:618-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian, L., J. D. Van Embden, A. G. Van Der Zanden, E. F. Weltevreden, H. Duanmu, and J. T. Douglas. 1999. Retrospective analysis of the Beijing family of Mycobacterium tuberculosis in preserved lung tissues. J. Clin. Microbiol. 37:471-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quy, H. T., F. G. Cobelens, N. T. Lan, T. N. Buu, C. S. Lambregts, and M. W. Borgdorff. 2006. Treatment outcomes by drug resistance and HIV status among tuberculosis patients in Ho Chi Minh City, Vietnam. Int. J. Tuberc. Lung Dis. 10:45-51. [PubMed] [Google Scholar]
- 17.Quy, H. T., D. T. Nhien, N. T. Lan, M. W. Borgdorff, and J. F. Broekmans. 2002. Steep increase in HIV prevalence among tuberculosis patients in Ho Chi Minh City. AIDS 16:931-932. [DOI] [PubMed] [Google Scholar]
- 18.Rad, M. E., P. Bifani, C. Martin, K. Kremer, S. Samper, J. Rauzier, B. Kreiswirth, J. Blazquez, M. Jouan, D. van Soolingen, and B. Gicquel. 2003. Mutations in putative mutator genes of Mycobacterium tuberculosis strains of the W-Beijing family. Emerg. Infect. Dis. 9:838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satishchandra, P., A. Nalini, M. Gourie-Devi, N. Khanna, V. Santosh, V. Ravi, A. Desai, A. Chandramuki, P. N. Jayakumar, and S. K. Shankar. 2000. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989-96). Indian J. Med. Res. 111:14-23. [PubMed] [Google Scholar]
- 20.Silber, E., P. Sonnenberg, K. C. Ho, H. J. Koornhof, S. Eintracht, L. Morris, and D. Saffer. 1999. Meningitis in a community with a high prevalence of tuberculosis and HIV infection. J. Neurol. Sci. 162:20-26. [DOI] [PubMed] [Google Scholar]
- 21.Thwaites, G. E., N. Duc Bang, N. Huy Dung, H. Thi Quy, D. Thi Tuong Oanh, N. Thi Cam Thoa, N. Quang Hien, N. Tri Thuc, N. Ngoc Hai, N. Thi Ngoc Lan, N. Ngoc Lan, N. Hong Duc, V. Ngoc Tuan, C. Huu Hiep, T. Thi Hong Chau, P. Phuong Mai, N. Thi Dung, K. Stepniewska, C. P. Simmons, N. J. White, T. Tinh Hien, and J. J. Farrar. 2005. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with tuberculous meningitis. J. Infect. Dis. 192:2134-2141. [DOI] [PubMed] [Google Scholar]
- 22.Thwaites, G. E., N. T. Lan, N. H. Dung, H. T. Quy, T. T. Oanh Do, N. T. Thoa, N. Q. Hien, N. T. Thuc, N. N. Hai, N. D. Bang, N. N. Lan, N. H. Duc, V. N. Tuan, C. H. Hiep, T. T. Chau, P. P. Mai, N. T. Dung, K. Stepniewska, N. J. White, T. T. Hien, and J. J. Farrar. 2005. Effect of antituberculosis drug resistance on response to treatment and outcome in adults with tuberculous meningitis. J. Infect. Dis. 192:79-88. [DOI] [PubMed] [Google Scholar]
- 23.Thwaites, G. E., D. B. Nguyen, H. D. Nguyen, T. Q. Hoang, T. T. Do, T. C. Nguyen, Q. H. Nguyen, T. T. Nguyen, N. H. Nguyen, T. N. Nguyen, N. L. Nguyen, H. D. Nguyen, N. T. Vu, H. H. Cao, T. H. Tran, P. M. Pham, T. D. Nguyen, K. Stepniewska, N. J. White, T. H. Tran, and J. J. Farrar. 2004. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 351:1741-1751. [DOI] [PubMed] [Google Scholar]
- 24.Tsenova, L., E. Ellison, R. Harbacheuski, A. L. Moreira, N. Kurepina, M. B. Reed, B. Mathema, C. E. Barry III, and G. Kaplan. 2005. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J. Infect. Dis. 192:98-106. [DOI] [PubMed] [Google Scholar]
- 25.Tsolaki, A. G., S. Gagneux, A. S. Pym, Y. O. Goguet de la Salmoniere, B. N. Kreiswirth, D. Van Soolingen, and P. M. Small. 2005. Genomic deletions classify the Beijing/W strains as a distinct genetic lineage of Mycobacterium tuberculosis. J. Clin. Microbiol. 43:3185-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.UNAIDS. 2005. AIDS epidemic update: Asia. UNAIDS, Geneva, Switzerland.
- 27.van Soolingen, D., L. Qian, P. E. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]