Abstract
Brugian filariasis (caused by the nematodes Brugia malayi and B. timori) is an important cause of disability in Southeast Asia. Improved diagnostic tests are needed for filariasis elimination programs (to identify areas of endemicity and to monitor progress) and for diagnosis of the disease in infected individuals. We have developed and evaluated two real-time PCR assays for detecting Brugia DNA in human blood and compared the results of these assays to those of “gold standard” assays. One assay uses a TaqMan probe (TaqM) to amplifiy a 320-bp “HhaI repeat” DNA sequence. The other assay uses a minor groove binding probe (MGB) and modified nucleotides in primers (Eclipse MGB) to amplify a 120-bp fragment of the HhaI repeat. This assay detects 22 copies of the target sequence, and it is more sensitive than the TaqM assay. Both assays were evaluated with human blood samples from two different areas of endemicity. The MGB assay was as sensitive as membrane filtration and microscopy for the detection of B. malayi infection in 57 blood samples recovered at night from patients in Sulawesi, Indonesia. The MGB assay also detected parasite DNA in 17 of 31 (55%) of microfilaria-negative day blood samples from these subjects. This test was more sensitive than the conventional and the TaqM PCRs (and was almost as sensitive as night blood membrane filtration) for the detection of infection in 52 blood samples recovered at night from individuals in an area of B. timori endemicity on Alor Island, Indonesia, where microfilaria-positive individuals had low densities after mass treatment. Thus, the Eclipse MGB real-time PCR assay is a sensitive means of detecting Brugia parasite DNA in human blood.
PCR-based assays have become the preferred methods for the diagnosis of many parasitic infections in diagnostic reference laboratories for several reasons. First, these assays do not require extensive training, experience, or skills in microscopy. Second, the equipment and practical skills required to perform PCR are now widespread and are also increasingly widespread in the developing world. In recent years, real-time PCR has increasingly replaced conventional PCR (C-PCR) for technical reasons (improved sensitivity) and practical reasons (faster results with less labor). In addition, real-time PCR assays have the potential to be used as high-throughput diagnostic tools for the screening of blood donors, for epidemiological studies, or for the monitoring of control programs.
Brugian filariasis is a mosquito-borne illness that is caused by the parasitic nematodes Brugia malayi and Brugia timori; approximately 13 million people are infected, mainly in India and Southeast Asia. In 2000, the Global Program to Eliminate Lymphatic Filariasis was implemented with the primary goal of interrupting filariasis transmission by using mass drug administration (MDA) with microfilaricidal drugs (9, 21, 30). Improved methods for the diagnosis of filariasis are urgently needed to monitor the impact of MDA on infection rates and to detect a resurgence of infection after MDA is discontinued.
The traditional method for the diagnosis of brugian filariasis is the detection of microfilariae (mf) in blood samples recovered at night (night blood samples) by microscopy with a thick smear, the modified Knott's test, or membrane filtration. The disadvantages of mf detection by microscopy are low sensitivity when small amounts of blood are examined, the high costs of membrane filters, the need for specially trained microscopists, and the cumbersome assessment of individual slides. A rapid-format antigen detection test (Filariasis Now; Binax, Inc., Portland, ME) is available for the detection of infections with the closely related filarial parasite Wuchereria bancrofti, and this test is widely used for mapping and monitoring the impact of mass treatment programs (28). However, the card test does not detect Brugia infections. Several recombinant B. malayi antigens have been used in enzyme-linked immunosorbent assays (ELISAs) or rapid-format assays to detect filaria-specific immunoglobulin G4 antibodies (17). A disadvantage of antibody detection is that it is an indirect diagnostic approach which relies on the host's immune response; positive antibody test results do not necessarily indicate current infection.
Several C-PCR assays have been developed for the molecular diagnosis of filarial infections. These assays detect repeated parasite DNA sequences in human blood and mosquito vectors (18, 33). These methods detect DNA of W. bancrofti or B. malayi and B. timori in blood samples or in mosquitoes with high sensitivities and specificities (2, 4-6, 8, 10, 14, 22, 29). However, they require cumbersome methods for the detection of PCR products by agarose gel electrophoresis, Southern blotting, or ELISA or with expensive DNA detection test strips. C-PCR assays based on gel electrophoresis have subjective endpoints and they are difficult to standardize; they are also prone to contamination because they require the manipulation of PCR products.
Real-time PCR is a powerful and relatively new tool for high-throughput detection of parasite DNA and is increasingly replacing C-PCR and microscopy for the diagnosis of several parasitic infections (1, 19, 25). Real-time PCR has been used in filariasis research for gene expression studies and to quantify Wolbachia endobacteria, but it has not been used extensively for the molecular diagnosis of filarial infections (11, 12, 15).
The purpose of the present study was to develop real-time PCR assays for the detection of Brugia DNA and to compare the performance of these assays with those of C-PCR and with blood filtration as the “gold standard” for the parasitological detection of B. malayi. We have developed two assay formats and evaluated these with parasite DNA samples and human blood samples from areas in Indonesia where filariasis is endemic. Our results show that real-time PCR is superior to C-PCR for the detection of Brugia DNA in human blood samples.
MATERIALS AND METHODS
Parasite DNA and HhaI plasmid.
Adult B. malayi worms were obtained from experimentally infected jirds (Mongolian gerbils [Meriones unguiculatus]). The worms were washed three times with RPMI 1640 medium and frozen in phosphate-buffered saline at −70°C until use. Adult Brugia pahangi worms were generously provided by T. R. Klei (School of Veterinary Medicine, Louisiana State University). W. bancrofti microfilariae were isolated from human blood by membrane filtration.
A control plasmid was constructed by cloning a PCR product of the B. malayi HhaI repeat (20) into the pCR4-TOPO vector (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. The plasmid was propagated in Escherichia coli and purified by using a QIAprep spin miniprep kit (QIAGEN, Valencia, CA). The identity of the cloned HhaI repeat was confirmed by DNA sequencing. The size of the plasmid was 4,281 bp (including the 320-bp HhaI sequence). One microgram of the plasmid contains approximately 3.6 fmol plasmid DNA; 1 fg of plasmid DNA contains 216 copies of the HhaI sequence. The concentration of the plasmid was measured with a GeneQuant instrument (Pharmacia Biotech, Cambridge, United Kingdom).
Human blood samples.
Blood samples from individuals living in areas highly endemic for B. malayi or B. timori were collected during field surveys in eastern Indonesia in 1998, 2003, and 2004 (5, 27). Venous nighttime blood samples were collected between 7 p.m. and 11 p.m. in EDTA-coated vacuum tubes in central Sulawesi (B. malayi) and on Alor Island (B. timori). In addition, in the area where B. malayi is endemic, blood samples were collected during the day (day blood samples), between 9 a.m and noon, from some of the microfilaremic individuals who had provided night blood samples. Blood donors on Sulawesi were untreated. Most blood donors on Alor Island had been treated with two to three rounds of diethylcarbamazine combined with albendazole. Blood samples from Alor Island were collected 10 months after the last round of MDA; and mf densities were relatively low, with a few exceptions for individuals who had not participated in MDA. Microfilaria densities were assessed by filtration of 1 ml night blood, as described previously (27). Blood samples from Sulawesi were kept at ambient temperatures in the field for 1 to 2 days and were then transported to a central laboratory and stored at −20°C until use in DNA studies. The B. timori samples from Alor Island were preserved in the field by adding 500 mM EDTA up to a final concentration of 100 mM; these samples were stored for up to 3 months at ambient temperatures, and they were later stored at −20°C. Use of a high concentration of EDTA inhibits DNases and enables storage at ambient temperatures. However, EDTA can inhibit the PCR if it is not totally removed during DNA extraction.
The project was approved by the ethical review board of the University of Indonesia in Jakarta. Informed consent was obtained from all individuals who participated in the study. Blood samples from 3 patients infected with W. bancrofti, 3 patients infected with Onchocerca volvulus, 18 individuals from tropical Africa (where is Brugia not endemic) without clinical signs of disease, and 6 healthy Europeans available from previous studies were used as controls.
DNA extraction.
Total genomic DNA was extracted from adult worms by using a QIAamp DNA extraction kit (QIAGEN) and eluted in 200 μl of sterile water. Total DNA from thawed blood samples was prepared by using the QIAamp blood DNA extraction kit (QIAGEN). DNA was extracted from 100 μl of night blood or from 200 μl of day blood. For each DNA extraction, at least three negative control blood samples (human blood) and one microfilaria-positive sample (human or jird blood) were included. In order to detect potential PCR inhibitors in the DNA extracts, PCR-negative samples were spot-checked by adding Brugia DNA and were retested by C-PCR or real-time PCR, but no significant inhibition was detected. DNA samples were kept frozen at −20°C until use. The technicians who performed the DNA extractions and subsequent real-time PCR testing did not know the infection status of the blood samples being tested.
Design of real-time PCR assays.
The HhaI repeat (GenBank accession nos. M12691 and AF499109 to AF499129) was used as the target sequence for several reasons. This sequence is arranged as a tandem repeat and is present at several thousand copies per haploid genome (7). Furthermore, most C-PCR assays for Brugia have used this target, and no consistent sequence variation has been reported in various strains of B. malayi and B. timori (3, 6). The HhaI repeat is AT rich, and closely related sequences are present in zoonotic filarial species such as Brugia pahangi; this makes it difficult to design primers and probes specific for the Brugia species that are parasitic in humans (B. malayi and B. timori). In addition, even within one parasite, the numbers of copies of the HhaI repeat are not identical, and there are conserved and more variable areas. Conserved regions rarely span more than 30 bp, and selection of long primers and probes can decrease detection sensitivity. Primer Express software for TaqMan (TaqM) assays (Applied Biosystems, Foster City, CA) recommended the use of primers and a probe that were 28 to 40 bp long. Therefore, we decided to use the published primers and probe sequences for the conventional PCR in a TaqM assay (5, 18).
The use of modified nucleotides (“supernucleotides”) together with minor groove binding probe (MGB) probes increases the melting temperatures of oligonucleotides to complementary sequences. This enables the design of short and specific primers and probes for AT-rich target sequences. Therefore, an MGB Eclipse assay was designed by using specific software (Nanogen, Bothell, WA) to amplify a 118-bp fragment of the HhaI repeat (Table 1).
TABLE 1.
Nucleotide sequences of the oligonucleotide primers and probes used to amplify the HhaI repeat by either TaqM assay or Eclipse MGB assay
| Name (position)a | Sequence (5′-3′)b | Reporter | Quencher | Passive reference | Data collection |
|---|---|---|---|---|---|
| T-HhaI-For (1) | GCGCATAAATTCATCAGC | ||||
| T-HhaI-Rev (301) | GCGCAAAACTTAATTACAAAAGC | ||||
| T-HhaI-probe (238) | ACGTGAATTGTACCAGTGCTGGTCG | FAMc (5′) | TAMRAd (3′) | ROXe | Extension |
| MGB-HhaI-For (253) | GCAATATACGACCAGCAC | ||||
| MGB-HhaI-Rev (139) | ACA*TTAGA*CAAGGAAATTGGTT | ||||
| MGB-HhaI-probe (192) | TTTTTAGTAGTTTTGGC | FAM (3′) | Eclipse dark (5′) | None | Annealing |
T-HhaI, TaqM assay; MGB-HhaI, Eclipse MGB assay. The nucleotide position refers to the HhaI sequence in GenBank with accession number M12669.
A star denotes incorporation of a supernucleotide to the left.
FAM, 6-carboxyfluorescein.
TAMRA, 6-carboxytetramethylrhodamine.
ROX, carboxy-X-rhodamine.
TaqM real-time PCR.
The primers and probe were synthesized by Integrated DNA Technologies Inc., Coralville, IA (for sequence and labeling information, see Table 1). The primers were purified by the supplier by standard desalting, while the labeled probes were purified by dual high-pressure liquid chromatography. Real-time PCR mixtures included 12.5 μl of Universal TaqMan master mixture (Applied Biosystems), 400 nM each primer, and 125 nM probe in a final volume of 25 μl. This master mixture contained dUTP and uracil N-glycosylase (UNG) to prevent contamination with previously amplified PCR products. One microliter of template was added to 24 μl PCR master mixture in 96-well MicroAmp optical plates (Applied Biosystems). Thermal cycling and data analysis were done with an ABI Prism 7000 or 7300 instrument (Applied Biosystems). Cycling conditions included 2 min of incubation at 50°C for UNG performance, 10 min at 95°C for activation of Taq polymerase and deactivation of UNG, and 40 cycles of 10 s of denaturation at 95°C and 60 s at 60°C for combined annealing and extension. A three-step protocol with 10 s of denaturation at 95°C, 30 s of annealing at 55°C, and 30 s of extension at 72°C was evaluated; but that protocol was no more sensitive than the two-step protocol. All PCRs were carried out in duplicate. The cycle threshold (Ct) value was defined as the number of PCR cycles required for the fluorescence signal of the amplified products to exceed the detection threshold value. The Ct values reported for the samples are the arithmetic means for both duplicates. Preliminary studies showed that the assay-to-assay and person-to-person variations for identical samples were ±1 cycle. Therefore, samples were retested if only one of the duplicates produced a positive signal or if the Ct value was borderline (39 cycles). Water was used as a no-template negative control and 100 pg of B. malayi DNA served as a positive control in all real-time PCR runs.
Eclipse MGB real-time PCR.
The primers and probe were purchased from Nanogen and were delivered as 20× concentrates. The reaction mixture for the real-time PCR consisted of 12.5 μl of Eclipse MGB PCR reagent buffer containing 1.25 U Jumpstart Taq DNA polymerase (Sigma-Aldrich, St. Louis, MO), 1.25 μl primer mixture, 1.25 μl probe, 0.5 U UNG (Applied Biosystems), and water up to a final volume of 25 μl. Cycling conditions included a 20-min incubation at 37°C for UNG performance; 2 min at 95°C for activation of Taq polymerase and deactivation of UNG; and 40 cycles of 10 s at 95°C for denaturation, 30 s at 58°C for annealing, and 30 s at 76°C for extension. Data collection was performed at the annealing step (Table 1). When the ABI 7000 instrument was used, the cycling was programmed to start with the extension step, followed by denaturation and annealing, because this instrument automatically collects fluorescence data in the last step of each cycle.
C-PCR.
C-PCR was performed with all night blood samples. The HhaI repeat was amplified by using the primers and reaction conditions described previously (6, 18). Following PCR, 10 μl of the PCR product was electrophoresed on a 1.5% agarose gel containing ethidium bromide. Gels containing DNA bands were viewed with a UV light box and photographed. Samples showing DNA bands at the expected size of 320 bp were scored as C-PCR positive.
Data analysis.
Target sequence copy numbers were calculated from the Ct values by using the external standard curves generated with the TaqM or Eclipse MGB assays (16). The association between mf counts and Ct values or copy numbers was analyzed by the Spearman rank test.
RESULTS
Sensitivity of TaqM real-time PCR.
Serial dilutions (1 ng to 0.1 fg) of genomic B. malayi DNA extracted from adult female worms were tested to assess the sensitivity of the TaqM real-time PCR. The limit for reliable detection of the HhaI repeat in genomic DNA was about 100 fg of total DNA (Fig. 1). No fluorescence signal was obtained when purified DNA from W. bancrofti, B. pahangi, or humans was tested; this confirmed the specificity of the assay.
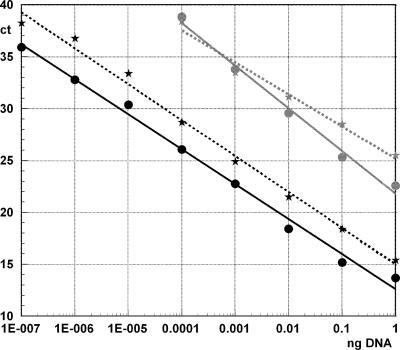
FIG. 1.
Sensitivities of the TaqM assay (gray lines) and Eclipse MGB (black lines) real-time PCR for detection of genomic Brugia malayi DNA (stars) or plasmid DNA (circles) containing the HhaI sequence. The amount of DNA (ng; x axis) is plotted against the cycle threshold values (y axis). Strong inverse correlations were observed between the amount of DNA and Ct values (both tests and both DNA samples; R values ranged from −0.9899 to −0.9964; P < 0.001).
For better standardization, the assay was also evaluated by using a plasmid containing the HhaI target sequence. The detection limit obtained with the plasmid was similar to that observed with genomic DNA, and no significant difference in Ct values was observed over the range of 1 ng to 100 fg (P = 0.145) (Fig. 1). We estimate that the TaqM real-time PCR assay has a detection limit of approximately 22,000 copies of the HhaI target. Quality control testing revealed that this assay had a mean efficiency of 101.5% ± 3.5% (cutoff of efficiency, 93 to 105%).
Sensitivity of Eclipse MGB real-time PCR.
Serial dilutions of genomic B. malayi DNA and plasmid DNA containing the HhaI target sequences were tested. The detection limit for genomic DNA was approximately 0.1 fg. This corresponds to 22 copies of the HhaI sequence in the plasmid DNA. Over the range from 1 ng to 0.1 fg, the Ct values for the plasmid were about 2 cycles lower than those for genomic DNA. This means that genomic DNA contains only about 25% of the number of copies of the target sequence in an equivalent amount of plasmid DNA (Fig. 1). Since 7.6% of the plasmid is the target sequence, it can be estimated that the target sequence detected in this assay accounts for approximately 1.9% of total genomic B. malayi DNA. Quality control testing showed that the assay had an average amplification efficiency of 98.5% ± 4.5% (cutoff of efficiency, 93 to 105%).
The MGB assay did not detect a signal with W. bancrofti, B. pahangi, or human DNA. Interestingly, the primers used in the TaqM assay amplify a HhaI target in B. pahangi (visible by agarose gel electrophoresis), but the TaqMan probe does not bind to this target. In contrast, the primers used in the Eclipse MGB assay do not amplify any target in B. pahangi DNA (data not shown).
Real-time PCR to detect Brugia malayi or B. timori DNA in night blood samples.
The operational sensitivity of the PCR assay may differ from the sensitivity assessed by using purified target DNA or plasmid DNA containing the target sequence. Therefore, the sensitivities of the TaqM assay and the MGB assay were evaluated with blood samples from humans living in areas highly endemic for brugian filariasis. These results were compared to those obtained by C-PCR with the same samples.
(i) B. malayi samples from Sulawesi.
We tested night blood samples from 36 microfilaremic and 21 microfilaria-negative individuals who lived in an area in central Sulawesi, Indonesia, where nocturnally periodic B. malayi is endemic. The geometric mean microfilaria density in 1 ml of positive blood samples was 63 mf/ml (range, 1 to 1,812 mf/ml). The PCR assays tested 1 μl of DNA template (1% of the DNA isolated from 100-μl blood samples per reaction mixture). The MGB assay was positive for all 36 samples, while both the TaqM and the C-PCR assays were positive for 33 samples (Table 2). The MGB and the C-PCR assays detected Brugia DNA in 2 of 21 samples of individuals who tested microfilaria negative by filtration, while the TaqM assay was positive for 1 of these samples. There was no significant difference in sensitivity between the three PCR assays (P > 0.05). These results show that in this area with moderate to high mf densities, the three PCR assays had sensitivities similar to the sensitivity of membrane filtration, although they sample a smaller quantity of blood.
TABLE 2.
Conventional and real-time PCR detection of HhaI repeat DNA in blood from Indonesian subjects living in areas highly endemic for Brugia malayi or Brugia timori
| No. of mf/mla |
Brugia malayi
|
Brugia timori
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of samples | No. (%) of samples positive byb:
|
No. of samples | No. (%) of samples positive by:
|
|||||
| C-PCR | TaqM assay | Eclipse MGB assay | C-PCR | TaqM assay | Eclipse MGB assay | |||
| 0 | 21 | 2 (10) | 1 (5) | 2 (10) | 21 | 0 (0) | 3 (14) | 7 (33) |
| 1-10 | 4 | 2 (50) | 4 (100) | 4 (100) | 15 | 9 (60) | 5 (33) | 10 (67) |
| 11-50 | 12 | 11 (92) | 10 (83) | 12 (100) | 8 | 6 (75) | 3 (38) | 8 (100) |
| >50 | 20 | 20 (100) | 19 (95) | 20 (100) | 8 | 7 (88) | 7 (88) | 8 (100) |
The mf density was estimated by membrane filtration of 1 ml of venous blood collected at night.
PCR was performed with DNA extracted from 100-μl night blood samples. B. malayi samples were from untreated subjects in Sulawesi with blood frozen shortly after collection. B. timori samples from Alor Island were mostly from previously treated subjects; blood was preserved in the field in EDTA and later frozen.
(ii) B. timori samples from Alor Island post-MDA.
The second set of samples was obtained from 31 microfilaremic and 21 microfilaria-negative individuals from an area on Alor Island, Indonesia, where B. timori is endemic. In contrast to the B. malayi samples, the B. timori samples were collected 10 months after two rounds of MDA of diethylcarbamazine with albendazole; subjects with persistent microfilaremia in this area generally had very low microfilaria densities (Table 2). The geometric mean microfilarial density in microfilaremic individuals was 13 mf/ml. C-PCR detected Brugia DNA in 22 of the 31 microfilaria-positive samples, the TaqM assay detected Brugia DNA in 17 samples, and the Eclipse MGB assay detected Brugia DNA in 26 samples. Among the 21 samples from microfilaria-negative individuals, 3 were positive by the TaqM assay and 7 were positive by the MGB assay. One of these real-time PCR-positive and filtration-negative samples was obtained from a person with filarial lymphoedema, while two other samples were collected from individuals who had tested microfilaria positive 1 year earlier.
(iii) Other filarial infections and control samples from areas of nonendemicity.
All samples from individuals infected with other filarial parasites, such as W. bancrofti (n = 3) or O. volvulus (n = 3), or from Africans (n = 18) or Europeans (n = 6) with no history of exposure to Brugia were negative by all three PCR assays, confirming the high specificities of the assays. No higher background was observed with these samples than with the negative no-template controls.
Real-time PCR to detect DNA of nocturnally periodic B. malayi in day blood.
We examined day blood samples from 31 individuals from Sulawesi who had B. malayi microfilaremia in their night blood, as determined with the Eclipse MGB assay, because this was the most sensitive of the three PCR assays tested. All of these samples were mf negative by microscopy. The MGB assay detected Brugia DNA in 17 of 31 samples (55%) (Table 3). The day blood samples that were negative by the MGB assay had a geometric mean mf density in night blood of 20 per ml, while the mf density in the night blood of people whose day blood was positive by the MGB assay was 136 per ml. This difference was statistically significant (P > 0.01). All six samples with 264 or more mf per ml night blood but with no mf in the day blood were MGB positive. These results indicate that the MGB assay often detected filarial DNA in day blood when no mf were detected by microscopy.
TABLE 3.
Sensitivity of Eclipse MGB real-time PCR for detection of nocturnally periodic Brugia malayi in daytime blood samples from Indonesia
| No. of mf/mla | No. of samples
|
No. (%) of samples Eclipse MGB PCR positiveb | |
|---|---|---|---|
| Night blood | Day blood | ||
| 1-10 | 3 | 0 | 0 |
| 11-50 | 11 | 0 | 5 (45) |
| >50 | 17 | 0 | 12 (71) |
The mf density was estimated by membrane filtration of 1 ml of venous blood. Night blood was collected between 7 p.m. and 11 p.m. Day blood was collected between 9 a.m. and noon.
A total of 200 μl of liquid blood was used for the DNA extractions.
Relation between HhaI copy numbers and parasite load. (i) Night blood samples.
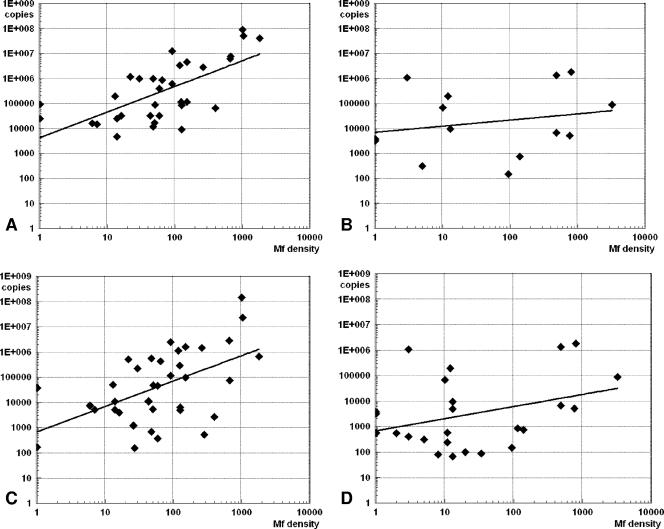
We used an external standard curve to estimate the HhaI copy numbers in blood samples based on the Ct values obtained by the TaqM assay or the Eclipse MGB real-time PCR. The estimated copy numbers were positively correlated with the mf densities for both assays (Fig. 2). The correlation was significant only for the B. malayi night blood samples from Sulawesi, which had a broader range of mf counts (TaqM assay R value, 0.767; Eclipse MGB assay R value, 0.42; P < 0.05). These results indicate that real-time PCR assays can provide information on mf densities without the need for plate-specific standard curves and internal control samples.
FIG. 2.
Sensitivity of real-time PCR for detection of the HhaI sequence in blood samples. The numbers of copies of the HhaI repeat were calculated by using external standard curves. Copy numbers were plotted against blood microfilaria counts (mf/ml) on a double logarithmic scale. (A) TaqM assay results for Brugia malayi blood samples from Sulawesi (R = 0.767; P < 0.05); (B) TaqM assay results for Brugia timori blood samples from Alor Island (correlation not significant); (C) Eclipse MGB assay results for B. malayi blood samples from Sulawesi (R = 0.42; P < 0.05); (D) Eclipse MGB assay results for B. timori blood samples from Alor Island (correlation not significant).
(ii) Day blood samples from Sulawesi.
The geometric mean copy number in the day blood samples was estimated to be 5.5 ×104 copies/μl (range, 1,400 to 1.7 ×107 copies/μl). For comparison, the corresponding night blood samples had a geometric mean copy number of 13 × 104 copies/μl (range, 530 to 1.4 × 107 copies/μl). Although the day blood samples had significantly lower copy numbers than the night blood samples (P < 0.05), the difference was not as high as expected from the mf densities. Day and night blood samples were tested in the same real-time PCR run and normalized because the DNA of the day blood samples was extracted from twice as much blood.
DISCUSSION
We developed and evaluated two sensitive real-time PCR methods for detecting Brugia DNA in blood samples. By using blood samples from microfilaremic individuals from Indonesia, we found that the detection of the HhaI repeat by real-time PCR can be as sensitive as the microscopic detection of mf, but with a much smaller amount of blood. The Eclipse MGB real-time PCR assay was more sensitive than the C-PCR or the TaqM assay, and it detected the DNA of nocturnally periodic B. malayi in day blood samples. This represents a significant improvement over previously available diagnostic methods.
The tandemly repeated 320-bp HhaI sequence of Brugia has been used as a target for PCR assays for many years (18). The present study shows that this sequence is also a useful target for real-time PCR. Early studies estimated that there were about 30,000 copies of the HhaI repeat per haploid genome of B. malayi (∼10% of the total DNA) (20). Shotgun sequencing of the B. malayi genome has shown that the HhaI repeat is less abundant than this and suggests that the HhaI repeat comprises only ∼1% of the haploid genome (7).
Conventional PCR assays by using the HhaI repeat as a target can detect ∼1 pg of B. malayi DNA, which is less than 1% of the DNA in one mf (18). The combination of C-PCR with ELISA or test strip detection of amplicons can detect 75 to 500 fg of DNA (5, 6). The TaqM real-time PCR that we developed also has a sensitivity in this range (detection limit, ∼100 fg).
The Eclipse MGB real-time PCR assay has a better sensitivity than the other PCR assays tested, with a detection limit of 0.1 fg, or approximately 22 HhaI copies. It is difficult to estimate the total copy number of the HhaI repeat in the Brugia genome on the basis of our data, because not all copies of the repeat sequence are identical and only a subset may be detected by the MGB assay. On the basis of the HhaI sequences for B. malayi and B. timori available in the public databases, we estimate that ∼70% of the HhaI sequences should be detected by the MGB assay. If we divide 1.9% (the estimated percentage of total genomic B. malayi DNA that comprises HhaI repeat sequences detected by the MGB assay) by 70% (the percentage of all HhaI sequences detected by the MGB assay), we estimate that HhaI repeat sequences account for 2.7% of the B. malayi genome. The haploid genome size of Brugia is about 90 Mb (7). Thus, the target sequence detected by the MGB assay comprises ∼1.71 Mb, or 5,300 copies per haploid genome. If a single Brugia cell is present in 100 μl blood, extracted with 50% efficiency, and eluted in 100 μl, the result would be 53 copies of the HhaI target sequence per μl of DNA template (the amount used in one real-time PCR). This is more than twice as much as our measured detection limit of 22 copies for the MGB assay.
Our field studies used membrane filtration of 1 ml blood with microscopy as the gold standard for evaluation of PCR assays. A disadvantage of filtration is that it requires the collection of venous blood by specially trained personnel and expensive supplies (filters, syringes, etc.). Therefore, the Global Program to Eliminate Lymphatic Filariasis recommends the use of thick blood smears for assessment of the prevalence and density of mf in populations (32). Although mf densities are higher in capillary blood than in venous blood, membrane filtration of venous blood is more sensitive than the use of thick smears for the detection of mf because of the large amount of blood tested by the filter method (1 ml versus 20 to 60 μl tested in thick smears) (13). All three PCR assays tested had sensitivities similar to the sensitivity of membrane filtration with blood samples from untreated patients in an area with moderate to high mf densities (Sulawesi). All of these tests are likely to be much more sensitive than the thick blood smear method. The increased sensitivity of the Eclipse MGB real-time assay would be especially important in areas with low mf densities following MDA (such as Alor Island).
The Eclipse MGB assay had a fairly good sensitivity for the detection of Brugia DNA in day blood samples from Sulawesi. Filariasis experts have requested the development of tests that can replace night blood examination for the detection of mf (31). The Eclipse MGB assay represents an important step toward this goal.
Real-time PCR provides an automated and objective means of detection of specific PCR products and is superior to other methods, such as agarose gel electrophoresis and Southern blotting. It also has significant advantages over previous methods that used a semiautomated quantitative PCR system or detection of PCR products by ELISA (5, 24). Our group has recently shown that real-time PCR is superior to C-PCR for detecting W. bancrofti DNA in blood and mosquito samples (23). The results from the present study, especially those obtained with the Eclipse MGB assay, confirm that real-time PCR is superior to C-PCR for the diagnosis of filariasis.
Although brugian filariasis is not common among travelers, the real-time PCR assay described here (together with similar assays for other helminth parasites) may be useful for the diagnosis of filariasis and other helminth infections in travelers and immigrants with eosinophilia or symptoms consistent with helminth infections. Since cases of brugian filariasis are rare in the United States, Europe, and other areas of nonendemicity, experience with the morphological identification of mf is limited in these areas and the establishment of an accurate diagnosis is often a problem. The assays described here should permit clinical or reference laboratories without specific expertise in medical helminthology to accurately detect B. malayi or B. timori in blood samples or biopsy specimens.
While the real-time PCR can be used to diagnose filariasis in individuals, the main practical importance of these assays will be as tools for the Global Program to Eliminate Lymphatic Filariasis. Real-time PCR can be used to screen mosquitoes or human blood samples to identify and map areas of endemicity. The assay should also be useful for assessing changes in transmission following MDA by molecular xenomonitoring (29). One problem with introducing more sensitive diagnostic assays during the course of ongoing control programs is the comparabilities of different tests. Real-time PCR may detect persistent infections or remnants of parasites that were not detectable by traditional methods, such as the thick smear method (human blood) or dissection (mosquitoes).
PCR assays have significant advantages over other methods used for the diagnosis of filariasis (greater sensitivity, lower labor requirements, and high-throughput capability). Since there is no post-PCR handling of the amplification products, real-time PCR assays are less prone to contamination than C-PCR assays that look at PCR products by agarose gel electrophoresis. Although real-time PCR instruments cost more than conventional thermocyclers, their prices are decreasing rapidly. Apart from the instrument, the materials costs per sample for C-PCR and real-time PCR are comparable; labor costs favor real-time assays, because they do not require a separate assay for the detection of PCR products. We estimate that the TaqM assay costs about $3.80 for supplies per sample, including blood collection, while the Eclipse MGB real-time assay costs about $4.40 per sample. The higher sensitivity and applicability for the diagnosis of filariasis with day blood make the Eclipse test the more promising diagnostic tool. For comparison, the supply costs for filtration of 1 ml blood, including venous blood collection, costs about $3.00 per sample. For large population surveys, the costs of PCR can be reduced by testing pooled blood samples (26). The Eclipse MGB real-time assay is slightly more expensive than the TaqM assay, but its higher sensitivity and applicability for diagnosis by the use of day blood make it the more promising diagnostic tool.
In conclusion, our studies have shown that real-time PCR can be used as a sensitive and specific means of detecting Brugia DNA in human blood samples. This technology can be used for the diagnosis of filariasis in individual patients, but it is likely to be more useful as a tool for the mapping and monitoring of brugian filariasis in the framework of the Global Program to Eliminate Lymphatic Filariasis.
Acknowledgments
We thank the filariasis field research team at the University of Indonesia in Jakarta for help during sample collection on Sulawesi and Alor Island. We also thank Laura Atkinson and Kurt Curtis for excellent technical assistance.
We have no conflict of interest to declare.
This work was supported in part by the National Institutes of Health grant AI-35855. Sample collection in Indonesia was supported in part by UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR) and by GlaxoSmithKline, United Kingdom.
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Bell, A. S., and L. C. Ranford-Cartwright. 2002. Real-time quantitative PCR in parasitology. Trends Parasitol. 18:337-342. [PubMed] [Google Scholar]
- 2.Bockarie, M. J., P. Fischer, S. A. Williams, P. A. Zimmerman, L. Griffin, M. P. Alpers, and J. W. Kazura. 2000. Application of a polymerase chain reaction-ELISA to detect Wuchereria bancrofti in pools of wild-caught Anopheles punctulatus in a filariasis control area in Papua New Guinea. Am. J. Trop. Med. Hyg. 62:363-367. [DOI] [PubMed] [Google Scholar]
- 3.Fischer, P., D. Boakye, and J. Hamburger. 2003. Polymerase chain reaction-based detection of lymphatic filariasis. Med. Microbiol. Immunol. (Berlin) 192:3-7. [DOI] [PubMed] [Google Scholar]
- 4.Fischer, P., X. Liu, M. Lizotte-Waniewski, I. H. Kamal, R. M. Ramzy, and S. A. Williams. 1999. Development of a quantitative, competitive polymerase chain reaction-enzyme-linked immunosorbent assay for the detection of Wuchereria bancrofti DNA. Parasitol. Res. 85:176-183. [DOI] [PubMed] [Google Scholar]
- 5.Fischer, P., T. Supali, H. Wibowo, I. Bonow, and S. A. Williams. 2000. Detection of DNA of nocturnally periodic Brugia malayi in night and day blood samples by a polymerase chain reaction-ELISA-based method using an internal control DNA. Am. J. Trop. Med. Hyg. 62:291-296. [DOI] [PubMed] [Google Scholar]
- 6.Fischer, P., H. Wibowo, S. Pischke, P. Ruckert, E. Liebau, I. S. Ismid, and T. Supali. 2002. PCR-based detection and identification of the filarial parasite Brugia timori from Alor Island, Indonesia. Ann. Trop. Med. Parasitol. 96:809-821. [DOI] [PubMed] [Google Scholar]
- 7.Ghedin, E., S. Wang, J. M. Foster, and B. E. Slatko. 2004. First sequenced genome of a parasitic nematode. Trends Parasitol. 20:151-153. [DOI] [PubMed] [Google Scholar]
- 8.Goodman, D. S., J. N. Orelus, J. M. Roberts, P. J. Lammie, and T. G. Streit. 2003. PCR and mosquito dissection as tools to monitor filarial infection levels following mass treatment. Filaria J. 2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyapong, J. O., V. Kumaraswami, G. Biswas, and E. A. Ottesen. 2005. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin. Pharmacother. 6:179-200. [DOI] [PubMed] [Google Scholar]
- 10.Helmy, H., P. Fischer, H. A. Farid, M. H. Bradley, and R. M. Ramzy. 2004. Test strip detection of Wuchereria bancrofti amplified DNA in wild-caught Culex pipiens and estimation of infection rate by a PoolScreen algorithm. Trop. Med. Int. Health 9:158-163. [DOI] [PubMed] [Google Scholar]
- 11.Higazi, T. B., A. Filiano, C. R. Katholi, Y. Dadzie, J. H. Remme, and T. R. Unnasch. 2005. Wolbachia endosymbiont levels in severe and mild strains of Onchocerca volvulus. Mol. Biochem. Parasitol. 141:109-112. [DOI] [PubMed] [Google Scholar]
- 12.Hoerauf, A., S. Mand, K. Fischer, T. Kruppa, Y. Marfo-Debrekyei, A. Y. Debrah, K. M. Pfarr, O. Adjei, and D. W. Buttner. 2003. Doxycycline as a novel strategy against bancroftian filariasis—depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production. Med. Microbiol. Immunol. (Berlin) 192:211-216. [DOI] [PubMed] [Google Scholar]
- 13.Kimura, E., L. Penaia, and G. F. Spears. 1984. Comparison of methods for the detection of microfilariae of Wuchereria bancrofti in Western Samoa. Southeast Asian J. Trop. Med. Public Health 15:167-174. [PubMed] [Google Scholar]
- 14.Kluber, S., T. Supali, S. A. Williams, E. Liebau, and P. Fischer. 2001. Rapid PCR-based detection of Brugia malayi DNA from blood spots by DNA detection test strips. Trans. R. Soc. Trop. Med. Hyg. 95:169-170. [DOI] [PubMed] [Google Scholar]
- 15.Kron, M., M. Petridis, Y. Milev, J. Leykam, and M. Hartlein. 2003. Expression, localization and alternative function of cytoplasmic asparaginyl-tRNA synthetase in Brugia malayi. Mol. Biochem. Parasitol. 129:33-39. [DOI] [PubMed] [Google Scholar]
- 16.Kuhne, B. S., and P. Oschmann. 2002. Quantitative real-time RT-PCR using hybridization probes and imported standard curves for cytokine gene expression analysis. BioTechniques 33:1078, 1080-1082, 1084. [DOI] [PubMed] [Google Scholar]
- 17.Lammie, P. J., G. Weil, R. Noordin, P. Kaliraj, C. Steel, D. Goodman, V. B. Lakshmikanthan, and E. Ottesen. 2004. Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis—a multicenter trial. Filaria J. 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lizotte, M. R., T. Supali, F. Partono, and S. A. Williams. 1994. A polymerase chain reaction assay for the detection of Brugia malayi in blood. Am. J. Trop. Med. Hyg. 51:314-321. [DOI] [PubMed] [Google Scholar]
- 19.Mangold, K. A., R. U. Manson, E. S. Koay, L. Stephens, M. Regner, R. B. Thomson, Jr., L. R. Peterson, and K. L. Kaul. 2005. Real-time PCR for detection and identification of Plasmodium spp. J. Clin. Microbiol. 43:2435-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McReynolds, L. A., S. M. DeSimone, and S. A. Williams. 1986. Cloning and comparison of repeated DNA sequences from the human filarial parasite Brugia malayi and the animal parasite Brugia pahangi. Proc. Natl. Acad. Sci. USA 83:797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottesen, E. A. 2006. Lymphatic filariasis: treatment, control and elimination. Adv. Parasitol. 61:395-441. [DOI] [PubMed] [Google Scholar]
- 22.Ramzy, R. M., H. A. Farid, I. H. Kamal, G. H. Ibrahim, Z. S. Morsy, R. Faris, G. J. Weil, S. A. Williams, and A. M. Gad. 1997. A polymerase chain reaction-based assay for detection of Wuchereria bancrofti in human blood and Culex pipiens. Trans. R. Soc. Trop. Med. Hyg. 91:156-160. [DOI] [PubMed] [Google Scholar]
- 23.Rao, R. U., L. J. Atkinson, R. M. Ramzy, H. Helmy, H. A. Farid, M. J. Bockarie, M. Susapu, S. J. Laney, S. A. Williams, and G. J. Weil. 2006. A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes. Am. J. Trop. Med. Hyg. 74:826-832. [PMC free article] [PubMed] [Google Scholar]
- 24.Rao, U. R., S. A. Williams, and T. R. Klei. 2002. Quantification of PCR amplification products of Brugia HhaI repeat DNA using a semiautomated Q-PCR system. Mol. Cell. Probes 16:13-23. [DOI] [PubMed] [Google Scholar]
- 25.Roy, S., M. Kabir, D. Mondal, I. K. Ali, W. A. Petri, Jr., and R. Haque. 2005. Real-time-PCR assay for diagnosis of Entamoeba histolytica infection. J. Clin. Microbiol. 43:2168-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supali, T., I. S. Ismid, H. Wibowo, Y. Djuardi, E. Majawati, P. Ginanjar, and P. Fischer. 2006. Estimation of the prevalence of lymphatic filariasis by a pool screen PCR assay using blood spots collected on filter paper. Trans. R. Soc. Trop. Med. Hyg. 100:753-759. [DOI] [PubMed] [Google Scholar]
- 27.Supali, T., H. Wibowo, P. Ruckert, K. Fischer, I. S. Ismid, Purnomo, Y. Djuardi, and P. Fischer. 2002. High prevalence of Brugia timori infection in the highland of Alor Island, Indonesia. Am. J. Trop. Med. Hyg. 66:560-565. [DOI] [PubMed] [Google Scholar]
- 28.Weil, G. J., P. J. Lammie, and N. Weiss. 1997. The ICT filariasis test: a rapid-format antigen test for diagnosis of bancroftian filariasis. Parasitol. Today 13:401-404. [DOI] [PubMed] [Google Scholar]
- 29.Williams, S. A., S. J. Laney, L. A. Bierwert, L. J. Saunders, D. A. Boakye, P. Fischer, D. Goodman, H. Helmy, S. L. Hoti, V. Vasuki, P. J. Lammie, C. Plichart, R. M. Ramzy, and E. A. Ottesen. 2002. Development and standardization of a rapid, PCR-based method for the detection of Wuchereria bancrofti in mosquitoes, for xenomonitoring the human prevalence of bancroftian filariasis. Ann. Trop. Med. Parasitol. 96(Suppl. 2):S41-S46. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. 2006. Global Programme to Eliminate Lymphatic Filariasis. Wkly. Epidemiol. Rec. 81:221-232. [PubMed] [Google Scholar]
- 31.World Health Organization. 1992. Lymphatic filariasis: the disease and its control. Fifth report of the WHO Expert Committee on Filariasis. WHO Tech. Rep. Ser. 821:1-69. [PubMed] [Google Scholar]
- 32.World Health Organization. 2000. Preparing and implementing a national plan to eliminate lymphatic filariasis. WHO/CDS/CPE/CEE/2000.16. World Health Organization, Geneva, Switzerland.
- 33.Zhong, M., J. McCarthy, L. Bierwert, M. Lizotte-Waniewski, S. Chanteau, T. B. Nutman, E. A. Ottesen, and S. A. Williams. 1996. A polymerase chain reaction assay for detection of the parasite Wuchereria bancrofti in human blood samples. Am. J. Trop. Med. Hyg. 54:357-363. [DOI] [PubMed] [Google Scholar]