Abstract
Fetal bovine serum (FBS) and adult bovine serum (BS) exhibited bactericidal activity against Helicobacter pylori at various levels, which were higher in BS than in FBS. The bactericidal activity was inactivated by heat treatment at 56°C for 30 min. Our results demonstrated that heat-treated BS is a useful serum source of H. pylori culture medium.
Helicobacter pylori is usually grown on media that contain blood or serum. Horse blood is most commonly used for agar plates because of its high growth-supporting ability. For liquid culture, fetal bovine serum (FBS) is used in most studies. In vitro growth is largely affected by culture media, and the serum used is one of the most influential factors. Differences in the growth-supporting ability of media are of deep concern in both clinical and research settings, in which stable bacterial growth is required. In addition, for economical reasons, a less expensive serum that gives optimum growth is desirable. In the present study we examined the growth-supporting property of different serum lots. We show that adult bovine serum (BS), which is far less expensive than FBS, could be a useful serum source for H. pylori culture media after heat treatment.
The growth-supporting property of three FBS lots and two BS lots was compared to H. pylori 26695(American Type Culture Collection CRL-700392). The serum-containing liquid media and agar media were made with 10% serum in brain heart infusion (BHI; Difco) and BHI agar (Difco), respectively. Heat treatment of serum was performed by incubating the serum at 56°C for 30 min. H. pylori was grown in an incubator (model 7000; NAPCO), in which the atmosphere was controlled at 5% O2 and 12% CO2, with 95% humidity and a temperature of 37°C.
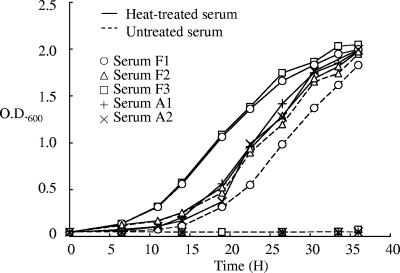
For analyses with liquid media, H. pylori was inoculated into each medium at a concentration of 6.5 × 106 CFU/ml in 10 ml of medium in a 9-cm dish and grown with rotary shaking at 60 rpm. The growth was monitored by measuring the optical density (OD) at 600 nm and the optical path length at 10 mm with a spectrophotometer (model U-3010; Hitachi, Japan). Measurement of bacterial growth by determining the OD should be applicable when bacterial cells are dispersed homogeneously in the culture. H. pylori strains often form aggregates in liquid culture, which impairs the reproducible quantification of bacterial cells. In this experiment all of the strains used grew homogeneously in liquid culture. When untreated serum was used (indicated by a dotted line in Fig. 1), there was obvious growth inhibition at various levels. Whereas two FBS lots (F1 and F2) yielded growth, no growth was obtained with one FBS lot (F3) and two BS lots (A1 and A2). When heat-treated serum was used (indicated by the solid line in Fig. 1), efficient growth was observed with all of the lots examined. With heat-treated serum F3, the doubling time of the bacteria was estimated to be ca. 3.1 h. These results indicate that each untreated serum contains a different level of growth-inhibiting activity, which is inactivated by heat treatment. With serum F1, the growth with untreated serum was slightly delayed compared to that using heat-treated serum, but the gradient of the plot was almost the same, suggesting that the growth inhibition is due to the reduction of live bacteria in the initial stage rather than suppression of the multiplication process during bacterial growth. Complement is a major bactericidal substance contained in serum, and complement-mediated bactericidal activity against H. pylori has been reported (6, 8). The characteristics of the bactericidal activity observed in this experiment agree with the general property of complement. In medium with untreated sera F3, A1, and A2, all inoculated cells would have been inactivated, resulting in complete inhibition of growth. Since the initial bacterial load was 6.5 × 106 CFU/ml in 10 ml of medium with 10% serum, the actual bacterial killing was estimated to be more than 6.5 × 107 CFU bacteria per ml of serum. The bactericidal effect was further confirmed with two additional strains, H. pylori NCTC11637 (ATCC 43504) and H. pylori 60190 (ATCC 49503). Untreated and heat-treated serum A2 was tested with these strains in the same manner. Whereas no growth was obtained with untreated serum, efficient growth was obtained with heat-treated serum, a finding consistent with the results for H. pylori 26695. These results indicate that even highly bactericidal serum lots yield efficient bacterial growth for H. pylori if heat treatment is performed in advance. Bacterial cultures of H. pylori 26695, NCTC11637, and 60190, with an OD of 1.0, contained 2.3 × 108, 1.1 × 108, and 0.9 × 108 CFU of bacterial cells/ml, respectively, suggesting that the growth of these strains was at similar levels in the liquid media examined.
FIG. 1.
Growth curve of H. pylori 26695 in BHI medium supplemented with FBS F1, FBS F2, FBS F3, BS A1, or BS A2. The results are representative of three independent experiments.
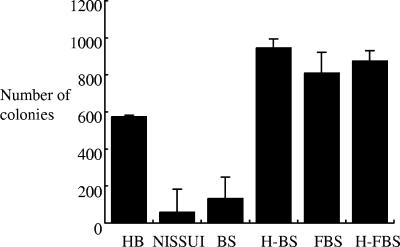
The growth-supporting property of different serum lots was further examined with agar medium. BHI agar medium containing either FBS F3, heat-treated FBS F3, BS A1, or heat-treated BS A1 and a commercial selective agar medium, NISSUI plate Helicobacter (Nissui, Japan), were tested for H. pylori growth. Horse blood agar medium was used as a reference. The bacterial suspension at an OD of 1.0 was diluted 104-fold with phosphate-buffered saline, and 25 μl of the diluted suspension was spread onto each agar medium. The number of colonies on each plate was counted after 4 days. The results are shown in Fig. 2. A growth-inhibitory effect was evident with BS but not with FBS. Although both untreated and heat-treated FBS efficiently yielded colonies, the number of colonies obtained with untreated BS was significantly lower than with the horse blood agar plate (132.3 ± 7.6 versus 574.0 ± 116.3; P < 0.01). When heat-treated BS was used, the number of colonies was ∼10-fold higher than with untreated BS (944.7 ± 123.6 versus 132.3 ± 7.6; P < 0.01). The difference in the number of colonies between untreated and heat-treated BS A1 media was approximately 8 × 102, as estimated from the data shown in Fig. 2. Since the agar plate was made with 2 ml of serum in 20 ml of medium, the actual killing of bacteria is estimated to be approximately 4 × 102 CFU per ml of serum, which is considerably lower than that observed with liquid culture. We suggest that, whereas complement is able to bind efficiently to bacterial cells in liquid medium, agar medium might be less feasible for complement molecules to migrate and reach bacterial cells that exist on the surface of agar, resulting in a reduction in actual bactericidal activity. The activity of complement contained in serum F3 might be insufficient to exert bactericidal activity with the agar medium. Our results indicate that heat-treated BS, which is usually unpopular as a serum source for H. pylori culture media, is a useful serum source for H. pylori culture. Colonies on NISSUI plate Helicobacter plates were fewer than on horse agar plates (58.7 ± 48.9 versus 574.0 ± 116.3; P < 0.01). NISSUI plate Helicobacter plates were kept sealed at 4°C after purchase until use; however, the surface of the medium seemed somewhat dry when the bacterial suspension was inoculated. This might lead to the formation of fewer colonies than seen with other agar media that were prepared and used freshly, since H. pylori requires high humidity for growth.
FIG. 2.
Colony formation of H. pylori 26695 on different agar media. HB, horse blood agar plate; NISSUI, NISSUI plate Helicobacter; BS, BHI agar with untreated BS; H-BS, BHI agar with heat-treated BS; FBS, BHI agar with untreated FBS; H-FBS, BHI agar with heat-treated FBS. The number of colonies on each agar plate was counted, and the results are expressed as means plus the standard deviation from three plates.
The usefulness of heat-treated BS was further examined with clinical specimens. Selective BHI agar (S-BHI agar) medium was made with 10% heat-treated BS and the following concentrations of antibiotics: 10 μg of vancomycin/ml, 5 μg of trimethoprim/ml, 5 μg of amphotericin B/ml, and 2.5 U of polymyxin B/ml. S-BHI agar medium was tested for H. pylori recovery from biopsy specimens in two clinical laboratories. Either a horse blood agar plate or a NISSUI plate Helicobacter plate was used as the reference medium. A microaerophilic condition was generated in a special jar with a gas-generating kit AnaeroPouch Helico (Mitsubishi-Gas Chemical, Japan), and a conventional incubator was used. Inoculation was done by smearing both the S-BHI agar plate and the reference agar plate with a single biopsy specimen. A urease test was performed on all isolates for identification. The addition of antibiotics in the medium did not cause a significant reduction in colony formation when examined with H. pylori 26695 (data not shown). When a horse blood agar plate was used as the reference medium, the results completely matched. S-BHI agar medium was able to detect H. pylori from all of the positive specimens (nine of nine specimens) but from none of the negative specimens (zero of five specimens). When the selective medium NISSUI plate Helicobacter plate was used as a reference medium, H. pylori growth was observed with 35 of 39 positive specimens, whereas no growth was obtained with 4 positive specimens using S-BHI agar medium. For three of the four specimens only a few colonies grew on NISSUI plate Helicobacter, suggesting that the false-negative result was ascribed to a very small number of bacteria contained in biopsy specimens rather than the aptness of the medium. From one specimen hundreds of colonies grew on NISSUI plate Helicobacter. In consideration of other observations that show the efficient growth-supporting ability of heat-treated BS, this false-negative result might be ascribed to a technical error that could have taken place during the procedure, such as inappropriate medium preservation, a gas leak, or insufficient humidity. There was no negative specimen determined using the NISSUI plate Helicobacter in this experiment. Taken together, the results indicate that heat-treated BS is useful for the primary culture of clinical specimens as well.
To date, a number of media containing serum, blood, blood derivatives, or egg yolk have been developed for H. pylori culture (2, 5, 12). Comparison studies have accordingly been done on various agar media for primary isolation using biopsy specimens (3, 4, 7, 9-11). For liquid culture it was reported that a lysed human erythrocyte-supplemented medium supported a remarkably rapid growth, with a doubling time of 50 min (1).
For practical use, materials to be used should be generally available, and the medium should be easily prepared. Thus, heat-treated BS is an attractive option. In the present study we showed that heat-treated BS is a useful serum source for both liquid and agar media for H. pylori culture. BS is usually far less expensive than FBS. In addition, BS can be stably preserved for a long time, in contrast to defibrinated blood, which should be consumed within a short time. Heat-treated BS would thus be a cost-effective choice as a serum source for H. pylori culture medium.
Acknowledgments
This study was supported by grant-in-aid 14770224 for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
We thank Atsushi Yuki and Naoko Ohmori for technical assistance. We thank Michio Ohta and Hidemi Gotoh for providing the facilities in the clinical study.
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Andersen, A. P., D. A. Elliott, M. Lawson, P. Barland, V. B. Hatcher, and E. G. Puszkin. 1997. Growth and morphological transformations of Helicobacter pylori in broth media. J. Clin. Microbiol. 35:2918-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, L. P., and T. Wadstrom. 2001. Basic bacteriology and culture, p. 27-38. In H. L. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori. ASM Press, Washington, D.C. [PubMed]
- 3.Ansorg, R., G. Von Recklinghausen, R. Pomarius, and E. N. Schmid. 1991. Evaluation of techniques for isolation, subcultivation, and preservation of Helicobacter pylori. J. Clin. Microbiol. 29:51-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuchi, E., M. Forne, and S. Quintana. 2002. Comparison of two transport media and three culture media for primary isolation of Helicobacter pylori from gastric biopsies. Clin. Microbiol. Infect. 8:609-610. [DOI] [PubMed] [Google Scholar]
- 5.Dent, J. C., and C. A. McNulty. 1988. Evaluation of a new selective medium for Campylobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 7:555-558. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Valencia, G., G. I. Perez-Perez, R. G. Washburn, and M. J. Blaser. 1996. Susceptibility of Helicobacter pylori to the bactericidal activity of human serum. Helicobacter 1:28-33. [DOI] [PubMed]
- 7.Hachem, C. Y., J. E. Clarridge, D. G. Evans, and D. Y. Graham. 1995. Comparison of agar based media for primary isolation of Helicobacter pylori. J. Clin. Pathol. 48:714-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korhonen, H., E. L. Syvaoja, H. Ahola-Luttila, S. Sivela, S. Kopola, J. Husu, and T. U. Kosunen. 1995. Bactericidal effect of bovine normal and immune serum, colostrum, and milk against Helicobacter pylori. J. Appl. Bacteriol. 78:655-662. [DOI] [PubMed] [Google Scholar]
- 9.Krajden, S., J. Bohnen, J. Anderson, J. Kempston, M. Fuksa, A. Matlow, N. Marcon, G. Haber, P. Kortan, M. Karmali, et al. 1987. Comparison of selective and nonselective media for recovery of Campylobacter pylori from antral biopsies. J. Clin. Microbiol. 25:1117-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccolomini, R., G. Di Bonaventura, D. Festi, G. Catamo, F. Laterza, and M. Neri. 1997. Optimal combination of media for primary isolation of Helicobacter pylori from gastric biopsy specimens. J. Clin. Microbiol. 35:1541-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tee, W., S. Fairley, R. Smallwood, and B. Dwyer. 1991. Comparative evaluation of three selective media and a nonselective medium for the culture of Helicobacter pylori from gastric biopsies. J. Clin. Microbiol. 29:2587-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westblom, T. U., E. Madan, and B. R. Midkiff. 1991. Egg yolk emulsion agar, a new medium for the cultivation of Helicobacter pylori. J. Clin. Microbiol. 29:819-821. [DOI] [PMC free article] [PubMed] [Google Scholar]