Abstract
Sixty-eight drug-resistant Mycobacterium tuberculosis isolates (44.2% of all resistant cases) were analyzed by IS6110 restriction fragment length polymorphism fingerprinting and spoligotyping to provide a deeper insight into the status of drug-resistant tuberculosis in Hungary. A total of 54.4% of the drug-resistant cases and 75% of the multidrug-resistant cases could be clustered. Analysis of the spoligotyping patterns of the strains revealed a high rate (66.2%) of infection by the Haarlem genotype, while none of the patients were infected by the Beijing genotype. The magnitude and the dynamics of drug-resistant tuberculosis are underestimated in Hungary.
One of the greatest concerns of tuberculosis control programs is the emergence and spread of drug-resistant and multidrug-resistant (MDR) (resistance at least to isoniazid [INH] and rifampin [RIF]) tuberculosis. In Hungary in 2003, there were 93 (7.9%) patients with drug-resistant tuberculosis (including MDR tuberculosis) and 16 (1.4%) patients with MDR, and in 2004, the number of drug-resistant and MDR cases was 61 (5.1%) and 9 (0.75%), respectively (8, 9). However, it is important that the rate of culture-confirmed cases was only 42.7% in 2003 and 48.6% in 2004. Moreover, mandatory susceptibility testing was performed for only 48.8% of the culture-positive isolates in 2003 and 48.5% of the culture-positive strains in 2004 (8, 9). Consequently, the actual extent and type of drug-resistant tuberculosis in Hungary are unknown.
Therefore, a retrospective population-based study was performed to provide a molecular insight into the extent of drug-resistant tuberculosis in Hungary using DNA fingerprinting analysis. For this purpose, all drug-resistant Mycobacterium tuberculosis complex strains (68 isolates) that were identified in the Hungarian Reference Laboratory for Mycobacteria at the Koranyi National Institute for Tuberculosis and Respiratory Medicine in 2003 and 2004 were analyzed by IS6110 restriction fragment length polymorphism (RFLP) fingerprinting and spoligotyping. These isolates were submitted to the reference center for susceptibility testing from all over Hungary and represented 44.2% of all drug-resistant tuberculosis cases reported in Hungary in 2003 and 2004 (8, 9).
Testing of all isolates for susceptibility to INH, RIF, ethambutol, and streptomycin (SM) was carried out by the proportion method on Löwenstein-Jensen medium as described previously by Canetti et al. (2). Conventional epidemiologic data were obtained from the database of the National Tuberculosis Surveillance System (NTSC) as described elsewhere previously (13).
IS6110 RFLP fingerprinting were performed in line with a standardized protocol as described previously (3, 14, 17). Spoligotyping was performed with a commercially available kit (Isogen Bioscience BV, Maarssen, The Netherlands) according to the instructions of the manufacturer (10). The IS6110 fingerprint and spoligotype patterns of the examined strains were analyzed using Bionumerics software, version 3.5 (Applied Maths, Sint-Martens-Latem, Belgium), as described previously (3, 14, 17). Clusters were defined as groups of patients with M. tuberculosis strains showing identical IS6110 RFLP (same number of IS6110 bands at identical positions [position tolerance, 1.2%]) and spoligotype patterns.
The chi-square test and the Mann-Whitney U test were employed to evaluate differences in demographic, epidemiologic, and drug resistance characteristics between clustered and nonclustered patients. Values of P of less than 0.05 were considered significant.
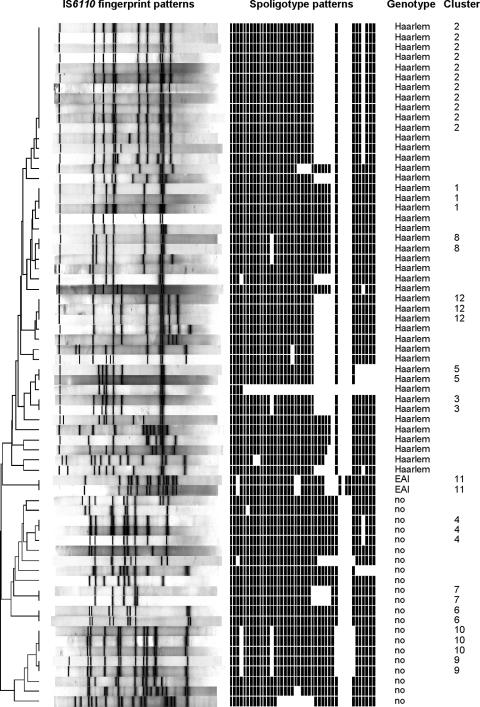
In order to display the degree of relatedness of the isolates, a dendrogram was generated by applying the Dice coefficient and the unweighted-pair group method with arithmetic mean (Fig. 1). Thirty-one isolates (45.6%) showed unique fingerprint patterns, while 37 isolates (54.4%) had an IS6110 RFLP pattern and a spoligotyping pattern identical to those of at least one other isolate and were thus grouped into 12 different clusters (Fig. 1). Eleven (16.2%) patients belonged to the largest cluster (cluster 2), 12 (17.6%) patients belonged to four smaller clusters with 3 patients each (clusters 1, 4, 10, and 12), and 14 (20.6%) patients belonged to seven clusters with 2 patients each (clusters 3, 5, 6, 7, 8, 9, and 11) (Fig. 1).
FIG. 1.
IS6110 fingerprint and spoligotyping patterns of the 68 patients with drug-resistant tuberculosis. EAI, East African-Indian.
Analysis of the spoligotyping patterns revealed that a remarkably high number (45 [66.2%]) of patients were infected with strains of the Haarlem genotype, while two (2.9%) patients were infected with an East African-Indian genotype strain, and no classification of major genotypes was possible for 21 (30.9%) isolates (Fig. 1). It is noteworthy that none of the patients were infected by strains of the Beijing genotype.
The characteristics of the 68 human immunodeficiency virus-negative patients with drug-resistant tuberculosis are summarized in Table 1. Statistical analysis did not find any significant difference between clustered and nonclustered patients with regard to their demographic characteristics or their medical and social risk factors.
TABLE 1.
Characteristics of the 68 clustered and nonclustered patients from Hungary with drug resistant-tuberculosis
| Characteristic | No. (%) of specimens
|
P valuea | ||
|---|---|---|---|---|
| Clustered (n = 37) | Nonclustered (n = 31) | Total (n = 68) | ||
| Gender | 0.4951 | |||
| Male | 30 (81.1) | 23 (74.2) | 53 (77.9) | |
| Female | 7 (18.9) | 8 (25.8) | 15 (22.1) | |
| Mean age (yr) | 48.7 | 53.7 | 50.9 | 0.1360b |
| Male | 47.5 | 51.9 | 49.4 | |
| Female | 53.9 | 58.9 | 56.5 | |
| Treatment history | 0.9637 | |||
| No | 30 (81.1) | 25 (80.6) | 55 (80.9) | |
| Yes | 7 (18.9) | 6 (19.4) | 13 (19.1) | |
| Smear microscopy | 0.5148 | |||
| Positive | 21 (56.8) | 20 (64.5) | 41 (60.3) | |
| Negative | 16 (43.2) | 11 (35.5) | 27 (39.7) | |
| Alcohol abuse | 11 (29.7) | 9 (29.0) | 20 (29.4) | 0.9499 |
| Homeless | 9 (24.3) | 4 (12.9) | 13 (19.1) | 0.2329 |
| Contact | 1 (2.7) | 1 (3.2) | 2 (2.9) | 0.8988 |
| Immigrant | 1 (2.7) | 1 (3.2) | 2 (2.9) | 0.8988 |
| Resident of congregate facility | 1 (2.7) | 0 | 1 (1.5) | 0.3565 |
Chi-square test.
Mann-Whitney U test.
A review of the database of the NTSC revealed that many of the related isolates were from patients located in similar geographic regions. For example, of the 11 patients of cluster 2 (the largest cluster), 5 (2 homeless) were from neighboring downtown districts of Budapest (the capital), and 3 were residents of two adjacent, high-incidence, northeast counties (Heves and Nógrád Counties). Similar coincidences of genotype and geographic location were observed for patients in all other clusters: two of the three patient in cluster 1, both patients in cluster 3, all three patients in cluster 4, both patients in cluster 5, both patients in cluster 6, both patients in cluster 7, both patients in cluster 8, two of the three patients in cluster 10, both patients in cluster 11, and two of the three patients in cluster 12 were from the same geographic location. Three patients with MDR tuberculosis were not reported to the NTSC.
Results of susceptibility testing of the 68 resistant isolates by the proportion method are presented in Table 2. Notably, 21 (75.0%) of the MDR strains were clustered. Comparison of susceptibility patterns of clustered and nonclustered patients revealed a statistically significant difference for mono-SM-resistant (P = 0.0374) and MDR (P = 0.0043) patients. In addition, in several clusters, a good correlation was found between the IS6110 fingerprints and the drug susceptibility patterns, implying a close relationship between these strains (data not shown).
TABLE 2.
Drug susceptibility patterns of M. tuberculosis strains isolated from the 68 clustered and nonclustered patients from Hungary with drug-resistant tuberculosis
| Drug susceptibility | No. (%) of specimens
|
P value | ||
|---|---|---|---|---|
| Clustered (n = 37) | Nonclustered (n = 31) | Total (n = 68) | ||
| Mono-INH resistant | 6 (16.2) | 7 (22.6) | 13 (19.1) | 0.5062 |
| Mono-RIF resistant | 1 (2.7) | 1 (3.2) | 2 (2.9) | 0.8988 |
| Mono-SM resistant | 2 (5.4) | 7 (22.6) | 9 (13.2) | 0.0374 |
| Polyresistant (not MDR) | 7 (18.9) | 9 (29.0) | 16 (23.5) | 0.3275 |
| MDR | 21 (56.8) | 7 (22.6) | 28 (41.2) | 0.0043 |
The present study is the first to provide a molecular epidemiological insight into the patterns and transmission dynamics of drug-resistant tuberculosis in Hungary. The strains that were included in the study exhibit a medium degree of DNA polymorphism (43 different RFLP patterns in 68 isolates). Since strain diversity is inversely associated with the incidence of the disease, this observation may indicate that the incidence of drug-resistant tuberculosis is actually underestimated by presently available conventional epidemiologic data (7).
The rate of clustered cases (54.4%) was much higher than the rate of clustered drug-resistant cases observed in studies performed in Poland (38.9%), Belgrade, Central Serbia (43.5%), and Germany (33%), while it was lower than that in Estonia (67.2%) (11, 14, 15, 19). The high rate of clustered cases and the high rate (81.1%) of new cases among clustered cases indicate that a significant portion of the drug-resistant cases resulted from recent transmission. This result and the fact that 75% of the MDR cases were also clustered show that the monitoring and control of drug-resistant tuberculosis is rather inadequate in Hungary (Table 2). The rate of clustered MDR cases was lower in Poland (50.7%) and Germany (49.4%), was similar in Belgrade (70%), and was higher in Estonia (95.8%) (3, 11, 12, 15, 19).
Although statistical analysis did not reveal any statistically significant association between clustering and the various demographic and epidemiologic characteristics, it is noteworthy that nearly one-third of the clustered patients were alcohol abusers, and almost one-quarter of them were homeless (Table 1). Moreover, since 28.6% of the MDR cases were homeless and 61% of the homeless cases were infected by MDR strains, it is clear that, as in other countries (The Netherlands and France), homelessness plays a major role in the transmission of drug-resistant tuberculosis in the capital, where all these cases were found (6, 18).
Since contact tracing in Hungary is focused mainly on transmission between close family contacts of diseased individuals, the present study could not determine relationships among all clustered patients. However, the overall correlation of residence and drug resistance profile of patients with clustering strongly supports the close relationship of the strains within a particular cluster.
Another troubling finding of this study was that 3 (10.7%) of the 28 MDR cases were not reported to the NTSC. According to the NTSC, in 2003 and 2004, less than 40% of the newly diagnosed tuberculosis patients received the mandatory four-drug regimen from their physicians (8, 9). This nonadherence of clinicians to national regulations could also contribute to the increase and transmission of drug-resistant tuberculosis. As the number of patients with drug-resistant tuberculosis in a particular hospital can be low, the lack of familiarity with treatment of patients with drug-resistant tuberculosis might also worsen the situation. Indeed, the importance of this problem was underlined by a recent survey conducted in France. That study revealed a 59% treatment failure rate for 51 patients with drug-resistant tuberculosis that were treated in a total of 42 different clinical sites, only 35 of which were managed by a respiratory disease specialist (4).
Some M. tuberculosis genotypes, like the W-Beijing and Haarlem families, received special clinical and public health attention because of their greater ability to be transmitted (1, 5). Beijing strains showed a high prevalence in Russia and Estonia (30 to 50% of all cases), while the Haarlem genotype is more prevalent in northern Europe (20%) (11, 16). Analysis of the spoligotyping patterns of the strains revealed that a remarkably high number (66.2%) of the patients were infected by strains of the Haarlem genotype, while none of the patients were infected by the Beijing genotype (Fig. 1). It is noteworthy that the presence of the Beijing genotype was observed in every European country from which genotyping results are available (1). These data, and the fact that all but two patients were Hungarian, indicate that in contrast to other European Community countries (i.e., Germany, The Netherlands, and Poland), drug-resistant tuberculosis in Hungary is the result of active in-country circulation of historical clones with European descent (Haarlem family) and not the result of the importation of strains (i.e., Beijing family) from neighboring countries (Romania and former republics of the Soviet Union) with a high rate of Beijing genotype and/or drug-resistant tuberculosis cases.
In conclusion, the results of this study indicate that more effective control steps are needed to detect and intercept the transmission of drug-resistant tuberculosis in Hungary. One solution to the problem could be the introduction of directly observed therapy. The other solution would be the introduction of real-time cohort analysis of cases by the NTSC in order to increase the adherence of clinicians to national guidelines. In addition, the establishment of reference centers, or teams with expert physicians and microbiologists, for consultation and treatment of drug-resistant cases may also be helpful.
Acknowledgments
C. Ködmön and S. Niemann contributed equally to this study.
Á. Somoskövi was supported by grant D43TW00233 from the Fogarty International Center, National Institutes of Health, Bethesda, MD.
We thank Keith Derbyshire for critical review of the manuscript.
Footnotes
Published ahead of print on 13 September 2006.
REFERENCES
- 1.Brudey, K., J. R. Driscoll, L. Rigouts, W. M. Prodinger, A. Gori, S. A. Al-Hajoj, C. Allix, L. Aristimuno, J. Arora, V. Baumanis, L. Binder, P. Cafrune, A. Cataldi, S. Cheong, R. Diel, C. Ellermeier, J. T. Evans, M. Fauville-Dufaux, S. Ferdinand, D. Garcia de Viedma, C. Garzelli, L. Gazzola, H. M. Gomes, M. C. Gutierrez, P. M. Hawkey, P. D. van Helden, G. V. Kadival, B. N. Kreiswirth, K. Kremer, M. Kubin, S. P. Kulkarni, B. Liens, T. Lillebaek, H. M. Ly, C. Martin, C. Martin, I. Mokrousov, O. Narvskaia, Y. F. Ngeow, L. Naumann, S. Niemann, I. Parwati, M. Z. Rahim, V. Rasolofo-Razanamparany, T. Rasolonavalona, M. L. Rossetti, S. Rusch-Gerdes, A. Sajduda, S. Samper, I. Shemyakin, U. B. Singh, A. Somoskovi, R. Skuce, D. van Soolingen, E. M. Streicher, P. N. Suffys, E. Tortoli, T. Tracevska, V. Vincent, T. C. Victor, R. Warren, S. F. Yap, K. Zaman, F. Portaels, N. Rastogi, and C. Sola. 2006. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canetti, G., W. Fox, A. Khomenko, N. Mahler, N. K. Menon, D. A. Mitchison, N. Rist, and N. A. Smeley. 1969. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull. W. H. O. 41:21-43. [PMC free article] [PubMed] [Google Scholar]
- 3.Diel, R., S. Schneider, K. Meywald-Walter, C. M. Ruf, S. Rusch-Gerdes, and S. Niemann. 2002. Epidemiology of tuberculosis in Hamburg, Germany: long-term population-based analysis applying classical and molecular epidemiological techniques. J. Clin. Microbiol. 40:532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flament-Saillour, M., J. Robert, V. Jarlier, and J. Grosset. 1999. Outcome of multi-drug-resistant tuberculosis in France: a nationwide case-control study. Am. J. Respir. Crit. Care Med. 160:587-593. [DOI] [PubMed] [Google Scholar]
- 5.Glynn, J. R., J. Whiteley, P. J. Bifani, K. Kremer, and D. van Soolingen. 2002. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg. Infect. Dis. 8:843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutiérrez, M. C., V. Vincent, D. Aubert, J. Bizet, O. Gaillot, L. Lebrun, C. Le Pendeven, M. P. Le Pennec, D. Mathieu, C. Offredo, B. Pangon, and C. Pierre-Audigier. 1998. Molecular fingerprinting of Mycobacterium tuberculosis and risk factors for tuberculosis transmission in Paris, France, and surrounding area. J. Clin. Microbiol. 36:486-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermans, P. W., F. Messadi, H. Guebrexabher, D. van Soolingen, P. E. de Haas, H. Heersma, H. de Neeling, A. Ayoub, F. Portaels, D. Frommel, et al. 1995. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia, and The Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J. Infect. Dis. 171:1504-1513. [DOI] [PubMed] [Google Scholar]
- 8.Jónás, J., K. B. Fodor, P. Kiss, M. P. Türgyei, and L. Nyári. 2004. Annual report of the Hungarian medical care centers in respiratory medicine, 2003. Korányi National Institute for Tuberculosis and Respiratory Medicine, Budapest, Hungary.
- 9.Jónás, J., K. B. Fodor, P. Kiss, M. P. Türgyei, and L. Nyári. 2005. Annual report of the Hungarian medical care centers in respiratory medicine, 2004. Korányi National Institute for Tuberculosis and Respiratory Medicine, Budapest, Hungary.
- 10.Kremer, K., D. van Soolingen, R. Frothingham, W. H. Haas, P. W. Hermans, C. Martin, P. Palittapongarnpim, B. B. Plikaytis, L. W. Riley, M. A. Yakrus, J. M. Musser, and J. D. van Embden. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 37:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krüüner, A., S. E. Hoffner, H. Sillastu, M. Danilovits, K. Levina, S. B. Svenson, S. Ghebremichael, T. Koivula, and G. Källenius. 2001. Spread of drug-resistant pulmonary tuberculosis in Estonia. J. Clin. Microbiol. 39:3339-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubica, T., S. Rusch-Gerdes, and S. Niemann. 2004. The Beijing genotype is emerging among multidrug-resistant Mycobacterium tuberculosis strains from Germany. Int. J. Tuberc. Lung Dis. 8:1107-1113. [PubMed] [Google Scholar]
- 13.Mester, J., I. Vadasz, G. Pataki, L. Parsons, T. Fodor, M. Salfinger, and A. Somoskovi. 2002. Analysis of tuberculosis surveillance in Hungary in 2000. Int. J. Tuberc. Lung Dis. 6:966-973. [PubMed] [Google Scholar]
- 14.Niemann, S., S. Rusch-Gerdes, and E. Richter. 1997. IS6110 fingerprinting of drug-resistant Mycobacterium tuberculosis strains isolated in Germany during 1995. J. Clin. Microbiol. 35:3015-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sajduda, A., A. Brzostek, M. Poplawska, N. Rastogi, C. Sola, E. Augustynowicz-Kopec, Z. Zwolska, J. Dziadek, and F. Portaels. 2004. Molecular epidemiology of drug-resistant Mycobacterium tuberculosis strains isolated from patients with pulmonary tuberculosis in Poland: a 1-year study. Int. J. Tuberc. Lung Dis. 8:1448-1457. [PubMed] [Google Scholar]
- 16.Toungoussova, O. S., P. Sandven, A. O. Mariandyshev, N. I. Nizovtseva, G. Bjune, and D. A. Caugant. 2002. Spread of drug-resistant Mycobacterium tuberculosis strains of the Beijing genotype in the Archangel Oblast, Russia. J. Clin. Microbiol. 40:1930-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Soolingen, D., M. W. Borgdorff, P. E. de Haas, M. M. Sebek, J. Veen, M. Dessens, K. Kremer, and J. D. van Embden. 1999. Molecular epidemiology of tuberculosis in The Netherlands: a nationwide study from 1993 through 1997. J. Infect. Dis. 180:726-736. [DOI] [PubMed] [Google Scholar]
- 19.Vuković, D., S. Rüsch-Gerdes, B. Savić, and S. Niemann. 2003. Molecular epidemiology of pulmonary tuberculosis in Belgrade, Central Serbia. J. Clin. Microbiol. 41:4372-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]