Abstract
We utilized denaturing gradient gel electrophoresis profiling to survey stool microbiota in 175 persons with diarrhea and 113 asymptomatic persons in a diarrhea surveillance study. Compared with healthy controls, the microbiota profiles in diarrhea cases more frequently exhibited decreased diversity and strong bands indicating the overgrowth of selected bacteria or bacterial groups.
Food-borne diseases continue to present a large population burden even in industrialized countries. There are an estimated 76 million illnesses, 325,000 hospitalizations, and 5,000 deaths per year due to food-related pathogens in the United States (6). Of these food-related illnesses, approximately 62 million are due to undetermined pathogens. Studies of diarrhea etiology frequently fail to detect a viral, bacterial, or parasitic pathogen in the stools of a significant proportion of subjects that present with diarrhea indicative of an infectious origin (8, 10). For bacterial pathogens, such failures might be due to the inability to cultivate the causative organism or the fact that cultivatable microbes are not recognized as potential diarrhea pathogens. Little is known about how diarrhea is associated with overall distortions in the normal intestinal microbiota composition; distortions in diversity have been associated with intestinal disease and, specifically, Crohn's disease (5).
From 2002 to 2006, we performed a multiple-site diarrhea surveillance study in which we attempted to determine the prevalence of known diarrhea pathogens in symptomatic participants that presented to an emergency department or medical clinic with a self-identified complaint of diarrhea. Control subjects without diarrhea were derived from the general population and included healthy volunteers, individuals presenting to clinics for routine health maintenance visits, and individuals presenting to emergency departments with minor complaints not including diarrhea. These subjects were matched to cases by location and age (≤5 years, 6 to 17 years, 18 to 64 years, and ≥65 years). This research was approved by the institutional review boards of all participating institutions. To identify the etiologic agent of diarrhea for each subject and compare the prevalence with that for controls, we utilized a complex study protocol that included (i) standard culture techniques for bacterial pathogens, (ii) standard staining for parasitic pathogens, (iii) PCR (PCR/reverse transcription-PCR) detection of viruses and bacterial virulence factors including Escherichia coli virulence factors (Eae, Eagg, IpaH, LT1, and ST1), and (iv) enzyme immunoassays for bacterial toxins. Blinded laboratory personnel analyzed fresh fecal samples from patients and controls in parallel. Details regarding the surveillance study have been reported elsewhere previously (7). Despite our comprehensive approach for the detection of pathogens, we were unable to determine a microbial cause of diarrhea for approximately 65% of cases that participated in the study. This overall finding was not surprising; other studies have reported similarly low success rates (8, 10).
Here, we report on 16S rRNA gene-based profiling of the overall fecal microbiota in a subset of 175 diarrhea cases and 113 controls enrolled at the University of Maryland study site to explore the utility of denaturing gradient gel electrophoresis (DGGE) in diarrhea surveillance. Bacterial community DNA was extracted from diarrhea and normal stool samples, and PCR products were generated with primers directed against the variableV6-to-V8 (V6-V8) regions of the 16S rRNA gene as described previously (4). The V6-V8 primer set amplifies a large 457-bp fragment offsetting the advantage of higher resolution with the V3 primer set (11), which generates a <200-bp fragment. PCR products were separated on 8% polyacrylamide gels with a 40 to 50% top-to-bottom denaturing gradient run for 16 h at 65 V, followed by SYBR gold staining. Images were digitalized and then analyzed using Quantity One and Diversity Database software (Bio-Rad, Hercules, CA). DGGE allows the distinction of 30 to 45 PCR products with different denaturing characteristics in the fecal microbiota profile of an individual. Each lane in the DGGE gel contains the microbiota profile derived from community DNA extracted from a single stool sample and thus reflects overall microbiota composition. Although all the bands represent a PCR product of equivalent length (with our primer set of 457 bp), the products are separated by the denaturing gradient due to differences in denaturing characteristics based on nucleotide sequence (mainly GC content). Bands generally represent different bacterial groups or species. However, species that are closely related in the 16S rRNA gene at the investigated region (V6-V8 in our study), such as Escherichia coli and Shigella spp., cannot be separated by this method.
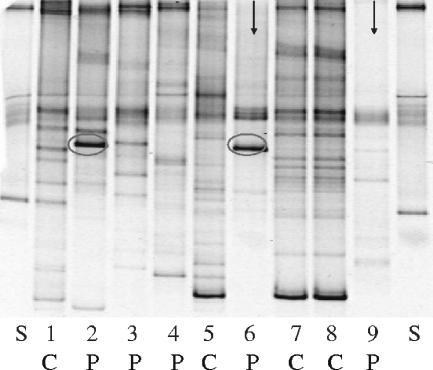
For this analysis, we defined a distorted microbiota profile, based on healthy subjects in this and a previously published feeding study (4), as one in which we could detect no more than eight distinguishable bands. We defined a strong band as any band that was at least 50% stronger (relative intensity, >4%) than the strongest band in a normal profile, excluding those at the top or bottom of a gel that might represent multiple molecular species not separated by the gradient. A representative DGGE gel that shows distorted profiles (lanes 6 and 9) as well as a strong band (lanes 2 and 6) is shown in Fig. 1. In each lane, multiple PCR products are detectable, but the total number of distinguishable bands varies between subjects. Among the 288 subjects that we analyzed by DGGE, we detected a distorted microflora profile in 25% of diarrhea cases and 9% of control subjects (P < 0.001). The mean Shannon-Wiener diversity index in subjects with a distorted profile was significantly lower than that in subjects with a normal profile (1.87 versus 3.76). A strong band, indicating overgrowth of a particular bacterial group or species, was detected in 17% of cases and 5% of the controls (P < 0.001) (Table 1). In both cases and controls, the majority of profiles showing a strong band also exhibited distorted diversity. The proportions of cases in which we detected a distorted profile were similar in subjects in which we identified a diarrhea pathogen and in subjects in which we did not. Principal-component analysis of the DGGE profiles generated in this study failed to identify distinct clusters. We also determined the prevalence of a specific band that was determined by (i) a known Escherichia coli control fragment and (ii) sequencing studies to represent Escherichia coli, Shigella, and related bacteria. This band was detected with a similar prevalence of 15% in diarrhea cases and 16% in normal controls (P = 0.13), refuting the hypothesis that the mere presence of such a band might be associated with diarrhea. Although Escherichia coli is a member of the normal commensal microbiota in most healthy individuals, its numbers are often too low, relative to the large amounts of other microbes, for detection of a distinct DGGE band.
FIG. 1.
DGGE gel showing representative fecal microbiota profiles of diarrhea patients (P) and controls (C). S, standard. Strong bands (lanes 2 and 6) are circled. Strong bands on the bottom of the gradient (lanes 5, 7, and 8) are not considered strong bands, as such bands frequently contain multiple molecular species that do not separate under the DGGE conditions used. Distorted DGGE profiles (at most eight detectable bands) are indicated by arrows (lanes 6 and 9). Lanes 7 and 8 show the DGGE profile from the stool of the same control subject for which DNA extraction and PCR amplification were done on different days indicating negligible experimental variation.
TABLE 1.
Proportion of diarrhea patients and controls for which DGGE detected a distorted profile, a strong band, or an E. coli/Shigella band
| Band type | No. (%)
|
P valued | |
|---|---|---|---|
| Diarrhea patients (n = 175) | Control subjects (n = 113) | ||
| Distorted profilea | 43 (25) | 10 (9) | <0.001 |
| Strong bandb | 30 (17) | 6 (5) | <0.001 |
| E. coli/Shigella bandc | 26 (15) | 18 (16) | 0.13 |
Distorted profiles are defined as profiles having no more than eight distinct bands.
Strong bands are bands with high intensity as described in the Fig. 1 legend.
Band indicating the presence of E. coli/Shigella and related bacteria.
Significance was calculated using Fisher's exact test.
To explore whether the detection of a DGGE band can aid in the identification of pathogens, we sequenced a strong band from a subject from whom we detected the presence of Shigella by conventional methods. Sequence analysis of the strong band eluted from the DGGE gels confirmed the presence of a molecular signature for Escherichia coli, Shigella, and related bacteria. Some of the strong bands detected in diarrhea cases might represent novel pathogens, as we detected such bands in subjects for which we did not identify a diarrhea pathogen by our conventional protocol. However, the numbers of observations of each such individual band were too infrequent for a meaningful analysis. Future studies would benefit from novel high-throughput molecular methods that yield a truly unique molecular signature for known or unknown pathogens.
We are not aware of any prior studies that have described differences between diarrhea cases and healthy controls in overall stool microbiota profiles as determined by any culture-independent molecular method. The finding that the overall microbiota complexity is reduced in some diarrhea cases is not surprising but suggests that such changes might contribute to the observed symptoms. However, because we did not test serial samples from patients, we are unable to distinguish if distortions in microflora profiles are the cause of the diarrhea or a result thereof. It is likely that during episodes of diarrhea, conditions in the colon change in such a way that many of the members of the normal commensal microflora are washed out and are unable to maintain residence. It is also plausible that a distorted microflora profile allows bacteria with pathogenic potential to occupy an ecological niche in the intestine that would normally not be available for colonization. DGGE profiling could potentially identify subjects at increased risk for antibiotic-induced or recurrent diarrhea. Antibiotics have been reported to affect microbiota DGGE profiles in healthy subjects for at least the time that they were administered (2), although distorted profiles or the emergence of prevalent bands as reported here have not been described previously. Using conventional microbial identification techniques, various studies have previously described effects of antibiotic administration on intestinal microbiota (9). It is unlikely that antibiotic use contributed significantly to the changes in microbiota profiles that we report here, as only a few subjects in this study took antibiotics during the week preceding the sample collection. Furthermore, in a previously reported study in which we administered enrofloxacin to sheep to induce phage movement (1), we failed to detect distorted microbiota profiles or strong prevalent bands (unpublished data). Some smaller changes in DGGE profiles have been reported in ileal chicken contents after antibiotic administration (3). Prior studies of the commensal microbiota have been limited in size, and our knowledge of normal microbiota complexity is still limited (12). This study, using a combined total of 288 subjects, adds some insight into the normal degree of microbiota diversity and variation among individuals. Our studies suggest some potential of microbiota profiling for the surveillance of known, as well as the detection of novel, diarrhea pathogens. The observation that a large proportion of patients with diarrhea appear to have a decreased diversity in intestinal microbiota composition should be confirmed by more sensitive approaches such as extensive sequencing of 16S rRNA gene libraries. However, because of our currently limited knowledge, any studies attempting to associate microbiota distortions with disease will depend on a better understanding of microbiota diversity and variation in a large cohort of healthy subjects.
Acknowledgments
This study was supported by grant U01CI000296 from the National Center for Infectious Diseases of the Centers for Disease Control and Prevention and grant MM0210-02/02 from the American Association of Medical Colleges. Work in the laboratory of V. Mai is supported by ACS grant MRSGT CCE-107301.
Footnotes
Published ahead of print on 4 October 2006.
REFERENCES
- 1.Cornick, N. A., A. F. Helgerson, V. Mai, J. M. Ritchie, and D. W. Acheson. 2006. In vivo transduction of an Stx-encoding phage in ruminants. Appl. Environ. Microbiol. 72:5086-5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De La Cochetiere, M. F., T. Durand, P. Lepage, A. Bourreille, J. P. Galmiche, and J. Dore. 2005. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J. Clin. Microbiol. 43:5588-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knarreborg, A., M. A. Simon, R. M. Engberg, B. B. Jensen, and G. W. Tannock. 2002. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl. Environ. Microbiol. 68:5918-5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mai, V., H. A. Katki, H. Harmsen, D. Gallaher, A. Schatzkin, D. J. Baer, and B. Clevidence. 2004. Effects of a controlled diet and black tea drinking on the fecal microflora composition and the fecal bile acid profile of human volunteers in a double-blinded randomized feeding study. J. Nutr. 134:473-478. [DOI] [PubMed] [Google Scholar]
- 5.Manichanh, C., L. Rigottier-Gois, E. Bonnaud, K. Gloux, E. Pelletier, L. Frangeul, R. Nalin, C. Jarrin, P. Chardon, P. Marteau, J. Roca, and J. Dore. 2006. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 55:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nataro, J. P., V. Mai, J. Johnson, W. C. Blackwelder, R. Heimer, S. Tirrell, S. C. Edberg, C. R. Braden, M. J. Glenn, Jr., and J. M. Hirshon. 2006. Diarrheagenic Escherichia coli infection in Baltimore, Maryland, and New Haven, Connecticut. Clin. Infect. Dis. 43:402-407. [DOI] [PubMed] [Google Scholar]
- 8.Slutsker, L., A. A. Ries, K. D. Greene, J. G. Wells, L. Hutwagner, and P. M. Griffin. 1997. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann. Intern. Med. 126:505-513. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan, A., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]
- 10.Talan, D., G. J. Moran, M. Newdow, S. Ong, W. R. Mower, J. Y. Nakase, R. W. Pinner, and L. Slutsker. 2001. Etiology of bloody diarrhea among patients presenting to United States emergency departments: prevalence of Escherichia coli O157:H7 and other enteropathogens. Clin. Infect. Dis. 32:573-580. [DOI] [PubMed] [Google Scholar]
- 11.Yu, Z., and M. Morrison. 2004. Comparisons of different hypervariable regions of rrs genes for use in fingerprinting of microbial communities by PCR-denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 70:4800-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoetendal, E. G., C. T. Collier, S. Koike, R. I. Mackie, and H. R. Gaskins. 2004. Molecular ecological analysis of the gastrointestinal microbiota: a review. J. Nutr. 134:465-472. [DOI] [PubMed] [Google Scholar]