Abstract
Cefoxitin is increasingly recommended for detection of methicillin resistance in Staphylococcus aureus (MRSA) when using disk diffusion testing. In this study, 95 mecA-negative S. aureus isolates and a highly genetically diverse collection of mecA-positive S. aureus types (n = 50) were used to investigate the influence of technical factors such as disk potency, incubation time, and temperature on Mueller-Hinton agar. The use of cefoxitin MIC testing by Etest for the same purpose was investigated under similar conditions. For disk diffusion, the accuracy was high at both 35°C and 36°C using overnight incubation, while incubation at 30°C or 37°C was associated with slightly lower accuracy. Increasing incubation times from 18 to 24 h did not improve accuracy at either temperature. Cefoxitin Etest MICs for mecA-positive strains were 6 mg/liter or higher, while cefoxitin Etest MICs for mecA-negative strains were ≤4 mg/liter. Our findings suggest that the current CLSI zone diameter breakpoints should be adjusted from resistance (R) ≤ 19 mm to R ≤ 21 mm. In conclusion, cefoxitin disk diffusion testing and Etest MIC testing can accurately predict the presence of the mecA gene in S. aureus. Testing can be reliably performed using incubation temperatures of 35 to 36°C and incubation times of 18 to 22 h. We suggest MRSA interpretive criteria of susceptible (S) ≤ 4 mg/liter and R > 4 mg/liter, corresponding to S ≥ 22 mm and R ≤ 21 mm for the 30-μg disk and S ≥ 17 mm and R ≤ 16 mm for the 10-μg cefoxitin disk. These criteria resulted in only one mecA-positive isolate being misclassified as susceptible.
Infections due to methicillin-resistant Staphylococcus aureus (MRSA) are an increasing problem worldwide inside and outside of hospitals (3).
Phenotypic detection of MRSA has been problematic ever since its discovery in the early 1960s. The emergence of low-level-resistant MRSA clones acquired in the community has only added to these difficulties.
Detection of the mecA gene or its product, penicillin binding protein (PBP2a), is considered the gold standard (5) for MRSA confirmation. Recent investigations suggest that disk diffusion using cefoxitin is superior to most previously recommended phenotypic methods, including oxacillin disk diffusion and oxacillin screen agar testing (8, 14, 19, 21). In 2005, the Clinical and Laboratory Standards Institute (CLSI) published zone diameter (6) breakpoint guidelines for cefoxitin. However, a number of technical issues remain regarding the use of cefoxitin as a predictor for methicillin resistance. The CLSI M100-S15 document stipulates an incubation time of 24 h unless the isolate has a zone diameter of ≤19 mm (i.e., resistant), in which case it can be reported after 18 h of incubation. However, in a recent publication, performance was equally good at 18 h of incubation (21).
For methicillin and oxacillin, incubation temperature is known to affect the test results (12, 17). For oxacillin, a maximum of 35°C for testing of staphylococci is specified by CLSI. For cefoxitin, high accuracy has been found using standard incubation temperatures, i.e., 35 to 37°C (8, 10, 19-21), but one study showed relatively low sensitivity at 37°C (2). In a recent investigation, zone diameters obtained on IsoSensitest agar with semiconfluent inoculum were dependent on whether the plates were incubated at 35°C or 36°C (R. Skov, unpublished results). Two studies have shown marginally improved accuracy when tests were performed at 30°C compared to 35°C and 37°C (4, 8). The 10-μg cefoxitin disk has been shown to be superior to the 30-μg disk with IsoSensitest agar and semiconfluent growth (20).
The CLSI M100-S15 document recommends oxacillin MIC testing for MRSA detection by the MIC method, and no criteria are as yet available for the use of cefoxitin as an alternative (6).
In this study, the influence of incubation time (18 h and 24 h) and temperatures (30°C, 35°C, 36°C, and 37°C) on the performance of 10- and 30-μg cefoxitin disks and cefoxitin Etest on Mueller-Hinton agar were evaluated for mecA-positive and mecA-negative S. aureus.
MATERIALS AND METHODS
Strains.
A total of 146 S. aureus strains were included in the study. All isolates were tested for the presence of the mecA gene by the EVIGENE MRSA Detection kit (18) (SSI Diagnostika, Statens Serum Institut, Copenhagen, Denmark) using the manufacturer's instructions. The strains selected from a previously tested collection (20) consisted of 95 mecA-negative consecutive blood culture isolates and 51 mecA-positive isolates from different patients. Only mecA-positive isolates which previously had produced inhibition zones with cefoxitin and had distinctly different pulsed-field gel electrophoresis (PFGE) patterns (one or more visible band differences) were included. Two recent clinical mecA-positive isolates with large cefoxitin inhibition zones and three isolates particularly sensitive to small variations in temperature, kindly provided by Derek Brown, Addenbrooke Hospital, Cambridge, United Kingdom, were also included.
One mecA-positive isolate was later excluded from the investigation as population analysis showed it to be a susceptible phenotype (see the population profile analysis), leaving a total of 95 mecA-negative and 50 mecA-positive isolates.
Three reference strains were included for quality control: one mecA-positive strain (S. aureus ATCC 43300) and two mecA-negative strains (S. aureus ATCC 25923 and ATCC 29213).
All strains stored at −80°C were subcultured on two consecutive days using 5% Danish blood agar (SSI Diagnostika) prior to use.
spa typing and MLST.
spa typing and multilocus sequence typing (MLST) were performed as previously described (7, 11). The spa types (t) and MLST sequence types (ST) were assigned through the Ridom (http://www.ridom.de) and MLST databases (http://www.mlst.net), respectively. Based on spa and/or sequence types, the isolates were given a predicted clonal complex annotation.
Susceptibility testing.
In the first phase of the study (phase I), all 145 strains were tested by disk diffusion using cefoxitin and Etest (AB Biodisk, Solna, Sweden) using cefoxitin and oxacillin. All methodological variants were assessed using the same inoculum. The inoculum was standardized to 0.5 McFarland turbidity. Disk diffusion was done with 10-μg and 30-μg disks (Oxoid, Basingstoke, United Kingdom) using Mueller-Hinton BBL II agar (Becton Dickinson, Heidelberg, Germany). Agar plates were incubated overnight (18 to 19 h) in ambient air at 30°C, 35°C, and 36°C in stacks no higher than five plates.
Inhibition zone diameters were read from the back of the agar plate using reflected light and calipers to read to the nearest millimeter at the inner zone edge. For isolates with a zone size of >19 mm after 18 to 19 h for the 30-μg cefoxitin disk at 35°C, all plates were further incubated and read after 24 h as specified in M100-S15 (6).
Etest oxacillin MIC testing was performed according to the manufacturer's instructions using Mueller-Hinton BBL II agar supplemented with 2% NaCl (wt/vol) and incubation at 35°C for a full 24 h. Etest cefoxitin MIC testing was done using Mueller-Hinton BBL II agar without NaCl supplementation. Plates were incubated in ambient air at 30°C, 35°C, and 36°C. Cefoxitin MICs were read after 18 to 19 h of incubation. Isolates for which cefotixin MICs were ≤4 mg/liter were incubated further and read after a total of 24 h.
The influence of incubation at 37°C was investigated in phase II using a subset of 54 strains (35 mecA-negative and 19 mecA-positive strains) incubated simultaneously at 35°C and 37°C. For phase II we selected the most challenging strains, those with the largest cefoxitin zone diameters and those that during phase I had shown results influenced by variations in incubation temperature. For phase II, MICs and zone diameters were read after 18 h and 24 h of incubation.
In both phase I and phase II, S. aureus strains ATCC 29213, ATCC 25923, and ATCC 43300 were included for quality control on all test runs. The temperature in the incubators was monitored at 10-min intervals for the entire incubation period using TinytagPlus digital thermometers with accompanying software (Gemini Data Loggers Ltd., Chichester, West Sussex, United Kingdom). The temperature curves showed that with empty incubators the maximum variation during a 12-h test period was ±0.25°C (data not shown). With plates on the shelves, the time to reach the preset temperature on each shelf was dependent on the number of plates placed on the shelf. In phase I, when shelves were full, the temperature only reached the preset temperature at the end of the incubation time. This was true for both temperatures. Each strain was subjected to the same conditions for each of the two temperatures. The temperature at the end of incubation was 34.6 to 34.8°C and 35.6 to 36.4°C when incubators were set at 35°C and 36°C, respectively. In phase II the preset temperatures were reached on all shelves within 4 h, and the temperatures were stationary between 34.7 to 35.6°C and 36.8 to 37.1°C when incubators were set at 36°C and 37°C, respectively (detailed data not shown).
Intraassay variation was studied using S. aureus ATCC 25923, ATCC 29213, and ATCC 43300 tested for 10 days using two different batches of Mueller-Hinton II agar (BBL) and two different batches of 10-μg and 30-μg disks (Oxoid). Each agar plate was read independently by five technologists, giving a total of 50 measurements per Mueller-Hinton agar/disk combination.
Population analysis.
Two mecA-positive isolates obtained from Norway (strains 9-8 and 10-22), previously undetectable by any phenotypic cefoxitin method (19, 20), were tested by population analysis profile using various concentrations of cefoxitin and oxacillin in agar. Colonies from a fresh overnight culture were inoculated into 5 ml tryptic soy broth (SSI Diagnostika) and incubated overnight at 35°C. Tenfold dilutions of the culture were prepared in 0.9% NaCl, and 20 μl from each dilution was spot inoculated in duplicate onto Mueller-Hinton agar plates containing twofold dilutions of oxacillin (0.25 to 128 mg/liter) and cefoxitin (0.25 to 128 mg/liter). The agar plates were incubated a full 24 h at 35°C, and colonies were counted to plot the population analysis profile. Strain 10-22 showed a highly heterogeneous resistance pattern against oxacillin and displayed colonies of up to 64 mg/liter (data not shown). Since strain 9-8 failed to show any colonies at 4 mg/liter in repeat experiments, even in the undiluted samples, it was concluded that this mecA-positive isolate was an oxacillin-susceptible phenotype, and it was omitted from further analysis. This is a well-described phenomenon that can be caused by a defect in one or more enzymes needed for an isolate to express oxacillin resistance to, e.g., one of the fem genes (1).
Statistics.
Statistical differences in MICs obtained at different temperatures and/or incubation times were analyzed by Wilcoxon's matched pairs test, with P < 0.05 considered significant. Distributions were reported by medians and ranges. For statistical analyses, the Statistica software program (version 7.0; Stat Soft Inc., Tulsa, TX) was used.
RESULTS
Genotypes.
The 50 mecA-positive S. aureus isolates represented 28 different spa types and 12 different clonal complexes/ST groups, as shown in Table 1.
TABLE 1.
Predicted clonal complexes (CC) based on spa type of the 50 mecA-positive isolates
| CC | No. of isolates | spa type(s) |
|---|---|---|
| ST1 | 2 | t127 |
| CC5 | 5 | t002, t005 |
| CC8 | 13 | t008, t024, t037, t051, t190, t211 |
| ST15 | 1 | t084 |
| CC22 | 4 | t022, t354, t431, t790 |
| CC30 | 6 | t018, t019, t318, t1209 |
| CC45 | 5 | t015, t065, t126 |
| CC59 | 3 | t216, t437 |
| CC78 | 2 | t186, t1339 |
| CC80 | 7 | t044, t131 |
| CC97 | 2 | t365 |
MIC.
Etest oxacillin MIC distributions at 35°C after 24 h of incubation and cefoxitin MIC distributions at 30°C, 35°C, and 36°C after 18 h and 24 h of incubation are shown in Table 2. Oxacillin MICs for the mecA-negative strains were between 0.125 mg/liter and 4 mg/liter (the oxacillin MIC for one isolate was 4 mg/liter) and for mecA-positive strains was between 1 and >256 mg/liter, with a median of 64 mg/liter. For cefoxitin the range was 1 to 4 mg/liter (with only one isolate at 1 mg/liter) and for mecA-negative isolates was 4 to >256 mg/liter, with a median MIC of 32 mg/liter for mecA-positive isolates regardless of the incubation temperature or time. However, MICs were significantly lower at 36°C than for incubation at 35°C (P = 0.006). Similar results were found in phase II (35 to 37°C). There was no difference in MICs obtained at 35°C for incubation for 18 h or 24 h (not significant; P = 0.1). There was no difference between MICs obtained at 30°C and 35°C incubation (not significant; P = 0.64). Using an MRSA interpretive breakpoint of susceptible (S) ≤ 4 mg/liter and resistant (R) > 4 mg/liter, only one mecA-positive isolate (isolate 10-22 from Norway) was incorrectly categorized as non-MRSA after both 18 h and 24 h of incubation at all temperatures (see also data from the population profile analysis for this isolate in Material and Methods). The oxacillin MIC for this isolate was 4 mg/liter. Another mecA-positive isolate (isolate 1748) was phenotypically sensitive to oxacillin (MIC, 1 mg/liter) but was resistant to cefoxitin (MIC, 16 mg/liter).
TABLE 2.
Oxacillin Etest MIC at 35°C after 24 h of incubation and cefoxitin Etest MIC at 30, 35, and 36°C after 18 h and 24 h of incubation
| Drug concn (mg/liter) | No. of observations by test type, mecA status, time, and tempa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxacillin Etest (35°C, 24 h) |
Cefoxitin Etest |
||||||||||
| mecA negative (n = 95) | mecA positiveb (n = 49) | 30°C |
35°C |
36°C |
|||||||
|
mecA negativeb (n = 94) |
mecA positive (n = 50), 18 h |
mecA negative (n = 95) |
mecA positive (n = 50), 18 h |
mecA negative (n = 95) |
mecA positive (n = 50), 18 h | ||||||
| 18 h | 24 h | 18 h | 24 h | 18 h | 24 h | ||||||
| 0.125 | 2 | ||||||||||
| 0.25 | 13 | ||||||||||
| 0.5 | 50 | ||||||||||
| 1 | 26 | 1 | 1 | 1 | |||||||
| 2 | 3 | 10 | 10 | 5 | 3 | 6 | 5 | ||||
| 4 | 1 | 5 | 84 | 84 | 1c | 89 | 91 | 1c | 89 | 90 | 1c |
| 6d | 2 | 5 | 3 | 3 | |||||||
| 8 | 5 | 3 | 2 | 1 | |||||||
| 16 | 4 | 11 | 11 | 15 | |||||||
| 32 | 3 | 8 | 16 | 22 | |||||||
| 64 | 11 | 13 | 9 | 5 | |||||||
| 128 | 6 | 5 | 5 | 2 | |||||||
| ≥256 | 12 | 4 | 3 | 1 | |||||||
Values in columns are number of observations for each unique variable.
One strain did not grow.
Strain 10-22. After a full 24 h of incubation, the cefoxitin MIC was 4 mg/liter at 30°C, 35°C, and 36°C.
All intermediate results are reported to the nearest higher concentration except for 6 mg/liter.
Disk diffusion.
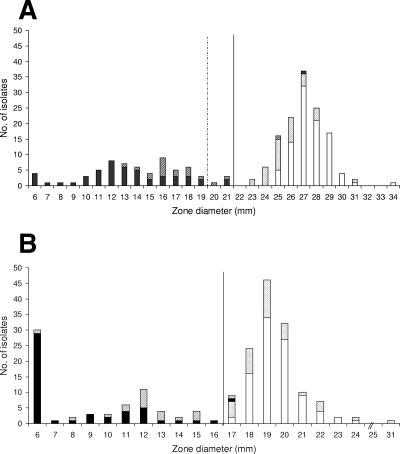
Results for cefoxitin 30-μg and the 10-μg disks incubated 18 to 20 h at 35°C are shown in Fig. 1. Except for one isolate (isolate 10-22), all mecA-positive isolates gave inhibition zone diameters of ≤21 mm for the 30-μg disk and ≤17 mm for the 10-μg disk in both phase I and phase II.
FIG. 1.
Zone diameter distribution for 30-μg cefoxitin disk (A) and 10-μg cefoxitin disk (B) against 145 S. aureus isolates (phase I) and repeat testing with 54 S. aureus isolates (phase II) at 35°C and 18 h of incubation. The current CLSI breakpoint for the 30-μg disk is shown by a dashed line, and the suggested breakpoints are shown by black lines in both figures. □, mecA negative, phase I; ░⃞, mecA negative, phase II; ▪, mecA positive, phase I; ▨, mecA positive, phase II.
For mecA-negative isolates, zone diameters for the 30-μg disk were ≥25 mm and ≥23 mm for phase I and II, respectively, and ≥17 mm for the 10-μg disk in both phases I and II.
Increasing the incubation time from 18 h to 24 h or the temperature from 35°C to 36°C did not affect inhibition zone diameter distributions for either disk for mecA-negative or mecA-positive isolates (data not shown).
Increasing the temperature from 35°C to 37°C was slightly more problematic; for the 30-μg disk, a mecA-negative strain was classified as positive, and with the 10-μg disk a mecA-positive strain was classified as susceptible. Decreasing the temperature from 35°C to 30°C made one strain falsely susceptible by both disks (data not shown).
Repeated testing of quality control strains.
The results for the 10-day repeated testing of S. aureus ATCC 29213 (i.e., CLSI quality control strain for routine MIC testing) and S. aureus ATCC 25923 (i.e., CLSI quality control strain for routine disk diffusion testing) are shown in Table 3 (all methodological variants were tested with the same inoculum suspension). S. aureus ATCC 25923, the quality control strain normally used by the CLSI, on one day (day 5) gave significantly different results from the rest of the nine days both for the 10-μg and the 30-μg disks. Omitting the outlier results from day 5, the following medians (ranges) were obtained for S. aureus ATCC 25923: 25 mm (22 to 28 mm) for the 30-μg disk and 20 mm (18 to 22 mm) for the 10-μg disk. For S. aureus ATCC 29213, tighter zone diameter ranges were obtained for both disks, giving medians (ranges) of 25 mm (23 to 27 mm) for the 30-μg disk and 18 mm (17 to 20 mm) for the 10-μg disk. There was no difference between batches of agar or disks (data not shown).
TABLE 3.
Intraassay variation for 10-μg and 30-μg cefoxitin disks for S. aureus ATCC 29213 and ATCC 25923
| S. aureus strain | Disk size (μg) | Temp (°C) | No. of observations with indicated zone diam (mm)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | |||
| ATCC 29213 | 10 | 35 | 16 (1) | 90 (4) | 70 (6) | 4 (9) | |||||||||
| 10 | 37 | 11 (1) | 85 (7) | 77 (11) | 7 (1) | ||||||||||
| ATCC 25923 | 10 | 35 | (1) | (4) | (9) | 19 (6) | 78 | 37 | 26 | ||||||
| 10 | 37 | (1) | 1 (6) | 35 (11) | 97 (2) | 32 | 15 | ||||||||
| ATCC 29213 | 30 | 35 | 3 | 27 (2) | 91 (11) | 55 (6) | 4 (1) | ||||||||
| 30 | 37 | 7 | 37 (8) | 89 (9) | 39 (3) | 8 | |||||||||
| ATCC 25923 | 30 | 35 | 0 (1) | 4 (5) | 14 (5) | 29 (4) | 39 | 37 | 15 | 42 (5) | |||||
| 30 | 37 | (2) | (1) | (4) | 6 (5) | 17 (1) | 33 (4) | 50 (3) | 41 | 20 | 9 | 4 | |||
Ten-day repeat testings were read by five persons using two batches of disks and media, respectively, i.e., 200 observations per variant. Numbers in parentheses refer to the number of observations on day 5 (see the text for an explanation).
DISCUSSION
Cefoxitin disk diffusion testing is now an accepted method for the detection of methicillin resistance in S. aureus by an increasing number of reference resistance groups, including CLSI. There are, however, still some unsolved issues, such as the optimal cefoxitin disk content and incubation temperature and time. In this study, a highly diverse collection of mecA-positive S. aureus isolates (12 different clonal complexes) was used to investigate these technical variants. To increase the challenge, mecA-positive strains without inhibition zones around the cefoxitin 10-μg disk were not included. Furthermore, only strains with unique PFGE patterns (one or more visible band differences) were included.
Results obtained suggest that both cefoxitin inhibition zone diameters and MICs for mecA-positive strains are influenced by incubation temperature, although they are influenced to a lesser degree than for oxacillin. Most importantly, detection of MRSA by the cefoxitin-based method was not affected by temperature variations between 35°C and 36°C, while at 37°C disk diffusion gave one false phenotypic categorization. In the International Organization for Standardization (ISO) methodology standard for MIC determinations currently under development (22), the incubation temperature is specified as 34 to 37°C. For oxacillin testing with staphylococci, there is a note stating that the temperature should not exceed 35°C. Although cefoxitin is influenced to a lesser degree, it may be appropriate to include the same note for cefoxitin testing. No advantage was seen with the 30°C incubation temperature as proposed by others (4, 8). Importantly, there was no benefit in extending the incubation time from 18 h to a full 24 h. This is in agreement with the findings of Swenson and Tenover (21). It is a major advantage to clinical laboratories that standard methodology (medium, inoculum, incubation time, and temperature) can be used for the detection of MRSA.
For the 30-μg disk, our findings suggest MRSA interpretive breakpoints of S ≥ 22 mm and R ≤ 21 mm, i.e., 2 mm larger than the criteria published by the CLSI (6). This proposal is supported by the results of several previous investigations (Table 4) in which mecA-negative isolates all have had zone diameters of 22 mm or more, and occasionally mecA-positive isolates exhibiting zone diameters of 20 and 21 mm have been reported (4, 9, 10, 16, 21, 23, 24).
TABLE 4.
Published zone diameter ranges (CLSI 30-μg cefoxitin disk) for mecA-positive and mecA-negative S. aureus isolates
| Reference | No. of isolates (mecA positive/ mecA negative) | Zone diam range (mm) for indicated isolate type |
|||
|---|---|---|---|---|---|
| mecA positive | mecA-positive outlier | mecA negative | mecA-negative outlier | ||
| Swenson and Tenover (21) | 202/309 | 6-19 | 21, 28 | 22->28 | |
| Fernandes et al. (9) | 180/418 | 6-17 | 22-35 | ||
| Velasco et al. (24) | 51/51 | 6-14 | ≥25 | 17 | |
| Pottumarthy et al. (16) | 103/100 | 6-17 | 23->35 | 21 | |
| Gueudet and Lemble (10) | 49/51 | 6-20 | 23-28 | ||
| Urbaskova et al. (23) | 218/534 | 6-19 | 23-35 | ||
| Cauweiler et al. (4) | 73/82 | 6-19 | 23 | 24-34 | |
For the 10-μg disk, the results support corresponding interpretive breakpoints of S ≥ 17 mm and R ≤ 16 mm. The contents of both disks had comparable sensitivity and specificity for detection of MRSA. The lower disk content produces smaller zones and thereby reduces the interference with results for other antibiotic disks tested on the same agar plate. The higher disk content may have a slightly higher specificity, as reflected by the larger gap between mecA-positive and mecA-negative zone diameter results.
In our study, cefoxitin MIC testing with Etest was accurate for MRSA detection. The results suggest interpretive breakpoints of S ≤ 4 mg/liter and R ≥ 8 mg/liter using CLSI terminology and S ≤ 4 mg/liter and R > 4 mg/liter in EUCAST (the European Committee of Antimicrobial Susceptibility Testing) terminology. However, the cefoxitin MIC mode for mecA-negative strains is close to the suggested breakpoints. The proposed breakpoints are supported by data published by other investigators (Felten et al. [8], Fernandes et al. [9], Swenson and Tenover [21], and Votta et al. [M. Votta, D. Turner, B. Turng, T. Wiles, J. Reuben, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. D-47, 2004]). However, in an investigation with many borderline oxacillin-resistant S. aureus strains by Swenson et al., cefoxitin breakpoints of S ≤ 6 mg/liter, instead of 4 mg/liter, and R ≥ 8 mg/liter were shown to have better specificity but a slightly lower sensitivity (J. Swenson, D. Lonsway, S. McAllister, A. Thompson, L. Jevitt, J. Patel, Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. D-1732, 2005). In this study, there was no difference in sensitivity or specificity using a breakpoint of S ≤ 6 mg/liter and R ≥ 8 mg/liter instead of S ≤ 4 mg/liter and R ≥ 8 mg/liter.
In conclusion, this study provides further evidence that cefoxitin is an accurate surrogate marker for the detection of MRSA in routine susceptibility testing for disk diffusion and MIC testing. Incubation temperature should not surpass 36°C. Incubating for a full 24 h did not improve results obtained after 18 h. Hence, standard conditions currently used by clinical laboratories for routine susceptibility testing as described by CLSI can be used, and the cefoxitin disk can be included among other disks relevant for susceptibility testing of Staphylococcus aureus. The current CLSI cefoxitin zone diameter breakpoints should be adjusted by a 2-mm increase.
Footnotes
Published ahead of print on 18 October 2006.
REFERENCES
- 1.Berger-Bachi, B., A. Strassle, J. E. Gustafson, and F. H. Kayser. 1992. Mapping and characterization of multiple chromosomal factors involved in methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 36:1367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boutiba-Ben Boubaker, I., R. Ben Abbes, H. Ben Abdallah, K. Mamlouk, F. Mahjoubi, A. Kammoun, A. Hammami, and S. Ben Redjeb. 2004. Evaluation of a cefoxitin disk diffusion test for the routine detection of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 10:762-765. [DOI] [PubMed] [Google Scholar]
- 3.Boyce, J. M., B. Cookson, K. Christiansen, S. Hori, J. Vuopio-Varkila, S. Kocagoz, A. Y. Oztop, C. M. Vandenbroucke-Grauls, S. Harbarth, and D. Pittet. 2005. Methicillin-resistant Staphylococcus aureus. Lancet Infect. Dis. 5:653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cauwelier, B., B. Gordts, P. Descheemaecker, and H. Van Landuyt. 2004. Evaluation of a disk diffusion method with cefoxitin (30 μg) for detection of methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 23:389-392. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, H. F. 1997. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 10:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing. 15th informational supplement M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felten, A., B. Grandry, P. H. Lagrange, and I. Casin. 2002. Evaluation of three techniques for detection of low-level methicillin-resistant Staphylococcus aureus (MRSA): a disk diffusion method with cefoxitin and moxalactam, the Vitek 2 system, and the MRSA-screen latex agglutination test. J. Clin. Microbiol. 40:2766-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes, C. J., L. A. Fernandes, and P. Collignon. 2005. Cefoxitin resistance as a surrogate marker for the detection of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 55:506-510. [DOI] [PubMed] [Google Scholar]
- 10.Gueudet, T., and C. Lemble. 2004. Comparison of five usual techniques for detection of methicillin-resistant Staphylococcus aureus. Pathol. Biol. (Paris) 52:617-621. [DOI] [PubMed] [Google Scholar]
- 11.Harmsen, D., H. Claus, W. Witte, J. Rothganger, H. Claus, D. Turnwald, and U. Vogel. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41:5442-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heneine, N., and P. R. Stewart. 1986. Physiological determination of methicillin resistance in Staphylococcus aureus: comparison of clinical and genetically derived isolates. J. Antimicrob. Chemother. 17:705-715. [DOI] [PubMed] [Google Scholar]
- 13.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mougeot, C., J. Guillaumat-Tailliet, and J. M. Libert. 2001. Staphylococcus aureus: new detection of intrinsic resistance using the diffusion method. Pathol. Biol. (Paris) 49:199-204. [DOI] [PubMed] [Google Scholar]
- 15.Murchan, S., M. E. Kaufmann, A. Deplano, R. de Ryck, M. Struelens, C. E. Zinn, V. Fussing, S. Salmenlinna, J. Vuopio-Varkila, N. El Solh, C. Cuny, W. Witte, P. T. Tassios, N. Legakis, W. van Leeuwen, A. van Belkum, A. Vindel, I. Laconcha, J. Garaizar, S. Haeggman, B. Olsson-Liljequist, U. Ransjo, G. Coombes, and B. Cookson. 2003. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J. Clin. Microbiol. 41:1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pottumarthy, S., T. R. Fritsche, and R. N. Jones. 2005. Evaluation of alternative disk diffusion methods for detecting mecA-mediated oxacillin resistance in an international collection of staphylococci: validation report from the SENTRY Antimicrobial Surveillance Program. Diagn. Microbiol. Infect. Dis. 51:57-62. [DOI] [PubMed] [Google Scholar]
- 17.Sabath, L. D., and S. J. Wallace. 1971. The problems of drug-resistant pathogenic bacteria. Factors influencing methicillin resistance in staphylococci. Ann. N. Y. Acad. Sci. 182:258-266. [DOI] [PubMed] [Google Scholar]
- 18.Skov, R., L. V. Pallesen, R. L. Poulsen, and F. Espersen. 1999. Evaluation of a new three hours hybridisation method for detection of the mecA gene in Staphylococcus aureus and correlation to existing genotypic and phenotypic susceptibility testing methods. J. Antimicrob. Chemother. 43:467-475. [DOI] [PubMed] [Google Scholar]
- 19.Skov, R., R. Smyth, M. Clausen, A. R. Larsen, N. Frimodt-Moller, B. Olsson-Liljequist, and G. Kahlmeter. 2003. Evaluation of a cefoxitin 30 μg disc on Iso-Sensitest agar for detection of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 52:204-207. [DOI] [PubMed] [Google Scholar]
- 20.Skov, R., R. Smyth, A. R. Larsen, N. Frimodt-Moller, and G. Kahlmeter. 2005. Evaluation of cefoxitin 5 and 10 μg discs for the detection of methicillin resistance in staphylococci. J. Antimicrob. Chemother. 55:157-161. [DOI] [PubMed] [Google Scholar]
- 21.Swenson, J. M., and F. C. Tenover. 2005. Results of disk diffusion testing with cefoxitin correlate with presence of mecA in Staphylococcus spp. J. Clin. Microbiol. 43:3818-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Technical Committee ISO/TC 212 and Technical Committee CEN/TC 140. 2006. Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices - part 1: reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. International Organization for Standardization, Geneva, Switzerland.
- 23.Urbaskova, P., O. Melter, B. Mackova, V. Jakubu, and M. Wunschova. 2004. Detection of MRSA in a group of 752 strains of S. aureus using a cefoxitin disk. Epidemiol. Mikrobiol. Imunol. 53:62-65. [PubMed] [Google Scholar]
- 24.Velasco, D., T. M. del Mar, M. Cartelle, A. Beceiro, A. Perez, F. Molina, R. Moure, R. Villanueva, and G. Bou. 2005. Evaluation of different methods for detecting methicillin (oxacillin) resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 55:379-382. [DOI] [PubMed] [Google Scholar]