Abstract
We present a PCR method targeting the 23S-5S internal transcribed spacer coupled with reverse line blotting that allows Rickettsia species detection and identification in a single step. The method is highly sensitive and specific in identifying Rickettsia species from both patient and environmental samples. The generic approach used allowed us to identify new pathogens.
Rickettsia spp. constitute a genus with an increasing number of species. As the methodologies for culture and molecular detection improve, the association between Rickettsia species found only in arthropods or reservoirs and human disease is on the rise (12-19).
Their obligate intracellular life cycle and the small number of organisms present in clinical samples, as well as serological cross-reactions between species, make rickettsiae difficult to diagnose and to identify at the species level (19). Although the treatment guidelines for suspected rickettsiosis are well known, an accurate determination of the rickettsial species causing human disease is clinically and epidemiologically relevant, and methods for its molecular identification should be implemented.
Available methods for molecular diagnosis of rickettsioses require a two-step methodology that includes sequencing of the amplified fragments and an accurate methodology for phylogenetic analysis and species identification that are not available at all laboratories. This hampers the acquisition of global data on rickettsia species circulating in vectors and reservoirs and on those that cause human disease.
Recently, the comparison of highly variable intergenic spacers has been proposed as an accurate typing method within bacterial species (5). Also, conserved regions in the rRNA intergenic spacers have been used in other bacterial genera for detection and differentiation among species (2, 3, 6, 8, 20, 21). The structure of the spacers within the rRNA operon, with interspecies hypervariable central regions flanked by conserved ends, makes them powerful tools for the design of generic methods for molecular detection.
Here, we present a method for the detection of Rickettsia based on a PCR that amplifies a fragment of the 23S-5S intergenic spacer and subsequent hybridization by reverse line blotting (RLB) (22). Briefly, in this methodology, group- and species-specific probes are covalently linked to activated membranes in lines using a slotted miniblotter. To test PCR products from samples for reactivity to probes, the blot is returned to the blotter, but rotated 90°. Hybridizations with denatured PCR products are detected using chemiluminescence. For the design of primers and probes, available sequences (Table 1) were aligned by using ClustalX (23). Regions of interest, between 18 and 24 bp long with melting temperatures above 60°C, were identified by visual analysis, and their feasibility as primers and probes (Table 1) was checked with Oligo6 software (Molecular Biology Insights, Inc. West Cascade, CO). The Basic Local Alignment Search Tool (BLASTn) (1) was used for a preliminary assessment of oligonucleotide specificity.
TABLE 1.
Primers and probes
| Organism | Primers | Probes | Sequence (5′-3′) | Positiona |
|---|---|---|---|---|
| Rickettsia spp. | RCK/23-5-F | GATAGGTCRGRTGTGGAAGCAC | 1-22 (AY125012) | |
| RCK/23-5-R | TCGGGAYGGGATCGTGTGTTTC | 388-367 (AY125012) | ||
| Generic | GP-RICK | TAGCTCGATTGRTTTACTTTG | 51-71 (AY125012) | |
| SFG | GP-SFG | ACTCACAARGTTATCAGGT | 123-141 (AY125012) | |
| R. akari | P-AKA3 | GATCATGCAGCAATACATTAGC | 291105-291126 (AAFE01000001) | |
| R. belliii | P-BELL | GTGTTTATTCTATAATATGTCAG | 2721-2743 (U11015) | |
| R. slovaca | P-SLO | GTAGCCCCTGCCACGATA | 194-211 (AY125009) | |
| R. conorii | P-CON | GTTATATACTGTAGCCCTG | 186-204 (AY125012) | |
| R. aeschlimannii | P-AESCH | ATATTATACTGTATGTAGCCCC | 183-204 (AY125016) | |
| R. rickettsii-R. sibirica | P-RI/SI | GTTATACTGTAGTCCTGCAA | 2814-2833 (U11022) | |
| R. helvetica | P-HELV | CATGGCTTGATCCACGGTA | 360-342 (AY125017) | |
| R. felis | P-FEL | TAATGTTATACCGTGGTCCCGC | 186-207 (AM055829) | |
| R. australis | P-AUS | GACAAGTTTAGTTATGCAAT | 230-249 (AM055830) | |
| Typhus group | GP-TG | GTTATTCTATCGTTTTATGTYACG | 2804-2827 (U11018) | |
| R. prowazekii | P-PROW | TACGATTTGATAGTAAAGTTTTG | 2824-2846 (U11018) | |
| R. typhi (R. mooserii) | P-TYPHI | ATGTCACGATTTGACCGTAAGATC | 188-211 (AY125019) | |
| Cannabis sativa | P-CI2 | GTGGACACTTTAGTGGAGGAGG | 281-302 (AB183705) |
Accession numbers for the reference sequences are in parentheses.
Primers and probes were synthesized with 5′-biotin and C-12 aminolink modifications, respectively (Sigma-Aldrich Química, Tres Cantos, Madrid, Spain) (Table 1). PCRs were performed in a volume of 50 μl with 10 mM Tris-HCl, 50 mM KCl, 2 mM Cl2Mg, 200 μM of each deoxynucleoside triphosphate (Promega, Madison, WI), 2.5 U of Taq Gold DNA polymerase (Applied Biosystems, Branchburg, NJ), and 0.8 μg/μl of DNase-free bovine serum albumin (Amersham Biosciences, Barcelona, Spain), with primers used at a final concentration of 0.5 μM. PCR cycling included an initial denaturating step of 9 min at 94°C, followed by 40 cycles of 15 s at 94°C, 1 min at 60°C, and 4 min at 65°C, with a final elongation of 7 min at 65°C in an MJ Research PCT-200 (Ecogen, Barcelona, Spain). For the hybridization, a Biometra OV3 Mini Hybridization Oven (Cultek, S. L., Madrid, Spain) was used, and the RLB was performed as described previously (20) with modifications in the temperatures of the hybridization (52°C) and washing (48°C) steps. Probes were attached to the membrane by 10 min of incubation. Cross-titration of probes and PCR products showed that the best concentration of probes was 0.4 ng/μl for a PCR product diluted 1:15 (data not shown).
As positive controls, DNA was extracted with a QIAamp DNA Mini Kit (IZASA S.A., Barcelona, Spain) from different sources. Strains (Table 2) were grown in Vero cell monolayers under biosafety level 3 conditions as previously described (10); DNA of Rickettsia rickettsii was extracted from scraped slides for indirect immunofluorescence (Focus Diagnostics, Cypress, CA), and DNA of R. felis from infected Ctenocephalides felis specimens was collected from stray cats in La Rioja (Northern Spain). The empirical determination of the sensitivity of the technique with these DNAs was done by serial 10-fold dilutions of the stock (Fig. 1). When native material was not available, as for R. prowazekii, R. bellii, and R. aeschlimannii, a synthetic DNA fragment was constructed following available sequences with overlapping primers up to 75 bp long to be used in consecutive PCRs (data not shown). Then, the synthetic fragments were cloned in pGEM-T-easy vectors (Promega Biotech Ibérica, SL, Madrid, Spain) following the manufacturer's instructions and sequenced to check their fidelity. The plasmid copy numbers were quantified by spectrophotometry with a NanoDrop ND-1000 spectrophotometer (Nucliber, Madrid, Spain). One, 10, 102, and 103 copies of each plasmid per reaction were tested, and a sensitivity of 1 plasmid copy per reaction was observed for each species whose intergenic spacer was cloned (Fig. 1). The sensitivity of the test was also determined in the presence of foreign DNAs from different clinical and environmental samples. Under these conditions, with the amount of DNA that is usually included in each reaction (200 to 600 ng) and 10 and 1 copies of a plasmid containing the intergenic spacer of R. bellii per reaction, no diminution of sensitivity was observed (Fig. 2). This test was also performed with plasmids of R. prowazekii, and similar results were obtained (data not shown).
TABLE 2.
Samples and strains included in the studya
| Patient/arthropod/ strain no. | Sample | Origin | Region | Resultb |
|---|---|---|---|---|
| 1 | R. aeschlimanii | Synthetic DNA | + | |
| 2 | R. akari | Native DNA | + | |
| 3 | R. australis | Native DNA | + | |
| 4 | R. bellii | Synthetic DNA | + | |
| 5 | R. conorii | Native DNA | + | |
| 6 | R. felis | Stray cat flea | + | |
| 7 | R. helvetica | Native DNA | + | |
| 8 | R- rickettsii | IIF slidesc | + | |
| 9 | R. sibirica | Native DNA | + | |
| 10 | R. slovaca | Native DNA | + | |
| 11 | R. prowazekii | Synthetic DNA | + | |
| 12 | R. typhi | Native DNA | + | |
| 13 | Negative control | Water | Neg | |
| 14 | Rhipicephalus sanguineus | Questing tick | Castilla y León | R. conorii |
| 15 | R. sanguineus | Questing tick | Castilla y León | R. conorii |
| 16 | D. marginatus | Tick from patient | La Rioja | R. slovaca |
| 17 | D. marginatus | Tick from patient | La Rioja | R. slovaca |
| 18 | D. marginatus | Tick from patient | La Rioja | R. slovaca |
| 19 | Xenopsylla cheopis | Flea from goat herd | Canary Island | R. typhi |
| 20 | X. cheopis | Flea from goat herd | Canary Island | R. typhi |
| 21 | X. cheopis | Flea from goat herd | Canary Island | R. typhi |
| 22 | X. cheopis | Flea from goat herd | Canary Island | R. typhi |
| 23 | Blood | Murine typhus patient | Canary Island | R. typhi |
| 24 | Blood | Murine typhus patient | Canary Island | R. typhi |
| 25 | Ctenocephalides felis | Flea from stray cat | La Rioja | R. felis |
| 26 | Plasma | MSF patient | Castilla y León | R. conorii |
| 27 | Blood | MSF patient | Castilla y León | R. conorii |
| 28 | Blood | MSF patient | La Rioja | R. conorii |
| 29 | Blood | DEBONEL/TIBOLA patient | La Rioja | Neg |
| 30 | Blood | DEBONEL/TIBOLA patient | La Rioja | Neg |
| 31 | Blood | MSF-like patient | La Rioja | SFG |
| 32 | Blood | MSF-like patient | País Vasco | SFG |
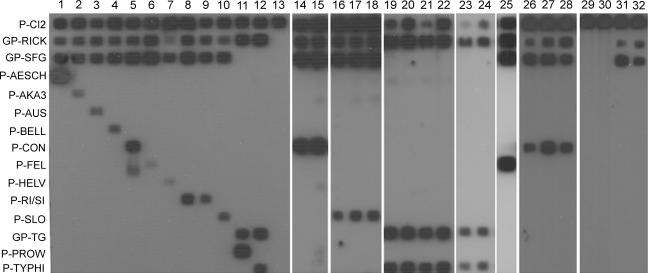
FIG. 1.
23S-5S PCR/RLB results for selected samples from this study. Lanes 1 to 13, positive controls: lane 1, R. aeschlimannii; lane 2, R. akari; lane 3, R. australis; lane 4, R. bellii; lane 5, R. conorii; lane 6, R. felis; lane 7, R. helvetica; lane 8, R. rickettsii; lane 9, R. sibirica subsp. mongolotimonae; lane 10, R. slovaca; lane 11, R. prowazekii; lane 12, R. typhi; lane 13, negative control. Lanes 14 to 32, environmental and human samples as listed in Table 2. The names of the probes used are shown on the left, as abbreviated in Table 1.
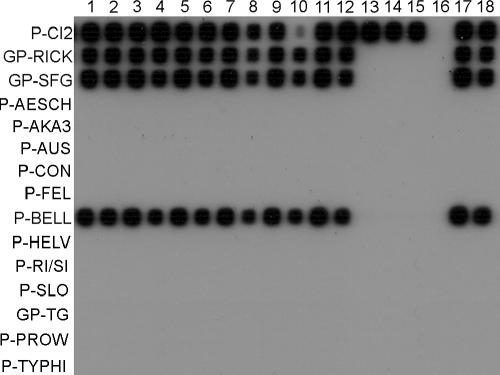
FIG. 2.
Sensitivities of the 23S-5S PCR/RLB in clinical and environmental samples spiked with an R. bellii 23S-5S cloned fragment. Lanes 1, 3, 5, 7, 9, and 11, samples spiked with 10 copies per reaction; lanes 2, 4, 6, 8, 10, and 12, samples spiked with 1 copy per reaction. Negative samples were human blood (lanes 1 and 2), human cerebrospinal fluid (lanes 3-4), and a Rhipicephalus sanguineus specimen (lanes 5 and 6). Positive samples were human blood positive for Coxiella burnetii (lanes 7 and 8), a human lymph node aspirate positive for Bartonella henselae (lanes 9 and 10), and a human swab specimen from a skin lesion positive for Francisella tularensis (lanes 11 and 12). Positive controls were 10 copies (lane 17) and 1 copy (lane 18) of an R. bellii cloned fragment without foreign DNA. Negative controls were distilled water without IAC (lane 16) and human samples positive for other pathogens without spiked DNA (human blood positive for C. burnetii [lane13], a lymph node aspirate positive for B. henselae [lane 14], and a human swab specimen from a skin lesion positive for F. tularensis [lane 15]).
The generic probes designed (Rickettsia catch-all, spotted fever group [SFG], and typhus group) and the specific probes for 11 Rickettsia species showed good specificity without cross-reactions (Table 2 and Fig. 1), allowing the individual identification of each of the species tested. In the cases of R. rickettsii and R. sibirica, due to the high homology of their rrf-rrl spacer sequences, a common probe for the two species was designed (Fig. 1). Sequencing of rrf-rrl allows differentiation between the two species. The specificity of detection for all positive samples presented here was confirmed by sequencing of ompA and/or gltA (data not shown).
As a negative control, 103 genome equivalents per reaction of Legionella pneumophila, Bartonella henselae, Bacillus cereus, Borrelia burgdorferi sensu stricto, and Mycoplasma pneumoniae was used as a target in the PCR. Also, Orientia tsutsugamushi and Anaplasma phagocytophilum were tested at a concentration 100 times higher than the last 10-fold dilution of the stock that yielded amplicons in specific PCR protocols to amplify the omp 47-kDa protein-coding gene (9) and msp2 (11), respectively. None of the heterologous bacterial species yielded a positive hybridization signal (data not shown). In addition, clinical samples positive for different agents were tested (Fig. 2, lanes 13 to 15), and all of them were negative.
Also, to assess the presence of PCR inhibitors in the samples, a synthetic internal amplification control (IAC) was constructed and cloned as described above, following the partial sequence of a gene of an unrelated organism (the Cannabis sativa tetrahydrocannabinolic acid synthase gene) (Table 1). Primers and a probe designed for IAC detection (Table 1) were used in combination with the Rickettsia-specific method at the same concentration described above, and 10 IAC plasmid copies per reaction were added at the moment of amplification. We observed the lack of PCR inhibitors, as IAC was detected in all the samples tested (Fig. 1, probe P-CI2).
For a preliminary validation of the technique, between 200 and 600 ng of DNA from several environmental samples, as well as human samples, was tested. For this purpose, different arthropods (ticks and fleas) were tested (Table 2). Specimens of questing Riphicephalus sanguineus reacted with the probe R-CON for R. conorii (Fig. 1, lanes 14 and 15). Questing Dermacentor marginatus specimens hybridized with the probe for R. slovaca (P-SLO) (Fig. 1, lanes 16 to 18). Also, specimens of Xenopsylla cheopis collected from areas where murine typhus cases had been diagnosed (7) hybridized with the probe P-TYPHI for R. typhi (Fig. 1, lanes 19 to 22) and C. felis specimens hybridized with P-FEL for R. felis (Fig. 1, lanes 25). As described above, all the positive samples were subjected to sequencing to confirm the results.
Among the human samples, patients suspected or confirmed to have Mediterranean spotted fever (MSF); Dermacentor-borne necrosis, erythema, and lymphadenopathy/tick-borne lymphadenopathy (DEBONEL/TIBOLA) (12, 15, 18); and murine typhus were tested (Table 2 and Fig. 1). Samples from two MSF patients from Castilla y León (Fig. 1, lanes 26 and 27) and La Rioja (Fig. 1, lane 28) reacted with the specific probe for R. conorii. Further identification of the subspecies involved in these cases is in progress. Also, two cases of murine typhus were analyzed, which hybridized with GP-TG and R. typhi probes as expected (Fig. 1, lanes 23 and 24). Two patients from La Rioja with clinically diagnosed DEBONEL/TIBOLA had negative results (Fig. 1, lanes 29 and 30), which was probably due to the low level of systemic dissemination observed for the pathogen involved. Moreover, the molecular diagnosis of this disease is known to have low efficiency with the available methodology (12, 18).
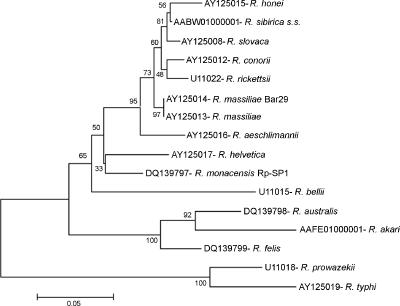
Interestingly, samples from two patients with clinically diagnosed MSF reacted only with the probes generic for all Rickettsia strains and for the SFG (Fig. 1, lanes 31 and 32). Surprisingly, no signal was obtained either with the R. conorii probe or with other SFG Rickettsia species probes. Consequently, we decided to sequence the amplicons of both samples, and identical sequences were obtained. In a phylogenetic tree, constructed as previously described (4), they placed close to R. helvetica but in a separate clade (Fig. 3), suggesting that a different Rickettsia strain was responsible for these cases. A further study of these cases had disclosed R. monacensis as the etiological agent of the processes (I. Jado, J. A. Oteo, M. Aldámiz, H. Gil, R. Escudero, V. Ibarra, J. Portu, A. Portillo, M. J. Lezaun, C. García-Amil, I. Rodríguez-Moreno, and P. Anda, submitted for publication). These results showed that the approach used for the design of this method, with both generic and specific probes, will be of interest when a new Rickettsia species in either human disease or environmental samples is involved. Also, the method is improvable by adding new specific probes as new Rickettsia species are described.
FIG. 3.
23S-5S phylogenetic analysis of the Rickettsia species detected in the sample Rp-SP1 from the blood of an MSF-like patient. The dendrogram was built using the neighbor-joining method, and Mega 3 software was used for the calculation of pairwise distances. The number above each node indicates the bootstrap value. A distance bar is shown at the bottom.
As a major conclusion, this new method has been proven to be a reliable and powerful tool for the identification of the etiology of human rickettsioses in a clinical setting, as well as for performing environmental studies. The proposed generic approach for amplification will also be useful for the establishment of new associations between Rickettsia species of unknown pathogenicity and human disease.
Nucleotide sequence accession number.
The Rickettsia sequences obtained in this study have been submitted to GenBank under accession number DQ139797.
Acknowledgments
This study was supported by Fondo de Investigación sanitaria “Red temática de Investigación Cooperativa EBATRAG (G03/057).”
We thank Michele Hernández Cabrera from the Hospital Insular de Gran Canaria, José Antonio Oteo from the Hospital de La Rioja, and José Ignacio Herrero from the Hospital Los Montalvos, Salamanca, Spain, for providing samples of human cases of murine typhus, DEBONEL/TIBOLA, and MSF, respectively. Special thanks are due to Fatima Bacellar from CEVDI, Aguas de Moura, Portugal, for providing us some Rickettsia strains. Specimens of Ctenocephalides felis obtained from stray cats were kindly provided by Santos Jiménez from Consejería de Salud, Consumo y Bienestar Social, Gobierno de la Rioja, Spain.
Footnotes
Published ahead of print on 11 October 2006.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Cardinale, M., L. Brusetti, P. Quatrini, S. Borin, A. M. Puglia, A. Rizzi, E. Zanardini, C. Sorlini, C. Corselli, and D. Daffonchio. 2004. Comparision of different primer sets for use in automated ribosomal intergenic spacer analysis of complex bacterial communities. Appl. Environ. Microbiol. 70:6147-6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalker, V. J., W. M. Owen, C. J. Paterson, and J. Brownlie. 2004. Development of a polymerase chain reaction for the detection of Mycoplasma felis in domestic cats. Vet. Microbiol. 100:77-82. [DOI] [PubMed] [Google Scholar]
- 4.Gil, H., M. Barral, R. Escudero, A. L. García-Pérez, and P. Anda. 2005. Identification of a new Borrelia species among small mammals in areas of Northern Spain where Lyme disease is endemic. Appl. Environ. Microbiol. 71:1336-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glazunova, O., V. Roux, O. Freylikman, Z. Sekeyova, G. Fournous, J. Tyczka, N. Tokarevich, E. Kovacava, T. J. Marrie, and D. Raoult. 2005. Coxiella burnetti genotyping. Emerg. Infect. Dis. 11:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González, N., J. Romero, and R. T. Espejo. 2003. Comprehensive detection of bacterial populations by PCR amplification of the 16S-23S rRNA spacer region. J. Microbiol. Methods 55:91-97. [DOI] [PubMed] [Google Scholar]
- 7.Hernández-Cabrera, M., A. Ángel-Moreno, E. Santana, M. Bolaños, A. Frances, M. S. Martín-Sánchez, and J. L. Pérez-Arellano. 2004. Murine typhus with renal involvement in Canary Islands, Spain. Emerg. Infect. Dis. 10:740-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen, W. A., M. Z. Fall, J. Rooney, D. L. Kordick, and E. B. Breitschwerdt. 2000. Rapid identification and differentiation of Bartonella species using a single-step PCR assay. J. Clin. Microbiol. 38:1717-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang, J., T. C. Chan, J. J. Temenak, G. A. Dasch, W. M. Ching, and A. L. Richards. 2004. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am. J. Trop. Med. Hyg. 70:351-356. [PubMed] [Google Scholar]
- 10.La Scola, B., and D. Raoult. 1997. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J. Clin. Microbiol. 35:2715-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massung, R. F., and K. G. Slater. 2003. Comparison of PCR assays for detection of the agent of human granulocytic ehrlichiosis, Anaplasma phagocytophilum. J. Clin. Microbiol. 41:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oteo, J. A., V. Ibarra, J. R. Blanco, V. Martínez de Artola, F. J. Márquez, A. Portillo, D. Raoult, and P. Anda. 2004. Dermacentor-borne necrosis erythema and lymphadenopathy: clinical and epidemiological features of a new tick-borne disease. Clin. Microbiol. Infect. 10:327-331. [DOI] [PubMed] [Google Scholar]
- 13.Paddock, C. D., J. W. Sumner, J. A. Comer, S. R. Zaki, C. S. Goldsmith, J. Goddard, S. L. McLellan, C. L. Tamminga, and C. A. Ohl. 2004. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 38:805-811. [DOI] [PubMed] [Google Scholar]
- 14.Parola, P., and D. Raoult. 2001. Tick-borne bacterial diseases emerging in Europe. Clin. Microbiol. Infect. 7:80-83. [DOI] [PubMed] [Google Scholar]
- 15.Raoult, D., P. Berbis, V. Roux, W. Xu, and M. Maurin. 1997. A new tick-transmitted disease due to Rickettsia slovaca. Lancet 350:112-113. [DOI] [PubMed] [Google Scholar]
- 16.Raoult, D., P. E. Fournier, P. Abboud, and F. Caron. 2002. First documented human Rickettsia aeschlimannii infection. Emerg. Infect. Dis. 8:748-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raoult, D., P. E. Fournier, F. Fenollar, M. Jensenius, T. Prioe, J. J. de Pina, G. Caruso, N. Jones, H. Laferl, J. E. Rosenblatt, and T. J. Marrie. 2001. Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N. Engl. J. Med. 344:1504-1510. [DOI] [PubMed] [Google Scholar]
- 18.Raoult, D., A. Lakos, F. Fenollar, J. Beytout, P. Brouqui, and P. E. Fournier. 2002. Spotless rickettsiosis caused by Rickettsia slovaca and associated with Dermacentor ticks. Clin. Infect. Dis. 34:1331-1336. [DOI] [PubMed] [Google Scholar]
- 19.Raoult, D., and V. Roux. 1997. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 10:694-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rijpkema, S. G. T., M. J. C. H. Molkenboer, L. M. Schouls, F. Jongejan, and J. F. P. Schellekens. 1995. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J. Clin. Microbiol. 33:3091-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson, P. N., B. Heidrich, F. Tiecke, F. J. Fehrenbach, and A. Rolfs. 1996. Species-specific detection of Legionella using polymerase chain reaction and reverse dot-blotting. FEMS Microbiol. Lett. 140:111-119. [DOI] [PubMed] [Google Scholar]
- 22.Saiki, R. K., C. A. Chang, C. H. Levenson, T. C. Warren, C. D. Boehm, H. H. Kazazian, and H. A. Erlich. 1988. Diagnosis of sickle cell anemia and β-thalassemia with enzymatically amplified DNA and nonradioactive allele-specific oligonucleotide probes. N. Engl. J. Med. 319:537-541. [DOI] [PubMed] [Google Scholar]
- 23.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmoungin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]