Abstract
Surveillance for methicillin-resistant Staphylococcus aureus (MRSA) at the University Hospital of Heidelberg revealed an increase in the numbers of newly detected MRSA isolates in recent years. We conducted a study to assess the dynamics of the changes in the MRSA population. Pulsed-field gel electrophoresis (PFGE) typing of MRSA isolates from all patients at the University Hospital of Heidelberg collected between 1993 and 2004 was performed. The microbiology database contained 1,807 entries for newly detected MRSA isolates from 1,301 patients. A total of 1,252 isolates were available for PFGE typing. The isolates could be classified into 109 different PFGE types. Most PFGE types (n = 70) were detected less than five times and showed no evidence of transmission (sporadic strains). They accounted for 8.7% of all isolates, with few variations in frequency over the time. Thirty-seven PFGE types were clustered by time of detection, and transmission of the strains was likely (local epidemic strains). A total of 37.3% of the isolates belonged to this group of strains. The remaining 54.0% of the isolates belonged to only two further PFGE types (endemic strains). One endemic strain accounted for 5.0% of all isolates in 1994 and 68.2% in 2004. A second endemic strain was detected in 1.1% of all isolates in 1998 but in 12.4% in 2004. Statistical analysis of the associations between the kind of strain (sporadic, local epidemic, or endemic) and the patients' characteristics revealed a significant association for age and mode of acquisition. The remarkable increase in the rate of MRSA detection at the University Hospital of Heidelberg is mainly due to the dissemination of two different strains. Infection control measures seemed sufficient to prevent further transmission of some but not all of the strains.
Methicillin-resistant Stapyhlococcus aureus (MRSA) was first discovered in 1961 and has since become a major nosocomial pathogen (19). Transmission within hospitals, spread between different hospitals in the same country, and even the intercontinental spread of endemic strains have been described (7, 8). Certain strains can be found worldwide, and only a few strains are responsible for a large proportion of MRSA infections (23). In Germany, the proportion of MRSA isolates among all S. aureus isolates in clinical specimens rose from 8% in 1995 to 30% in 2003, an increase greater than the increases in nearly all other European countries (21). During the same time we observed an increase in the proportion of MRSA isolates in the University Hospital of Heidelberg from 2.7 to 12.3%. Nonsystematic, incidence-driven pulsed-field gel electrophoresis (PFGE) typing of MRSA isolates revealed that many isolates belonged to one strain. The aim of this study was to describe the molecular epidemiology of MRSA, to assess the frequencies of the strains detected as a reference for further typing, and to generate hypotheses about the mechanisms of transmission and the efficacy of prevention measures by typing one isolate from each MRSA carrier who was identified between 1993 and 2004 and comparison of the kind of MRSA strain (sporadic, local epidemic, or endemic) with the patient's data.
(This work was presented in part at the 57th Annual Meeting of the Deutsche Gesellschaft für Hygiene und Mikrobiologie, Göttingen, Germany, 25 to 28 September 2005 [S. Petersdorf, K. Oberdorfer, and C. Wendt, 57th Ann. Meet. Deutsche Gesellsch. Hyg. Mikrobiol., poster PIP003].)
MATERIALS AND METHODS
Hospital setting and bacterial isolates.
Heidelberg University Hospital is a 1,600-bed tertiary-care hospital that includes departments for internal medicine, surgery, neurology, orthopedics, gynecology, pediatrics, dermatology, and psychiatry. Clinical specimens are tested in the laboratories of the Institute of Hygiene. The proportion of MRSA isolates among all S. aureus isolates detected increased from 2.7% in 1993 to 12.3% in 2004. MRSA-positive cultures were derived from either clinical specimens or screening swabs. The infection control program implemented consists of screening of special patient populations, e.g., patients with skin defects or transplant patients, for MRSA.
The isolates were confirmed to be S. aureus by colony morphology as well as by the production of catalase, hyaluronidase, and coagulase. Oxacillin resistance was confirmed by an agar screen test, according to the criteria of the Clinical and Laboratory Standards Institute (formerly the National Committee for Clinical Laboratory Standards) (16). Since 1993 at least one MRSA isolate from each MRSA carrier has been stirred into a skim milk medium, frozen, and stored at −30°C.
Molecular typing.
One isolate from each MRSA-carrying patient who was identified between 1993 and 2004 was typed by PFGE. Whole chromosomal DNA, which was embedded in agarose, was digested with SmaI. Restriction fragments were separated in a CHEF DRII apparatus (Bio-Rad, Munich, Germany) at 6 V/cm with a switching time that ranged from 5 to 60 s, as described by Pfaller et al. (18). The gels were stained with ethidium bromide, illuminated under UV light, and photographed.
PFGE patterns were interpreted according to the criteria of Tenover et al. (29), with slight modifications. We distributed the isolates into three groups: (i) isolates with identical banding patterns belonged to the same clone and received the same letter or combination of letters; (ii) isolates with up to three differences in their PFGE patterns were designated subtypes of a certain clone; and (iii) in the case of more than three differences in the PFGE patterns, the isolate was designated a different clone. For better visual comparison, we produced reference gels which showed one strain of each different PFGE type that served as a reference for the identification of the following PFGE patterns. If it was doubtful whether two PFGE patterns on different gels were identical, they were run again next to each other to guarantee that every strain was clearly appointed to a certain PFGE type. For validation of the visual results, the PFGE patterns were analyzed by the use of GelCompar software (Applied Maths, Kortrijk, Belgium). For generation of a dendrogram, the Dice coefficient and the unweighted pair grouping by mathematical averaging cluster algorithm with 1% tolerance was used.
Definitions.
Depending on how often isolates of the different PFGE types were identified during the test period, they were assorted into three different categories: PFGE types were designated “sporadic” if only one isolate showed a distinct pattern or if there where no more than five isolates of the same PFGE type and the isolates were isolated more than 3 months apart from each other. “Endemic” PFGE types were found continuously during the whole study period of 12 years. All other PFGE types, i.e., types that were identified more than five times or at least twice within 3 months were called “local epidemic” PFGE types.
Specimens were assorted into five different categories according to their sources: blood, tracheal secretions, wounds, urine, and others. Blood culture specimens included blood inoculated into aerobic and/or anaerobic blood culture medium. Tracheal secretion specimens included secretions from the trachea and bronchia and bronchoalveolar lavage fluid specimens. Wound specimens included surgical wound specimens but also specimens from ulcers, fistulae, abscesses, drainage fluids, catheters sites, percutaneous endoscopic gastrostomy insertion sites, and tracheostomas. Urine contained native urine from urinary catheters or bladder puncture and inoculated culture systems. Other specimens consisted of skin swabs specimens (from the nares, axilla, groin, or perianal area), as well as swab specimens of the throat, tonsils, eye, ear, and vagina; body liquids; puncture exudates; and swab specimens not further described.
The length of hospital stay until collection of the specimens was determined for inpatients. If MRSA was detected within the first 2 days after admission to the hospital, it was designated “community acquired”; if MRSA was detected on or later than the third day after admission, we assumed that the MRSA isolate was “hospital acquired.”
Antibiogram.
Antimicrobial resistance testing was performed by the microdilution test for each of the 109 distinct PFGE types. The susceptibilities of the isolates to the following antibiotics were tested: ciprofloxacin, clindamycin, erythromycin, gentamicin, rifampin, tetracycline, trimethoprim-sulfamethoxazole, and fusidic acid.
Statistical analysis.
The following patient data were collected from the microbiological database system: age, gender, medical department (internal medicine, surgery, orthopedics, gynecology, pediatrics, dermatology, psychiatrics, or ear-nose-throat and neurosurgery) and service (intensive care unit, normal care unit, or outpatient clinic) at the time of first identification of the MRSA isolate, and mode of acquisition (hospital or community acquired). All specimens from a patient that were examined in the laboratory of University of Heidelberg and that grew MRSA were distributed according to the body site into the categories blood, tracheal secretion, wounds, urine, and others. Associations between the kind of MRSA strain (sporadic, local epidemic, and endemic) and the patient's data were analyzed by the chi-square test or analysis of variance. A P value of <0.05 was considered statistically significant. Variables that were significant in the monovariate analyses were entered in a multimodal logistic regression model to identify factors that were independently associated with the kind of MRSA strain. All analyses were performed with SPSS 12.0G for Windows (SPSS Inc., Chicago, IL).
RESULTS
The MRSA strain collection of the Institute for Hygiene in Heidelberg, Germany, contained 1,807 strains from 1,301 different patients isolated between January 1993 and December 2004. Forty-nine isolates were not further evaluated for the following reasons: the MRSA carrier was not a patient at the University Hospital of Heidelberg, the misspelling of names had led to double entries, or retesting did not confirm methicillin resistance in the stored isolate. Thus, a total of 1,252 isolates were included in the study. The number of isolates per year rose continually during the study period; the smallest number of isolates were identified in 1994 (n = 20) and the highest number were identified in 2004 (n = 217).
PFGE results.
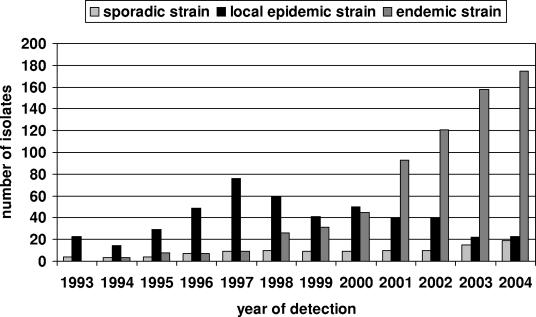
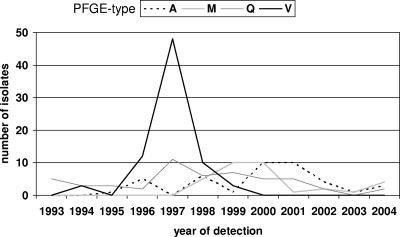
Among the 1,252 MRSA isolates typed, 109 distinct PFGE types could be identified. They appeared at very different frequencies; some occurred only once, and the one with the highest frequency occurred 527 times. Only two PFGE types, endemic PFGE types F and K, made up more than half (54.0%) of all MRSA isolates. In contrast, sporadic PFGE types, which represented two-thirds (64.2%) of the different PFGE types, accounted for only 8.7% of all isolates. Over the 12-year period the percentage of sporadic PFGE types remained relatively constant at about 10% (Fig. 1). In contrast, the proportion of local epidemic strains dramatically decreased, from 85% in 1993 to only 10% in 2004, whereas the proportion of epidemic strains continually increased, from 15% in 1994 to 80% in 2004. Most epidemic strains appeared and disappeared without directed interventions, e.g., PFGE type V (Fig. 2); but others were identified as outbreak strains, and special interventions were taken to prevent further transmission, e.g., eradication of nasal colonization in health care workers (Fig. 2, PFGE type M). The number of PFGE types that occurred for the first time ranged between 6 and 16 per year, but there was no clear trend in the numbers of new types recovered each year. The index of diversity, i.e., the number of different PFGE types in relation to the total number of isolates collected in the same year, showed a steady decrease, partly due to the presence of the predominant endemic strain, which increased the number of isolates without increasing the number of PFGE types.
FIG. 1.
Numbers of sporadic, local epidemic, and endemic strains during the study period by year of detection.
FIG. 2.
Frequency of isolation of local epidemic strains comprising ≥10 isolates in at least 1 year by year of detection.
According to the German National Reference Center for Staphylococci, several MRSA strains are endemic in Germany (20). Some of them were also detected in Heidelberg: the Vienna strain (sequence type 239 [ST239], Brazilian clone) occurred only once and, consequently, was categorized as sporadic. The North German strain (ST247, Iberian clone) accounted for 28 isolates, the South German strain (ST228) accounted for 51 isolates, and the Berlin strain (ST45) accounted for 16 isolates isolates. According to our definition, they belonged to the local epidemic strains in Heidelberg. The Barnim strain (ST22, epidemic MRSA type 15 [EMRSA-15]) and the Hannover strain (ST254, EMRSA-10) could not be identified at all. A comparison of our PFGE types and the international nomenclature and other typing methods is shown in Table 1. There are two endemic strains in Heidelberg: one endemic strain, the Rhine-Hessen-Epidemic strain (ST5, New York/Japan clone), accounted for 5.0% of all isolates in 1994 and 68.2% of all isolates in 2004. During this time this type developed more subtypes than any other type (Fig. 3A). A similar development became apparent with the second endemic strain, which comprised 1.1% of all isolates from 1998 but 12.4% from 2004 (Fig. 3B).
TABLE 1.
Comparison of PFGE types with the types in existing databases as well as with different methods of typinga
| Heidelberg PFGE type | German Reference Center for Staphylococci strain | International nomenclature | British endemic strain(s) | MLST type | spa typeb |
|---|---|---|---|---|---|
| F | Rhine-Hessen | EMRSA-3 | ST5 | t001, t002, t010, t045, t053, to62, t105, t178, t179, t187, t214, t319, t389, t443 | |
| F | New York, Japan | ||||
| F | Pediatric | ||||
| S | North German | Iberian | EMRSA-5 | ST247 | t008, t051, t052, t054, t200 |
| AI | Wienna | Brazilian | EMRSA-4/7 | ST239 | t037 |
| Q | South German | ST228 | t001, t188, t201 | ||
| Q | ST222 | ||||
| G | ST5 | ||||
| G | Berlin | ST45 | t004, t015, t031, t03, t050, t065, t204, t230, t3908 | ||
| AM | c-MRSA | ST80 | t044 | ||
| M | EMRSA-16 | ST36 | t018 | ||
| Not detected | Hannover | EMRSA-10 | ST254 | t008, t009 | |
| Not detected | Barnim | EMRSA-15 | ST22 | t005, t022, t030, t032, t223, t417 |
FIG. 3.
(a) Subtypes of PFGE type F (Rhine-Hessen epidemic strain/ST5/New York/Japan clone) isolated between 1994 and 2004. (b) PFGE type K with two suptypes, isolated between 1994 and 2004.
Patients.
The patients' ages ranged from 0 to 101 years, with an average age of 55.9 years. The majority of the patients were admitted to the surgery (n = 393), internal medicine (n = 237), orthopedic (n = 226), and neurology (n = 201) units. The mode of acquisition could be determined for 464 of the 969 inpatients. While the proportions of hospital- and community-acquired MRSA isolates were equal among the local epidemic strains and were similar among the endemic strains, the highest and the most statistically significant difference occurred among the sporadic strains, three-quarters of which were hospital acquired (Table 2). During the study period the proportion of hospital-acquired isolates dropped from about 80% in 1997 to less than 60% in 2003.
TABLE 2.
Association between type of strain (sporadic, local epidemic, or endemic) and characteristic of patients
| Characteristic | Sporadic | Local epidemic | Endemic | P |
|---|---|---|---|---|
| Age (mean [95% CIa]) | 50.9 (46.9-54.8) | 53.2 (51.2-55.1) | 58.7 (57.1-60.2) | <0.001 |
| No. of patients in the following category/total no. (%): | ||||
| Gender | ||||
| Male | 79/109 (64.2) | 325/467 (69.6) | 422/676 (62.4) | 0.043 |
| Female | 39/109 (35.8) | 142/467 (30.4) | 354/676 (37.6) | |
| Department | ||||
| Internal medicine | 11/109 (10.1) | 69/467 (14.8) | 157/676 (23.2) | <0.001 |
| Surgery | 30/109 (27.5) | 174/467 (37.2) | 189/676 (27.9) | |
| Neurology | 14/109 (12.8) | 69/467 (14.8) | 118/676 (17.4) | |
| Orthopedics | 32/109 (29.3) | 104/467 (22.3) | 90/676 (13.3) | |
| Dermatology | 13/109 (11.9) | 16/467 (3.4) | 64/676 (9.5) | |
| Gynecology | 3/109 (2.8) | 11/467 (2.3) | 24/676 (3.5) | |
| Pediatrics | 5/109 (4.6) | 23/467 (4.9) | 27/676 (4.0) | |
| Psychiatry | 1/109 (0.9) | 1/467 (0.2) | 7/676 (1.0) | |
| Specimen | ||||
| Blood | 4/86 (4.7) | 33/310 (10.6) | 46/576 (8.0) | 0.166 |
| Tracheal secretion | 17/86 (19.8) | 94/326 (28.8) | 96/577 (16.6) | <0.001 |
| Wounds | 36/87 (41.4) | 126/312 (40.4) | 263/577 (45.6) | 0.300 |
| Urine | 13/88 (14.8) | 59/319 (18.5) | 76/576 (13.2) | 0.105 |
| Others | 72/97 (74.2) | 304/382 (79.6) | 457/596 (76.7) | 0.412 |
| Service | ||||
| Outpatient | 27/108 (25.0) | 71/460 (15.4) | 173/672 (25.7) | <0.001 |
| Inpatient | 55/108 (50.9) | 243/460 (52.8) | 337/672 (50.1) | |
| ICUb | 26/108 (24.1) | 146/460 (31.7) | 162/672 (24.1) | |
| Mode of acquisitionc | ||||
| Community | 36/47 (76.6) | 94/189 (49.7) | 260/443 (58.7) | 0.003 |
| Hospital | 11/47 (23.4) | 95/189 (50.3) | 183/443 (41.3) |
CI, confidence interval.
ICU, intensive care unit.
Only for intensive care unit and inpatients with available data.
MRSA was detected in cultures of blood from 83 of 1,252 patients (6.6%). A total of 207 (16.5%) patients had a positive result for their tracheal secretions and 425 (33.9%) had a positive result for their wounds. A total of 148 (11.8%) of the patients had positive urine cultures, and 833 (66.5%) patients had positive cultures for specimens from other sites. There was no association between the location of MRSA detection and the category of the PFGE type (sporadic, local epidemic, and endemic), except for the category of tracheal secretions. Local epidemic strains were more often isolated from tracheal secretions than the other strain types were. The highest diversity of PFGE types was found among the blood cultures isolates (22 PFGE types among 83 isolates); the lowest diversity was found for the “other” specimen types (84 PFGE types among 883 isolates).
Multimodal logistic regression analysis of the 635 cases for which complete data sets were available revealed that age above 60 years and mode of acquisition were independently associated with the acquisition of a sporadic, local epidemic, or endemic strains.
Susceptibility patterns.
The proportion of gentamicin-resistant PFGE types among the newly detected strains was 60.6%, but the proportion showed a steady decrease from 1998 on.
Six strains were resistant to fusidic acid. All isolates tested were sensitive to vancomycin. The highest rate of antibiotic resistance was found among the local epidemic strains; they were resistant to 50% of the antibiotics tested, the lowest rate was found among the endemic strains (resistant to 33% of the antibiotics tested). Sporadic strains were resistant to 45% of the antibiotics tested.
DISCUSSION
Former studies of the epidemiology of MRSA were mostly restricted to shorter periods of time (4, 14). In other studies, isolates were only randomly typed (3, 12, 30) or, following certain criteria (24, 32), only selected isolates were typed. Our comprehensive study, which included all MRSA isolates detected from patients over 12 years at a university hospital, allowed us to create a unique picture of the changes in the local epidemiology. The existence of a predominant clone that accounted for roughly two-thirds of the isolates has been described previously (3, 12). Our data demonstrate that the time needed for a strain to become predominant can be as short as 4 to 5 years.
Although many studies of endemic strains have been performed, only a few have dealt with local epidemic and sporadic strains. We defined sporadic strains as those strains that were isolated in low numbers without an indication of transmission. Using this definition, we found a low percentage of sporadic strain with few variations over time. Whereas this is similar to the findings of Ip et al. (12), Salmenlinna and Vuopio-Varkila (25) found that in Finland 41% of the PFGE types were sporadic. The reasons for these different distributions may include the low incidence of MRSA in Finland, the fact that only a selection of strains was typed, and the fact that different epidemiological definitions were used. Similarly, Abb (1) found higher rates of sporadic MRSA strains, but she also typed selected isolates in small numbers, which may have distorted the results.
Our group of local epidemic strains is the most heterogeneous one and contains strains with very different patterns of distribution over time. They seem to have an advantage over the sporadic strains that enables them to be transmitted among patients, but they still lack the capacity to spread further like endemic strains do.
Wisplinghoff and coworkers (32) described a predominant clone that was present at any given time during their long-term study conducted over 14 years. They also found increasing genetic diversity among the selection of 101 strains that they typed. In Heidelberg the diversity decreased during the 12 years of the present study due to the predominant role of the endemic Rhine-Hessen strain (ST5, New York/Japan clone). It gained this predominant role not only by suppressing the other strains, as described for EMRSA-15 (13), but also by adding to the burden of MRSA infections. It shows an amazing number of 16 subtypes, as has similarly been described for other endemic strains (8, 13, 17).
There have been several explanations for the predominance of strains of some PFGE types.
Blanc et al. (5) suggested that sporadic strains are more often community acquired, whereas hospital-acquired MRSA strains are more often endemic. This theory is supported by the findings of our own study. Nevertheless, the classification “hospital acquired” must be seen critically: first, there is no unanimous definition, and the cutoff time between the patient's admission to the hospital and MRSA detection varies between 24 and 48 h (9, 15). Second, in our institution, patients were not screened on hospital admission during the study period, so colonization could have existed some time before detection and could have led to the wrong classification.
Apart from the mode of acquisition, the age of the patient was independently associated with the kind of MRSA strain. Patients who had acquired an epidemic strain were older than the patients who had acquired sporadic strains or a local epidemic strain. However, the difference in the ages between the patient groups was small, and the relevance of this finding needs to be confirmed in studies with other populations.
For certain strains, a higher affinity for a specific tissue has been described; i.e., Booth et al. (6) found a significant accumulation of certain PFGE types in tracheal secretions and blood cultures. This may be caused by special virulence factors as well as by the higher transmissibilities of certain strains. We found an association of PFGE types with specimens from the tracheobronchial system. However, this was not an independent risk factor and did not allow prediction of whether the strain was sporadic, local epidemic, or endemic.
Antibiotic resistance may be another reason for the predominance of strains. This may be supported by the fact that among the 109 PFGE types tested, the highest rate of antibiotic resistance (50%) was found among the local epidemic strains. Then again, the endemic strains present at the highest frequency were resistant to fewer antibiotics. An explanation could be that the high evolutionary cost of multiresistance lowers the fitness to survive (2).
Other explanations for the inferiority of sporadic strains in establishing themselves within hospitals (27) may be their low levels of resistance to environmental factors (31). Correlations between phenotypic characteristics—like the expression of protein A or coagulase (22), the number of spa repeats and the mucosal adhesion ability (10), or the importance of agr (17, 26, 28)—were discussed; but none of the data could sufficiently explain the predominance of a few strains.
Our data suggest that a new endemic strain is developing out of PFGE type K. Its frequency is not too high yet (8.3% of the isolates tested), but a striking similarity to the epidemiology of the Rhine-Hessen strain (ST5, New York/Japan clone) can be seen. The strain has existed in the environment for a long time; meanwhile, the number of isolations is second to that of the Rhine-Hessen strain (ST5, New York/Japan clone). Its repeated isolation from patients from the same ward within short time periods unquestionably proves its endemic potential. Future typing results may show its success in becoming the new endemic strain in Heidelberg.
Conclusion.
The remarkable increase in the rate of isolation of MRSA strains at the University Hospital of Heidelberg is mainly caused by the dissemination of two endemic strains. The Rhine-Hessen strain (ST5, New York/Japan clone) is so frequent that it is likely to be isolated from two different patients by chance alone. Infection control measures taken in the hospital were sufficient to terminate the transmission of some strains but not of the two endemic strains. The early identification of strains with the potential to become epidemic or even endemic would enable infection control personnel to concentrate their transmission termination measures on patients harboring these strains.
Footnotes
Published ahead of print on 4 October 2006.
REFERENCES
- 1.Abb, J. 2004. Frequency and diversity of molecular epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) isolates from patients of a South West German teaching hospital. J. Hosp. Infect. 56:232-235. [DOI] [PubMed] [Google Scholar]
- 2.Ala'Aldeen, D. 2002. A non-multiresistent community MRSA exposes its genome. Lancet 359:1791-1792. [DOI] [PubMed] [Google Scholar]
- 3.Beretta, A. L. R. Z., P. Trabasso, R. B. Stucchi, and M. L. Moretti. 2004. Use of molecular epidemiology to monitor the nosocomial dissemination of methicillin-resistent Staphylococcus aureus in a university hospital from 1991 to 2001. Braz. J. Med. Biol. Res. 37:1345-1351. [DOI] [PubMed] [Google Scholar]
- 4.Bertrand, X., M. Thouverez, and D. Talon. 2000. Antibiotic susceptibility and genotypic characterization of methicillin-resistant Staphylococcus aureus strains in eastern France. J. Hosp. Infect. 46:280-287. [DOI] [PubMed] [Google Scholar]
- 5.Blanc, D. S., D. Pittet, C. Ruef, A. F. Widmer, K. Mühlemann, et al. 2004. Molecular epidemiology of predominant clones and sporadic strains of methicillin-resistant Staphylococcus aureus in Switzerland and comparison with European epidemic clones. Clin. Microbiol. Infect. 8:419-426. [DOI] [PubMed] [Google Scholar]
- 6.Booth, M. C., L. M. Pence, P. Mahasreshti, M. C. Callegan, and M. S. Gilmore. 2001. Clonal associations among Staphylococcus aureus isolates from various sites of infection. Infect. Immun. 69:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deplano, A., W. Witte, W. van Leeuwen, Y. Brun, and M. J. Struelens. 2000. Clonal dissemination of epidemic methicillin-resistant Staphylococcus aureus in Belgium and neighboring countries. Clin. Microbiol. Infect. 6:239-245. [DOI] [PubMed] [Google Scholar]
- 8.De Sousa, M. A., S. Sanches, M. L. Ferro, M. J. Vaz, Z. Saraiva, et al. 1998. Intercontinental spread of a multidrug-resistant methicillin-resistant Staphylococcus aureus strain. J. Clin. Microbiol. 36:2590-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enright, M. C., N. P. Day, C. E. Davies, S. J. Peacock, and B. G. Spratt. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frenay, H. M., J. P. Theelen, L. M. Schouls, C. M. Vandenbroucke-Grauls, W. van Leeuwen, and F. R. Mooi. 1994. Discrimination of epidemic and nonepidemic methicillin-resistant Staphylococcus aureus strains on the basis of protein A gene polymorphism. J. Clin. Microbiol. 32:846-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmsen, D., H. Claus, W. Witte, J. Rothganger, H. Claus, D. Turnwald, and U. Vogel. 2003. Typing of methicillin-resistant Staphylococcus aureus in a hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41:5442-5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ip, M., D. Lyon, F. Chio, and A. F. Cheng. 2004. A longitudinal analysis of methicillin-resistant Staphylococcus aureus strains in a Hong Kong teaching hospital. Infect. Control Hosp. Epidemiol. 25:126-129. [DOI] [PubMed] [Google Scholar]
- 13.Jonas, D., K. Towner, M. Loerwald, L. Shunburne, T. Schmidt-Wieland, and F. Daschner. 2002. Diversity of Staphylococcus aureus strains isolated from two European regions with different prevalences of methicillin resistance. Eur. J. Clin. Microbiol. Infect. Dis. 21:880-883. [DOI] [PubMed] [Google Scholar]
- 14.Montesinos, I., E. Salido, T. Delgado, M. Cuervo, and A. Sierra. 2002. Epidemiologic genotyping of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis at a university hospital and comparison with antibiotyping and protein A and coagulase gene polymorphisms. J. Clin. Microbiol. 40:2119-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naimi, T., K. H. Le Dell, K. Como-Sabetti, S. M. Borschardt, D. J. Boxurd, et al. 2003. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infections. JAMA 290:2976-2984. [DOI] [PubMed] [Google Scholar]
- 16. National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Papakyriacou, H., D. Vaz, and A. Simor. 2000. Molecular analysis of the accessory gene regulator (agr) locus and balance of virulence factor expression in epidemic methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 181:990-1000. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller, M., R. Hollis, and H. Sader. 1992. PFGE of chromosomal DNA. American Society for Microbiology, Washington, D.C.
- 19.Robert Koch Institut. 2005. Fachtagung der AG Nosokomiale Infektionen am RKI zur Intensivierung der Umsetzung von Präventionsstrategien bei MRSA. Epidemiol. Bull. 5:31-38. [Google Scholar]
- 20.Robert Koch Institut. 2003. Staphylokokken-Infektionen in Deutschland im Jahr 2002. Epidemiol. Bull. 35:277-280. [Google Scholar]
- 21.Robert Koch Institut. 2004. Zur MRSA-Situation in Deutschland im Jahr 2003. Epidemiol. Bull. 42:358-359. [Google Scholar]
- 22.Roberts, J. I., and M. A. Gaston. 1987. Protein A and coagulase expression in epidemic and non-epidemic Staphylococcus aureus. J. Clin. Pathol. 40:837-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson, D. A., and M. C. Enright. 2004. Multilocus sequence typing and the evolution of methicillin-resistant Stapyhlococcus aureus. Clin. Microbiol. Infect. 10:92-97. [DOI] [PubMed] [Google Scholar]
- 24.Robinson, D. A., and M. C. Enright. 2003. Evolutionary models of the emergence of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3926-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salmenlinna, S., and J. Vuopio-Varkila. 2001. Recognition of two groups of methicillin-resistant Staphylococcus aureus strains based on epidemiology, antimicrobial susceptibility, hypervariable-region type, and ribotype in Finland. J. Clin. Microbiol. 39:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shopsin, B., B. Mathema, B. Alcabes, B. Said-Salim, G. Lina, A. Matsuka, J. Martinez, and B. N. Kreiswirth. 2003. Prevalence of agr specificity group among Staphylococcus aureus strains colonizing children and their guardians. J. Clin. Microbiol. 41:456-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sola, C., G. Gribaudo, A. Vindel, L. Patrito, J. L. Bocco, and the Cordoba MRSA Collaborative Study Group. 2002. Identification of a novel methicillin-resistant Staphylococcus aureus epidemic clone in Córdoba, Argentina, involved in nosocomial infections. J. Clin. Microbiol. 40:1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strommenger, B., C. Cuny, G. Werner, and W. Witte. 2004. Obvious lack of association between dynamics of epidemic methicillin-resistant Staphylococcus aureus in central Europe and agr specificity group. Eur. J. Clin. Microbiol. Infect. Dis. 23:15-19. [DOI] [PubMed] [Google Scholar]
- 29.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1985. Interpreting chromosomal DNA restriction pattern produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voss, A., D. Milatovic, C. Wallrauch-Schwarz, V. T. Rosdahl, and I. Braveny. 1994. Methicillin-resistant Staphylococcus aureus in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 13:50-55. [DOI] [PubMed] [Google Scholar]
- 31.Wagenvoort, J. H., W. Sluijsmans, and R. J. Penders. 2002. Better environmental survival of outbreak vs. sporadic MRSA isolates. J. Hosp. Infect. 45:231-234. [DOI] [PubMed] [Google Scholar]
- 32.Wisplinghoff, H., B. Ewertz, S. Wisplinghoff, D. Stefanik, G. Plum, F. Perdreau-Remington, and H. Seifert. 2005. Molecular evolution of methicillin-resistant Staphylococcus aureus in the metropolitan area of Cologne, Germany, from 1984 to 1998. J. Clin. Microbiol. 43:5445-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witte, W., C. Braulke, C. Cuny, B. Strommenger, G. Werner, et al. 2005. Emergence of methicillin-resistant Staphylococcus aureus with Panton-Valentine leukocidin genes in central Europe. Eur. J. Clin. Microbiol. Infect. Dis. 24:1-5. [DOI] [PubMed] [Google Scholar]