Abstract
Recently, three distinct genotypes of clinical herpes simplex virus type 1 (HSV-1) isolates were identified based on DNA sequence information and phylogenetic analysis of clinical isolates and laboratory strains. We utilized single-nucleotide polymorphism within the genes coding for glycoproteins G and I for rapid genotype classification by PCR and restriction enzyme cleavage. The method is suitable for high-scale genotyping of clinical HSV-1 isolates and for the detection of recombinants.
Herpes simplex virus type 1 (HSV-1) is a DNA virus that belongs to the Herpesviridae family and is one of eight herpesviruses that infect humans (9). HSV-1 causes lifelong infections and establishes latency in the sensory ganglia. Although the most common clinical symptoms are oral or genital lesions, more severe symptoms, such as keratitis, neonatal infection, facial palsy, and encephalitis, may occur (3, 5, 8, 14). Little information is available whether these conditions are results of genetic makeup of the host and/or of the virus or a result of random events.
The HSV-1 genome consists of a 152-kb linear duplex DNA molecule, and characterization of genetic variability of clinical HSV-1 isolates has become an area of growing interest. Restriction fragment length polymorphism analyses have been used earlier in an attempt to classify HSV-1 into different genotypes (10, 12, 13). However, these methods are not based on HSV-1 DNA sequencing data and phylogenetic relationships. Recently, three genotypes, clearly separated in phylogenetic trees, were described for clinical HSV-1 isolates (7). The genotypes were arbitrarily designated A, B, and C, and the classification was based on DNA sequencing of the US4, US7, and US8 genes coding for the glycoproteins G (gG), I (gI), and E (gE), all localized in the unique short region of the genome. In light of this newly described genetic diversity of HSV-1, the search for associations between specific viral genetic markers and clinical symptoms is of interest. Although DNA sequencing is the most accurate method for genotyping, the technique is time-consuming in the high-scale format. The aim of the present study was to develop a rapid and accurate method for genotyping of clinical HSV-1 isolates.
MATERIALS AND METHODS
The genotyping strategy was based on DNA sequence information presented recently on the gG and gI genes of 28 clinical HSV-1 isolates and the laboratory strains F, 17, and KOS321 (7). For the gG gene, more than 180 additional sequences were available from the GenBank and included in the selection of primers and restriction enzymes.
Viral DNA was prepared by using QIAmp blood kit (QIAGEN) as described recently (7). PCR primers were designed to target highly conserved regions within each gene amplifying regions containing single-nucleotide point mutations specific for each genotype. The selected PCR primers are listed in Table 1. The PCR for US4 and US7 started with denaturation for 5 min at 96°C, followed by 40 cycles as follows: denaturation for 45 s at 95°C, annealing for 45 s at 58°C, and elongation for 45 s at 72°C, with a 3-s extension per cycle. The amplicons were cleaved by restriction enzymes according to the manufacturer's recommendation (90 min at 37°C) and separated at 80 V for 2 h on a 3% Metaphor agarose gel (Cambrex). Restriction enzymes and recognition sequences are summarized in Table 1. To simplify the protocol for cleavage of the PCR products, the enzymes were selected for simultaneous use in the same buffer and temperature.
TABLE 1.
PCR primers and recognition sites for the restriction enzymes
| Gene or restriction enzyme | Primer orientation or genotype | Sequencea |
|---|---|---|
| Gene | ||
| gG | Forward | 5′-GACTCTCCCACCGCCATCAG-3′ |
| Reverse | 5′-TGTCTTCGGGCGACTGGTCT-3′ | |
| gI | Forward | 5′-CCTGCTTATTCTCGGGGAGCTTC-3′ |
| Reverse | 5′-AGCAGTTTCGGGTCGCAGGA-3′ | |
| Enzyme | ||
| PflMI | A | CCAAGTATCGG |
| B | CCAAGTATCGG | |
| C | CCAAGTATTGG | |
| DdeI | A | CCGGG |
| B | CTGAG | |
| C | CTGAG | |
| SacI | A | GAGCTG |
| B | GAGCTG | |
| C | GAGCTC | |
| PleI | A | TACTC |
| B | GACTC | |
| C | GACTC |
The sequence recognized by each enzyme is underlined.
RESULTS
Genotyping patterns.
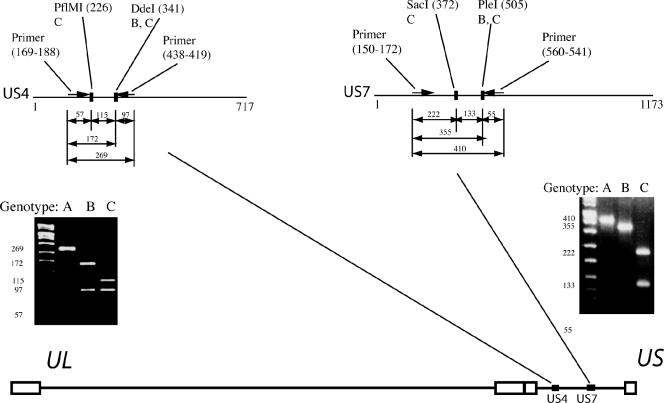
Primers amplifying the gG gene generated a 269-nucleotide (nt) PCR product, which was cleaved by the enzymes PflMI and DdeI (New England Biolabs). For isolates belonging to genotype A the amplicon was not cleaved, for isolates belonging to genotype B two fragments were generated (97 and 172 nt), and for isolates belonging to genotype C the amplicon was cleaved into three fragments (57, 97, and 115 nt). The classification of genotypes was based on the size of the two largest fragments since the smallest fragment (57 nt) was only visible as a faint band. The restriction enzymes SacI and PleI were used for cleavage of a 410-nt amplicon of the gI gene. Isolates belonging to the genotype A were not cleaved by either enzyme. Isolates belonging to genotype B were cleaved into two fragments (55 and 355 nt), and isolates belonging to genotype C were cleaved into three fragments (55, 133, and 222 nt), where the classification was based on the two longer fragments (Fig. 1).
FIG. 1.
Genotyping of HSV-1 based on the US4 and the US7 genes localized in the unique short (US) segment of the genome using restriction enzymes. Nucleotide positions are within parenthesis and refer to HSV-1 strain 17. The genotypes cleaved by each restriction enzyme are depicted below the respective enzyme. The cleavage products were separated on a 3% Metaphor agarose gel. UL, unique long segment.
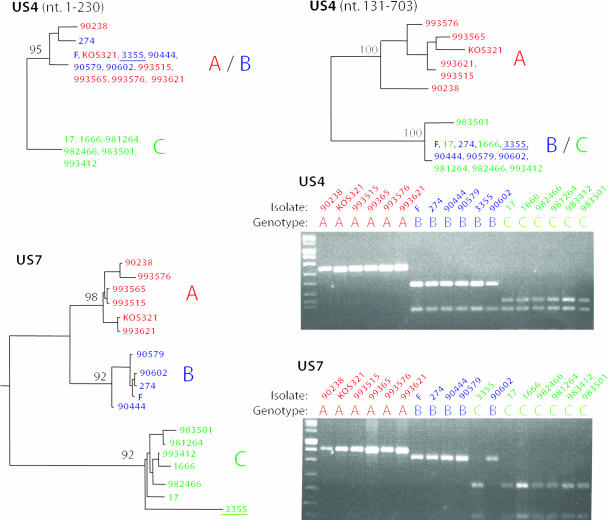
For the 28 clinical isolates and the three laboratory strains the following classification of genotypes was achieved. Based on the genotyping target in the gG gene, 8 isolates were classified as genotype A, 15 isolates were classified as genotype B, and 8 isolates were classified as genotype C. Based on the gI gene, 9 isolates were classified as genotype A, 12 isolates were classified as genotype B, and 10 isolates were classified as genotype C. Seven of the clinical isolates presented different genotype identities in the two genotyping targets and were therefore classified as recombinants. Two isolates belonged to genotype B in the gG gene and to genotype C in the gI gene, three isolates were classified into genotype B in the gG gene and switched to genotype C in the gI gene, and finally two isolates belonged to genotype A in the gG gene and to genotype B in the gI gene. The classification of these recombinants corresponded to results from HSV-1 DNA sequence analyses (7). Hence, recombinants with at least one recombination point located between the two genotyping targets and that belong to different genotypes in the gG and gI genes can be detected by these typing systems. Phylogenetic trees based on complete gene sequences, as well as the cleavage pattern for 15 of the clinical isolates and the three laboratory strains, are presented in Fig. 2.
FIG. 2.
Phylogenetic trees based on US4 and US7 genes, as well as cleavage patterns for the two genotyping targets for 15 clinical HSV-1 isolates and the three laboratory strains 17, F, and KOS321. The trees were calculated by using the maximum-likelihood method applied to 100 bootstrap replicates, and bootstrap values greater than 90 are shown. The US4 gene was analyzed in two segments due to a suggested ancient recombination event (7). The different genotypes—A, B, and C—are marked in red, blue, and green, respectively. One of the isolates (no. 3355) was detected as a recombinant and is underlined in the phylogenetic trees.
Performance of the PCR typing systems.
Recently, Namvar et al. (6) described a real-time TaqMan PCR system for the detection of HSV-1 based on a type-specific segment in the gB-1 gene. A plasmid (pUC57) containing the target sequence was constructed (GenScript) and amplified in Escherichia coli XL1-Blue, purified by HiSpeed Plasmid Maxi Kit (QIAGEN) and quantified by spectrophotometer analysis. Plasmid DNA and HSV-1 DNA from clinical isolates belonging to the genotypes A, B, and C were extracted in a MagnaPure LC robot (Roche) and amplified in a real-time PCR instrument ABI 7900 (Applied Biosystems). From the generated standard curve, the detection limits for the typing systems were calculated. To achieve clearly visible fragments in the gel, the sensitivity varied between different isolates in the range of 500 to 5,000 HSV-1 genome copies for both the gG and the gI systems.
The primers were selected not to amplify the homologous gG and gI gene sequences of HSV-2. Accordingly, five clinical HSV-2 isolates were negative in the PCRs (data not shown). The capacity of the method is highly dependent on the facilities of the laboratory. We easily processed 50 clinical HSV-1 isolates per day manually. By automation using robots, several hundreds of isolates can be processed simultaneously. Although DNA sequencing of the isolates can also be automated, the method described here is less expensive and faster since sequence analysis and the identification of informative sites specific for each genotype are not necessary.
DISCUSSION
We present here a rapid and accurate method for genotyping clinical HSV-1 isolates into three genotypes. The method may be a useful screening tool in searching for associations between genotype identity and a wide range of manifestations of HSV-1 infection such as clinical symptoms, immune responses of the host, the transmission of virus, virulence, and tropism. Furthermore, the method might also be of interest for epidemiological studies from different geographical regions to determine the genotype identity for a large number of clinical isolates. The gE and gI proteins have been shown to form a complex that is involved in cell-to-cell spread (2) and the binding of immunoglobulin G via the Fc receptor (4). The gG protein has been described as important for virus entry through the apical surfaces of polarized cells (11). The method can be used to study possible associations between genotype identity and the functions of the gG and gI proteins.
Although the typing method used here is based only on 31 HSV-1 gI gene sequences (7), we have since used the method for more than 200 clinical HSV-1 isolates. For a single isolate an atypical cleavage pattern was recognized. This isolate contained a point mutation T513→C (the nucleotide position refers to strain 17) within the restriction enzyme site (PleI) in the gI gene. This rare mutation created a typical PCR fragment of 222 nt that correctly classified the isolate to genotype C and an atypical uncleaved fragment of 188 nt instead of two fragments containing 55 and 133 nt (see Fig. 1). The genotyping system of the gG-1 gene is based on more than 180 HSV-1 sequences derived from Western and East Asia countries, and no ambiguous cleavage patterns were identified for the additional HSV-1 isolates examined. We and others have shown that homologous recombination is common in the HSV-1 genome (1, 7). Although we found that 7 of 28 clinical HSV-1 isolates were recombinants using a relatively short distance between the genotyping targets, it is possible that more recombinants could be detected if a longer distance between the genotyping targets is selected. Hence, based on phylogenetic and recombination analysis of other parts of the genome, e.g., the unique long segment, the use of such additional targets might be of interest.
Acknowledgments
This study was supported by grants from The Swedish Society of Medicine, the LUA Foundation at Sahlgrenska University Hospital, and The Swedish Research Council (grant 11225).
Footnotes
Published ahead of print on 11 October 2006.
REFERENCES
- 1.Bowden, R., H. Sakaoka, P. Donnelly, and R. Ward. 2004. High recombination rate in herpes simplex virus type 1 natural populations suggests significant coinfection. Infect. Genet. Evol. 4:115-123. [DOI] [PubMed] [Google Scholar]
- 2.Dingwell, K. S., L. C. Doering, and D. C. Johnson. 1995. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 69:7087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuta, Y., S. Fukuda, E. Chida, T. Takasu, F. Ohtani, Y. Inuyama, and K. Nagashima. 1998. Reactivation of herpes simplex virus type 1 in patients with Bell's palsy. J. Med. Virol. 54:162-166. [DOI] [PubMed] [Google Scholar]
- 4.Johnson, D. C., M. C. Frame, M. W. Ligas, A. M. Cross, and N. D. Stow. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimberlin, D. W., and R. J. Whitley. 2005. Neonatal herpes: what have we learned. Semin. Pediatr. Infect. Dis. 16:7-16. [DOI] [PubMed] [Google Scholar]
- 6.Namvar, L., S. Olofsson, T. Bergström, and M. Lind. 2005. Detection and typing of herpes simplex virus (HSV) in mucocutaneous samples by TaqMan PCR targeting a gB segment homologous for types 1 and 2. J. Clin. Microbiol. 43:2058-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norberg, P., T. Bergström, E. Rekabdar, M. Lindh, and J.-A. Liljeqvist. 2004. Phylogenetic analysis of clinical herpes simplex virus type 1 isolates identified three genetic groups and recombinant viruses. J. Virol. 78:10755-10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remeijer, L., A. Osterhaus, and G. Verjans. 2004. Human herpes simplex virus keratitis: the pathogenesis revisited. Ocul. Immunol. Inflamm. 12:255-285. [DOI] [PubMed] [Google Scholar]
- 9.Roizman, B. 1996. Herpesviridae, p. 2221-2230. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Virology. Lippincott-Raven, Philadelphia, Pa.
- 10.Sakaoka, H., K. Kurita, Y. Iida, S. Takada, K. Umene, Y. T. Kim, C. S. Ren, and A. J. Nahmias. 1994. Quantitative analysis of genomic polymorphism of herpes simplex virus type 1 strains from six countries: studies of molecular evolution and molecular epidemiology of the virus. J. Gen. Virol. 75:513-527. [DOI] [PubMed] [Google Scholar]
- 11.Tran, L. C., J. M. Kissner, L. E. Westerman, and A. E. Sears. 2000. A herpes simplex virus 1 recombinant lacking the glycoprotein G coding sequences is defective in entry through apical surfaces of polarized epithelial cells in culture and in vivo. Proc. Natl. Acad. Sci. USA 97:1818-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umene, K., T. Eto, R. Mori, Y. Takagi, and L. W. Enquist. 1984. Herpes simplex virus type 1 restriction fragment polymorphism determined using southern hybridization. Arch. Virol. 80:275-290. [DOI] [PubMed] [Google Scholar]
- 13.Umene, K., T. Inoue, Y. Inoue, and Y. Shimomura. 2003. Genotyping of herpes simplex virus type 1 strains isolated from ocular materials of patients with herpetic keratitis. J. Med. Virol. 71:75-81. [DOI] [PubMed] [Google Scholar]
- 14.Whitley, R. 2004. Neonatal herpes simplex virus infection. Curr. Opin. Infect. Dis. 17:243-246. [DOI] [PubMed] [Google Scholar]